Palmitoylated SCP1 is targeted to the plasma membrane and negatively regulates angiogenesis
Figures
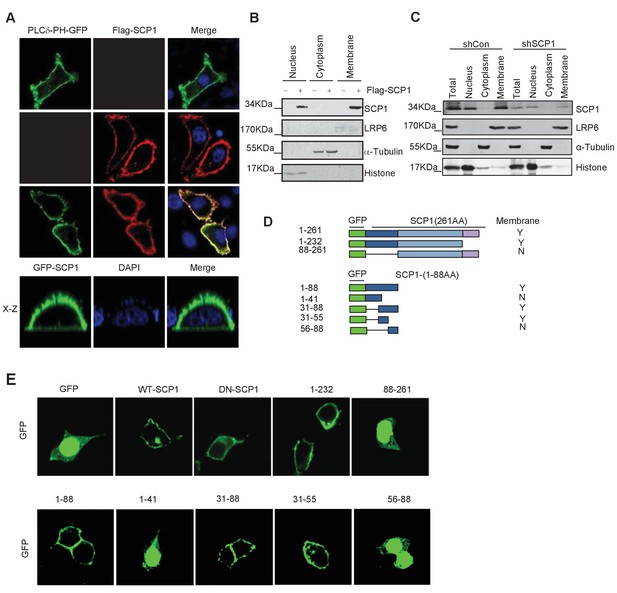
Membrane localization of SCP1
(A) SCP1 was co-localized with PLCδ-PH on the cell membrane. FLAG-SCP1 was transfected with or without PLCδ-PH-GFP in HeLa. The subcellular localization of SCP1 and PLCδ-PH-GFP was analyzed using immunofluorescence assay, and both the horizontal section (X–Y) and vertical section (X–Z) were photographed. (B) and (C) Subcellular localization of SCP1 in cells. HEK-293T cells were transfected and the subcellular localizations of transfected SCP1 (B) or endogenous SCP1 (C) were analyzed using western blotting. (D) Cartoon of different deletion mutations of SCP1. Yes (Y) and no (N) represent SCP1 or truncated mutant membrane localizations, respectively. (E) HeLa cells were transfected with GFP-SCP1 or its mutants for 24 h and then analyzed for their subcellular localization using immunofluorescence assays.
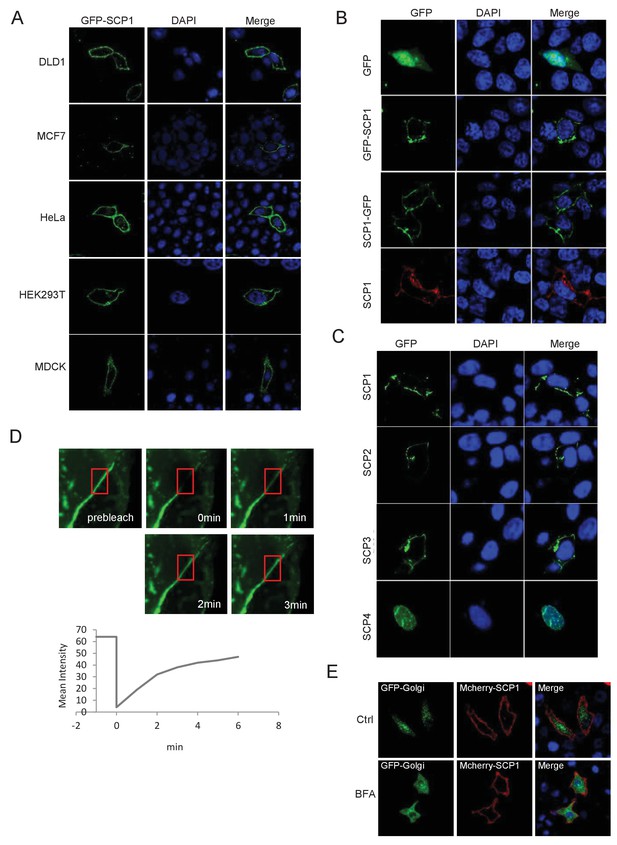
SCP1 is membrane localized.
(A) SCP1 was localized on the cell membrane in various cells. SCP1 was expressed in DLD1, MCF7, HeLa, HEK293T, or MCDK cells and the subcellular localization of SCP1 was analyzed using immunofluorescence assay. (B) HeLa cells were transfected and the subcellular localization of SCP1 was analyzed using immunofluorescence assay. (C) SCP1, SCP2, and SCP3, but not SCP4, were localized on the plasma membrane. HeLa cells were transfected with GFP-SCP1/SCP2/SCP3/SCP4, respectively. (D) Membrane-localized SCP1 was photobleached (as showed in the red frame), and the fluorescence was recovered for 3 min as indicated. (E) The SCP1 membrane distribution was not affected by the disruption of the Golgi. HeLa cells were transfected with GFP-Golgi and mCherry-SCP1, respectively,and treated with DMSO or brefeldin A (BFA; 5 μg/ml) for 6 h.
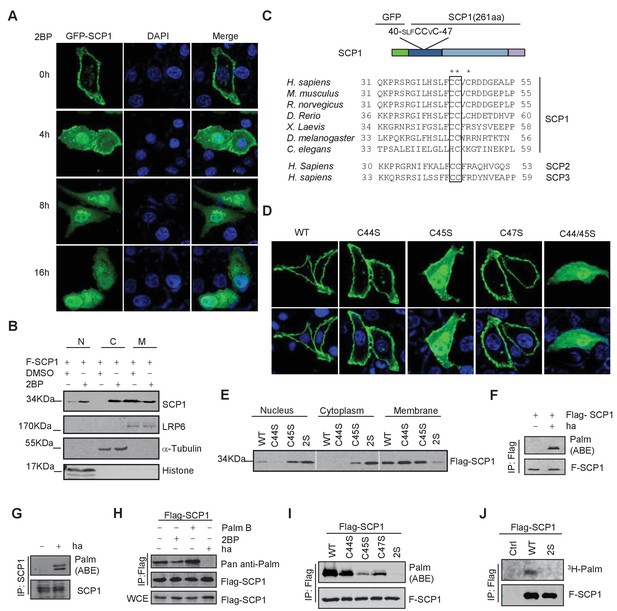
SCP1 was palmitoylated
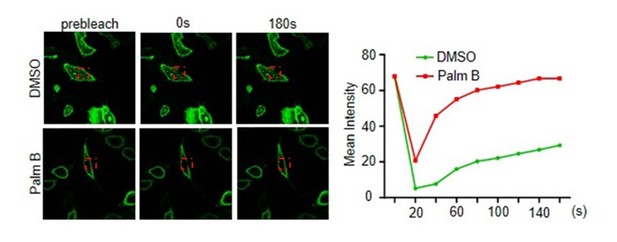
(A) Palmitoylation inhibitor 2-bromopalmitate (2BP) blocked the SCP1 membrane localization. HeLa cells were transfected and treated with 2BP (10 μM) for 4, 8, or 16 h or DMSO as a control. The subcellular localization of SCP1 was detected using immunofluorescence assay. (B) HEK293T cells were treated with DMSO or 2BP (10 μM) for 6 h and the subcellular location of SCP1 was detected using western blotting. (C) Potential palmitolylation sites Cys44 and Cys45 of SCP1 were evolutionarily conserved. Amino acids 33–55 of SCP1 in different species, ranging from Caenorhabditis elegans to Homo sapiens, 30–53 of SCP2 in H. sapiens, and 33–59 of SCP3 in H. sapiens are shown. (D) HeLa cells were transfected with WT-SCP1, C44S-SCP1, C45S-SCP1, C47S-SCP1, and C44/45S(2S)-SCP1 for 24 h. The subcellular localizations of WT-SCP1 and its mutants were detected using immunofluorescence assay. (E) HEK293T cells were transfected with WT-SCP1, C44S-SCP1, C45S-SCP1, and C44/45S(2S)-SCP1 for 24 h and cell fractions of were analyzed using western blotting. (F) FLAG-SCP1 was expressed in HEK293T cells, immunoprecipitated, and palmitoylation was detected using the acyl–biotin exchange (ABE) assay. (G) Palmitoylation of endogenous SCP1 in HEK293T cells was detected using the ABE assay. (H) FLAG-SCP1 was expressed in HEK293T cells for 24 h and treated with 2BP (10 μM) or palmostatin B (50 μM) for 12 h. Palmitoylation of SCP1 was detected using pan-palmitoylation antibody. (I) and (J) Identification of palmitoylation sites using the ABE assay (I) and the [3H] palmitate incorporation assay (J).
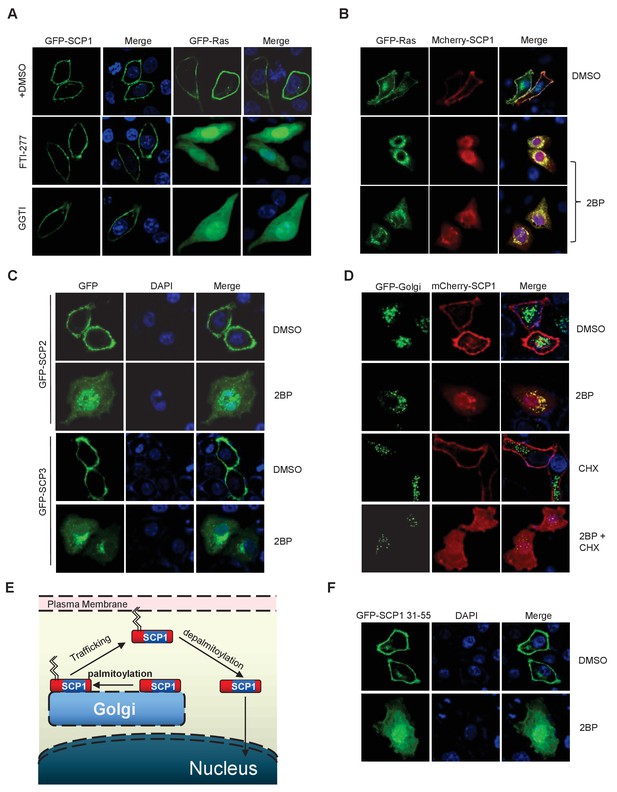
SCP1 membrane localization depends on its palmitoylation.
(A) The membrane localization of SCP1 was not affected by farnesyltransferase or prenyltransferase inhibitor. The transfected HeLa cells were treated with DMSO, FTI-277(10 μM), or GGTI (15 μM) for 8 h. (B) The membrane localization of SCP1 was blocked by palmitoyltransferase inhibitor. GFP-Ras and/or GFP-SCP1 were co-expressed in HeLa cells for 24 h. The transfected cells were treated with DMSO or 2BP (10 μM) for 8 h. (C) The membrane localizations of SCP2 and SCP3 were blocked by palmitoyltransferase inhibitor. HeLa cells were transfected with GFP-SCP2/SCP3 for 24 h. (D) The newly synthesized SCP1 was transported to the Golgi without palmitoylation and then translocated to the plasma membrane by palmitoylation. HeLa cells were transfected with GFP-Golgi and mCherry-SCP1 for 24 h and treated with 2BP (10 μM) or cycloheximide (15 μg/ml) for 8 h. (E) The working model for SCP1 palmitoylation and cell membrane location is shown. (F) Amino acid residues from 31 to 55 are important for SCP1 palmitoylation and cell membrane localization. HeLa cells were transfected with the truncated mutant of GFP-SCP1 31–55 for 24 h and treated with DMSO or 2BP (10 μM) for 8 h.
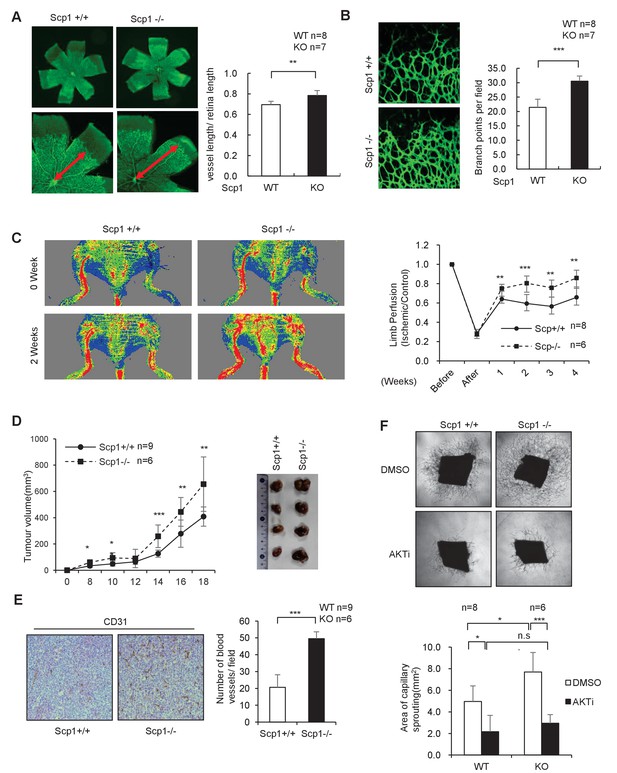
SCP1 knockout promoted angiogenesis
(A) SCP1 deletion impaired the development of retinal angiogenesis. Retinas of postnatal day 5 were isolated from littermates of wild-type (WT; n = 8) or SCP1-knockout (KO) mice (n = 7) and stained with Isolectin B4. Quantification of vessel length was measured, and the rate of vessel length/retina length was calculated. **p<0.01. (B) Loss of SCP1 reduced the branching of the vessels. The branching of vessels was counted. ***p<0.001. (C) SCP1 deficiency promoted the recovery of hind limb ischemia. The laser Doppler blood flowmetry ratio was significantly higher in SCP1-KO mice (n = 6) than in WT mice (n = 8). ***p<0.001, **p<0.01. (D) SCP1 KO promoted Lewis lung carcinoma cell (LLC) tumor bearing in C57 mouse. 2 × 105 LLC cells were injected into SCP1-WT or -knockdown littermates. The diameter of the tumor was measured every 2 days, and the volume of the tumor was calculated. ***p<0.001, **p<0.01, *p<0.05. (E) Angiogenesis was promoted in SCP1-KO mice. The angiogenesis in tumors was analyzed using immunohistochemistry by CD31 staining. (F) SCP1 deficiency promoted angiogenesis in an AKT-dependent manner. Segments (1 mm in length) of the aorta from SCP1-WT (n = 8) or SCP1-KO (n = 6) mice were embedded in Matrigel and treated with DMSO or AKT inhibitor (MK2206, 2 nM) for 6 days. Sprouting was observed and photographed by microscopy. The vascular area of each group was measured using Image J. ***p<0.001, **p<0.01, *p<0.05.
-
Figure 3—source data 1
SCP1 knockout promoted angiogenesis
(A) Retinas of postnatal day 5 mice were isolated from littermates of wild-type (n = 8) or SCP1-knockout mice (n = 7) and stained with Isolectin B4. Quantification of vessel length was obtained, and the ratio of vessel length/-retina length was calculated. (B) The branching of vessels was counted. (C) The laser Doppler blood flowmetry ratio was significantly higher in SCP1-knockout mice (n = 6) than in wild-type mice (n = 8). (D) 2 × 105 Lewis lung carcinoma cells were injected into SCP1-wild-type or -knockdown littermates. The diameter of the tumor was measured every 2 days, and the volume of the tumor was calculated. (E) The angiogenesis in tumors was analyzed using immunohistochemistry by CD31 staining. (F) Segments (1 mm in length) of the aorta from SCP1-wild-type (n = 8) or -knockout (n = 6) mice were embedded in Matrigel and treated with DMSO or AKT inhibitor (MK2206, 2 nM) for 6 days. Sprouting was observed and photographed by microscopy. The vascular area of each group was measured using Image J.
- https://doi.org/10.7554/eLife.22058.008
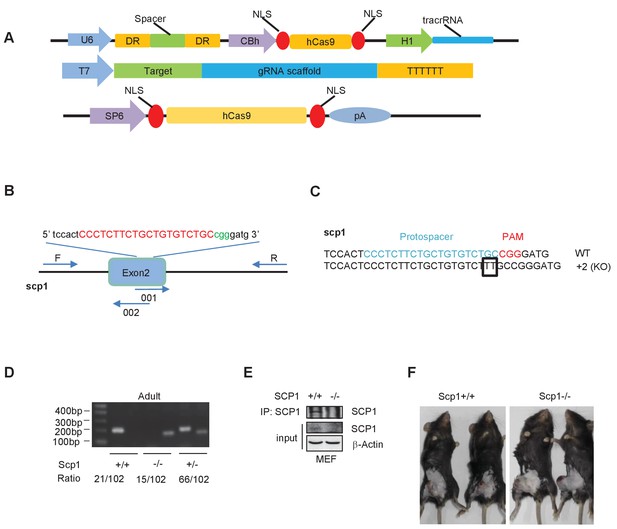
Generation and validation of SCP1-knockout mice.
(A) Constructs of the Cas 9/RNA system: DR, direct repeat to separate signal; NLS, nuclear localization signal. (B) Schematic overview of the strategy used to generate the SCP1-knockout mice. The sgRNA coding sequence is underlined, capitalized, and labeled in red. The protospacer-adjacent motif (PAM) sequence is labeled in green. (C) The DNA sequences of the Ctdsp1 genomic loci in the founders. (D) The genotyping of Ctdsp1+/+, Ctdsp1+/–, and Ctdsp1–/– mice. The ratio of Ctdsp1+/– offspring is listed. (E) SCP1 was analyzed by IP (Immunoprecipitation) and western blotting. (F) The Lewis lung carcinoma celltumor-bearing SCP1 wild-type and -knockout mice in Figure 3D are photographed.
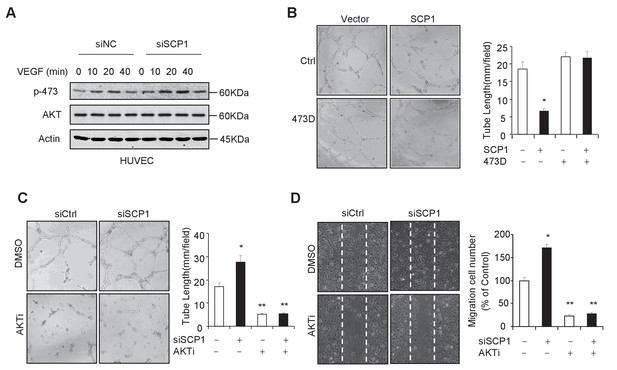
SCP1 inhibited AKT-mediated angiogenesis
(A) SCP1 deletion promoted VEGF-induced AKT activation in HUVECs. HUVECs were transfected with siNC (Small Interfering RNA for Normal Control) or siSCP1 (Small Interfering RNA for SCP1) for 72 h and stimulated with VEGF (100 ng/ml) as indicated after starvation for 8 h. (B) SCP1 impaired HUVEC tube formation through AKT. HUVECs were overexpressed with SCP1 with or without AKT-S473D. The cells were placed in plates coated with Matrigel and tubular structures were photographed after 6 h. The tube lengths were measured in each field. *p<0.05. (C) SCP1 depletion inhibited the tube formation of HUVECs through AKT. HUVECs were transfected with siSCP1 and treated with or without AKT inhibitor (AKTi; MK2206, 2 nM) for 5 days as indicated. The tube lengths were measured in each field. **p<0.01, *p<0.05. (D) SCP1 deletion promoted HUVEC migration through AKT. Cell migration was detected using a wound healing assay. HUVECs were transfected and treated with or without AKTi (MK2206, 2 nM). The migration cell number in each field was calculated. **p<0.01, *p<0.05.
-
Figure 4—source data 1
SCP1 inhibited AKT-mediated angiogenesis
(A) HUVECs were overexpressed with SCP1 with or without AKT-S473D. The cells were placed in plates coated with Matrigel and tubular structures were photographed after 6 h. The tube lengths were measured in each field. (B) HUVECs were transfected with siSCP1 and treated with or without AKT inhibitor (AKTi; MK2206, 2 nM) for 5 days as indicated. The tube lengths were measured in each field. (C) Cell migration was detected using a wound healing assay. HUVECs were transfected and treated with or without AKTi (MK2206, 2 nM). The migration cell number in each field was calculated.
- https://doi.org/10.7554/eLife.22058.011
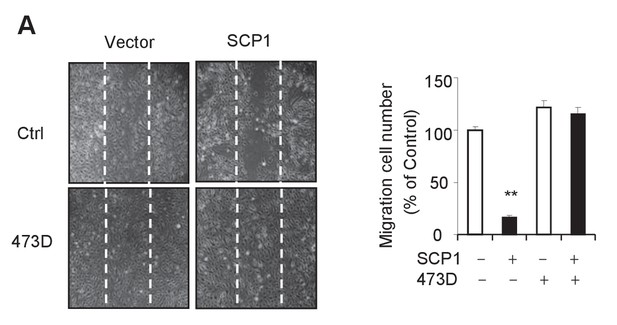
SCP1 inhibits HUVEC migration.
(A) SCP1 inhibited HUVEC migration. Cell migration was detected using a wound healing assay. Values represent mean ± SD (n = 3). **p<0.01, *p<0.05.
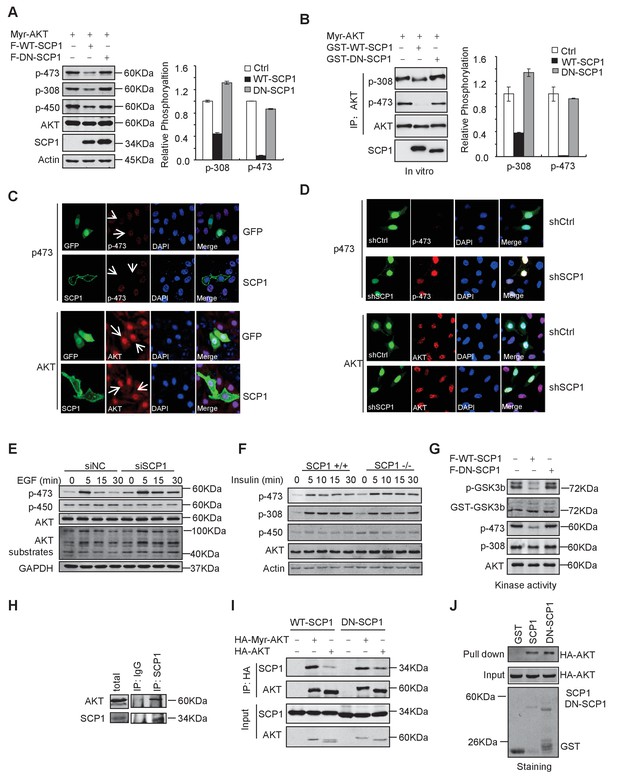
SCP1 dephosphorylated AKT
(A) Wild-type (WT)-SCP1 dephosphorylated AKT Ser473. Myr-AKT was co-expressed with vector, WT-SCP1, or DN-SCP1. The phosphorylations of p-Ser473-AKT, p-Thr308-AKT, and p-Thr450-AKT was analyzed using western blotting. The relative phosphorylations of p-Thr308-AKT and p-Thr450-AKT are displayed in the form of a histogram. (B) WT-SCP1 dephosphorylated AKT in vitro. HA-Myr-AKT was immunoprecipitated from HEK293T cells and incubated with purified GST, GST-WT-SCP1, or GST-DN-SCP1 for 30 min. The phosphorylations of p-Ser473-AKT and p-Thr308-AKT were analyzed using western blotting. (C) WT-SCP1 dephosphorylated AKT in HeLa cells. HeLa cells were transfected with GFP-SCP1 for 24 h. The phosphorylation of p-Ser473-AKT and total AKT was detected using immunofluorescence assay. (D) SCP1 knockdown promoted AKT Ser473 phosphorylation in HeLa cells. Control or Ctdsp1 shRNA was transfected into HeLa cells for 72 h. The phosphorylation of p-Ser473-AKT and total AKT was detected using immunofluorescence assay. (E) SCP1 knockdown promoted EGF-induced AKT activity. H1299 cells were transfected with control or Ctdsp1 siRNA for 72 h. The cells were stimulated with EGF (100 ng/ml) as indicated after 8 h of starvation, and phosphorylation of AKT was detected using immunofluorescence assay. (F) SCP1 depletion promoted insulin-stimulated AKT activation. Ctdsp1+/+ or Ctdsp1–/–MEFs (mouse embryonic fibroblast) were stimulated with insulin (1 mM) as indicated after 6 h of starvation. (G) WT-SCP1 decreased the AKT kinase activity. AKT was transfected into HEK293T cells with vector, WT-SCP1, or DN-SCP1, immunoprecipitated, and incubated with GST-GSK3β. The phosphorylation of GSK3β was measured using western blotting. (H) Endogenous AKT interacted with endogenous SCP1. Endogenous SCP1 was immunoprecipitated using an anti-SCP1 antibody and the associated AKT was detected using an anti-AKT antibody. (I) The interaction of SCP1 with WT or myristoylated AKT1 is independent of its phosphatase activity. (J) Purified GST, GST-WT-SCP1, and GST-DN-SCP1 were incubated with cell lysates overexpressing AKT. The interaction was detected using western blotting.
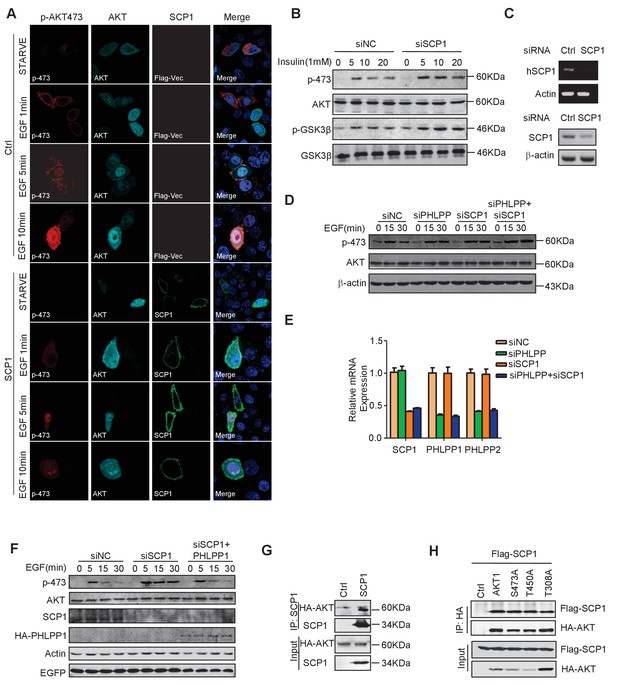
SCP1 negatively regulates AKT activation on membrane.
(A) SCP1 overexpression strongly suppressed EGF-induced AKT membrane activation. (B) SCP1 knockdown promoted insulin-stimulated AKT activation in H1299 cells. H1299 cells were transfected with control or Ctdsp1 siRNA for 72 h. The cells were stimulated with insulin (1 mM) as indicated after 6 h of starvation, and phosphorylation of AKT was detected using western blotting. (C) The knockdown efficiency of SCP1 was measured at the mRNA or protein level. (D) SCP1 or PHLPP knockdown promoted EGF-stimulated AKT activation in MEF cells. MEF cells were transfected with control or Ctdsp1 siRNA or PHLPP siRNA or Ctdsp1/PHLPP siRNA for 72 h. The cells were stimulated with EGF (100 ng/ml) as indicated after 8 h of starvation, and phosphorylation of AKT was detected using western blotting. (E) The knockdown efficiencies of SCP1 and PHLPP were measured at the mRNA level. (F) PHLPP rescued the EGF-stimulated AKT activation after SCP1 knockdown in HeLa cells. (G) SCP1 interacted with endogenous AKT. (H) SCP1 interacted with wild-type AKT and its mutants.
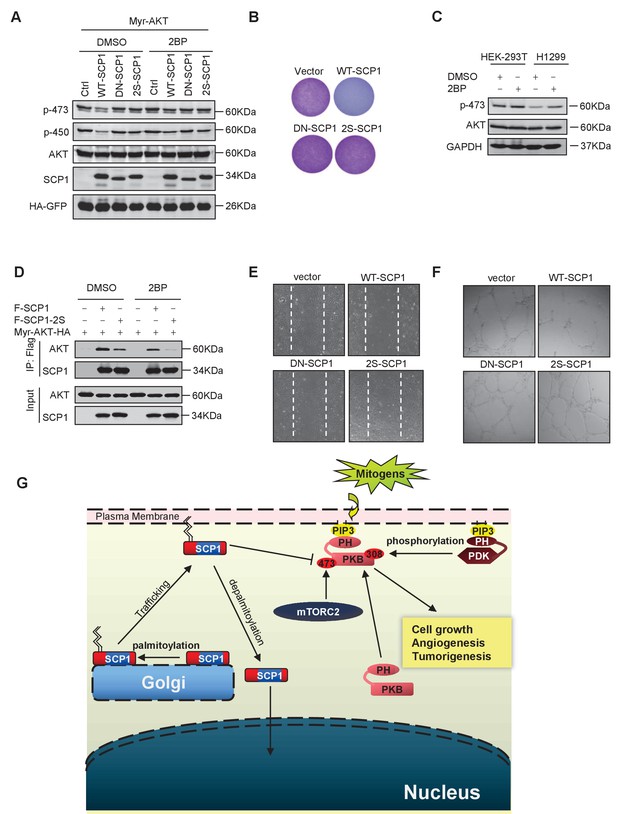
Palmitoylation was required for SCP1-mediated AKT inhibition
(A) Palmitoylation was required for SCP1 to dephosphrylate AKT Ser473. HEK293T cells were transfected with WT-SCP1, DN-SCP1, and 2S-SCP1 for 24 h and treated with DMSO or 2-bromopalmitate (2BP; 10 μM) for 6 h. The phosphorylations of p-Ser473-AKT and p-Thr450-AKT were detected using western blotting. (B) Palmitoylation of SCP1 at C44 and C45 was required for its suppression of cell proliferation. Values represent mean ± SD (n = 3) (C) Palmitoylation inhibition increased the phosphorylation levels of endogenous AKT Ser473. HEK293T cells and H1299 cells were treated with DMSO or 2BP (10 μM) for 6 h. The phosphorylation of p-Ser473-AKT was detected using western blotting. (D) Depalmitoylation blocked the interaction between SCP1 and AKT. HEK293T cells were transfected with Myr-AKT-HA and FLAG-SCP1 or FLAG-SCP1-2S for 24 h and treated with DMSO or 2BP (10 μM) for 6 h. The interaction between AKT and SCP1 or SCP1-2S was detected using western blotting. (E) SCP1 blocked HUVEC migration in a palmitoylation-dependent manner. HUVECs were transfected with vector, WT-SCP1, DN-SCP1, and 2S-SCP1, respectively. Cell migration was detected using a wound healing assay. (F) SCP1 blocked HUVEC tube formation in a palmitoylation-dependent manner. HUVECs were transfected with vector, WT-SCP1, DN-SCP1, and 2S-SCP1, respectively. Tube formation was detected using a tube formation assay. (G) The working model for SCP1 palmitoylation and cell membrane localization is shown.
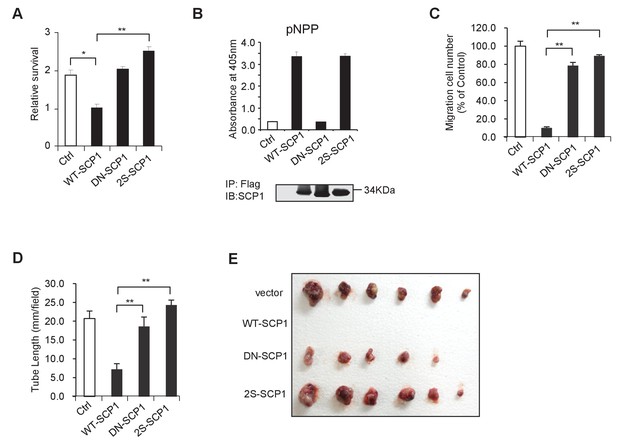
SCP1 suppresses AKT-mediated biological functions.
(A) The relative cell survival in Figure 6B was quantified. **p<0.01, *p<0.05. (B) The phosphatase activity of SCP1 was not affected by its palmitoylation status. The phosphatase activity was measured using a pNPP (p-nitrophenyl-phosphate) assay. (C) The migration was calculated, as shown in Figure 6E. **p<0.01, *p<0.05. (D) The tube length in Figure 6F was measured. **p<0.01, *p<0.05. (E) SCP1 impaired H1299 tumor bearing in nude mice in a palmitoylation-dependent manner. H1299 cells were infected with lentivirus with vector, WT-SCP1, DN-SCP1, or 2S-SCP1, and they were subcutaneously injected into nude mice (1 × 107 per mouse). The tumor was photographed after 42 days. WT: wild-type.