The PERK arm of the unfolded protein response regulates satellite cell-mediated skeletal muscle regeneration
Figures
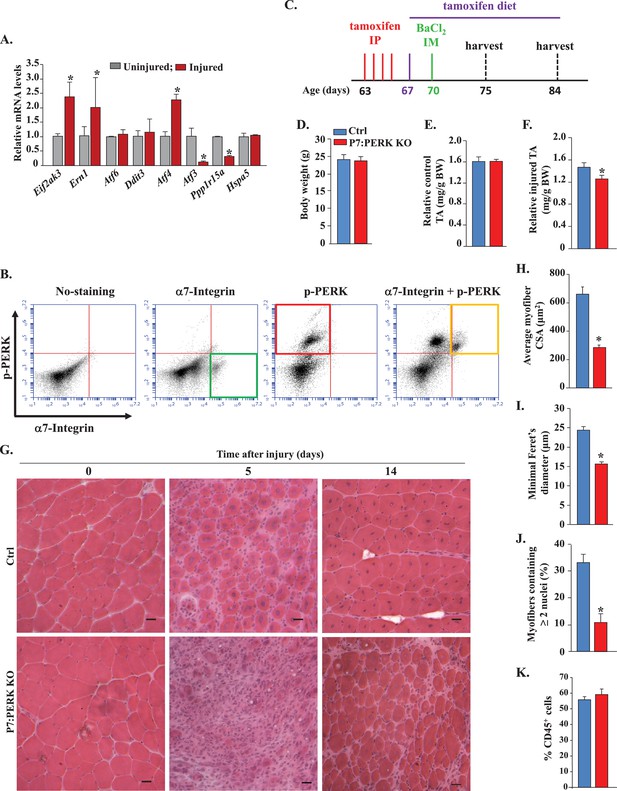
Role of PERK in satellite cell-mediated skeletal muscle regeneration.
(A) Primary mononucleated cells were isolated from uninjured and 5d-injured hind limb muscle of WT mice. Satellite cells from cellular mixture were purified by FACS technique and immediately frozen. RNA was extracted and the transcript levels of the indicated ER stress markers quantified by qRT-PCR. N = 3 mice in each group. Data are mean ± SD. *p<0.05, values significantly different from uninjured muscle by unpaired t-test. (B) Primary mononucleated cells were isolated from the hind limb muscle of WT mice 5d after BaCl2-mediated injury and subjected to FACS analysis for the expression of α7-integrin and phospho-PERK. Representative dot plots presented here demonstrate enrichment of phospho-PERK+ cells amongst α7-integrin+ population. N = 3 in each group. (C) Schematic representation of mice age and time of tamoxifen treatment and TA muscle injury and analysis. IP, intraperitoneal; IM, intramuscular. (D) Average overall body weight (BW) and (E) average uninjured TA muscle wet weight per gram BW of Ctrl and P7:PERK KO mice. TA muscle of Ctrl and P7:PERK KO mice were injured by intramuscular injection of 1.2% BaCl2 solution. The muscles were harvested after 5d or 14d of muscle injury. (F) Average wet weight per gram BW of 5d-injured TA muscle of Ctrl and P7:PERK KO mice. (G) TA muscle sections were stained with H&E dye. Representative photomicrographs of H&E-stained sections illustrating a severe regeneration defect in injured TA muscle of P7:PERK KO mice compared with Ctrl littermates at day 5 (N = 6) and 14 (N = 3) after BaCl2-mediated injury. Scale bar: 20 µm. Quantification of (H) average cross-sectional area (CSA) and (I) average minimal Feret’s diameter of regenerating myofibers. (J) Percentage of myofibers containing two or more centrally located nuclei per field at day 5 post injury. (K) Percentage of CD45+ cells in 5d-injured TA muscle of Ctrl (N = 4) and P7:PERK KO (N = 4) mice determined by FACS analysis. Data are mean ± SD. *p<0.05, values significantly different from corresponding Ctrl mice, as determined using unpaired Student’s t-test.
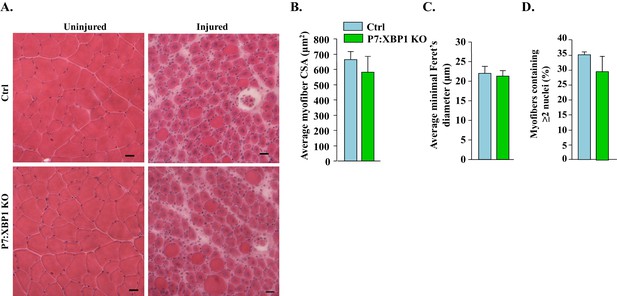
XBP1 is not required for satellite cell-mediated skeletal muscle regeneration.
(A) TA muscle of Ctrl and P7:XBP1 KO mice were injured by intramuscular injection of 1.2% BaCl2 solution. The muscles were harvested after 5d of muscle injury and sections were stained with H&E dye. Representative photomicrographs of H&E-stained sections suggesting no major effect on muscle regeneration between Ctrl and P7:XBP1 KO mice. Scale bar: 20 µm. Quantification of (B) average cross-sectional area (CSA), (C) average minimal Feret’s diameter of regenerating myofibers, and (D) percentage of myofibers containing two or more centrally located nuclei per field at day 5 post injury. No significant differences were observed by unpaired Student’s t-test between Ctrl and P7:XBP1 KO mice.
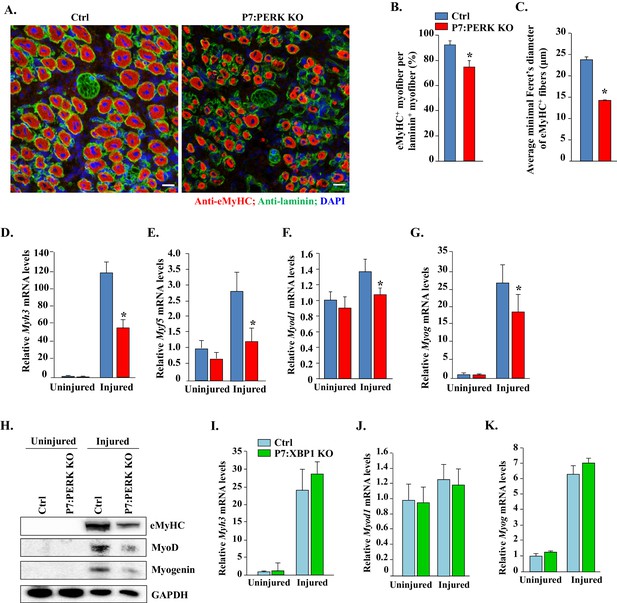
Deletion of PERK in satellite cells inhibits myofiber formation and expression of myogenic regulatory factors following injury.
(A) Representative photomicrographs of 5d-injured TA muscle sections of Ctrl and P7:PERK KO mice after immunostaining for eMyHC (red color) and laminin (green). Nuclei were identified by staining with DAPI. Scale bar: 20 μm. (B) Percentage of eMyHC+ myofiber with laminin in 5d-injured TA muscle of Ctrl and P7:PERK KO mice. (C) Average minimal Feret’s diameter of eMyHC+ myofibers of 5d-injured TA muscle of Ctrl and P7:PERK KO mice. Relative mRNA levels of (D) Myh3, (E) Myf5, (F) Myod1, and (G) Myog in uninjured and 5d-injured TA muscle of Ctrl and P7:PERK KO mice measured by performing qRT-PCR assay. N = 4 mice in each group for A-G. (H) Immunoblots presented here demonstrate the levels of eMyHC, MyoD, myogenin and an unrelated protein GAPDH in uninjured and injured TA muscle of Ctrl and P7:PERK KO mice. Relative mRNA levels of (I) Myh3, (J) Myod1, and (K) Myog in uninjured and 5d-injured TA muscle of Ctrl and P7:XBP1 KO mice measured by performing qRT-PCR assay. N = 4 mice in each group for each analysis. Data are mean ± SD. *p<0.05, values significantly different from corresponding injured TA muscle of Ctrl mice by unpaired t-test.
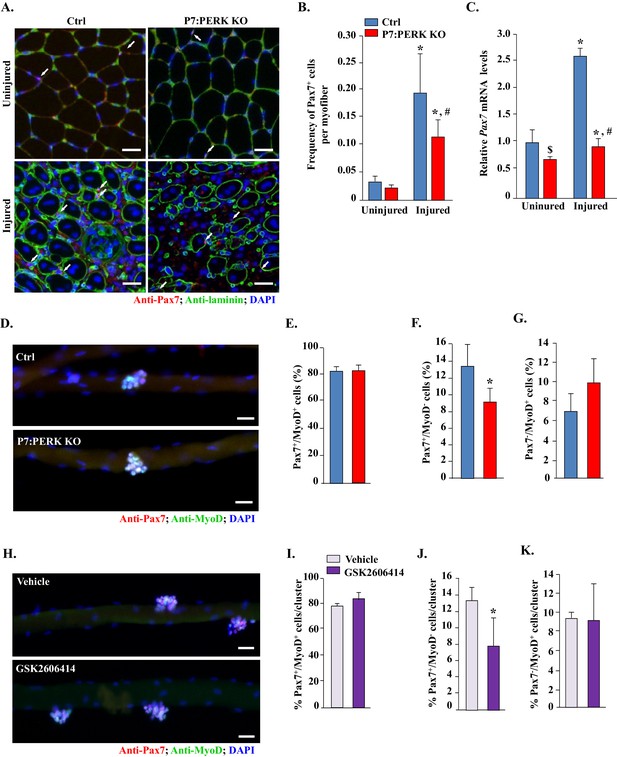
Inhibition of PERK reduces the number of Pax7+ cells during skeletal muscle regeneration and on cultured myofibers.
(A) TA muscle of Ctrl and P7:PERK KO mice were injured by intramuscular injection of BaCl2. After 5d, uninjured and injured TA muscles were isolated and transverse sections made were analyzed by immunostaining for Pax7 and laminin. Nuclei were identified by co-staining with DAPI. Representative photomicrographs are shown here. Arrows point to Pax7+ cells. Scale bar: 50 μm. (B) Quantification of the frequency of Pax7+ cells per myofiber in uninjured and injured TA muscle section of Ctrl and P7:PERK KO mice. (C) Relative mRNA levels of Pax7 in uninjured and 5d-injured TA muscle of Ctrl and P7:PERK KO mice measured by performing qRT-PCR. N = 4 in each group for A–C. *p<0.05, values significantly different from corresponding uninjured TA muscle of Ctrl or P7:PERK KO mice by unpaired t-test. #p<0.05, values significantly different from injured TA muscle of Ctrl mice by unpaired t-test. $p<0.05, values significantly different from uninjured TA muscle of Ctrl mice by unpaired t-test. (D) Single myofibers were isolated from EDL muscle of Ctrl and P7:PERK KO mice. After 72 hr of culturing, myofibers were collected and stained for Pax7 and MyoD. Representative merged images of cultured myofibers are presented here. Scale bars: 20 μm. Quantification of the percentage of (E) Pax7+/MyoD+, (F) Pax7-/MyoD+, and (G) Pax7+/MyoD- cells per myofiber in Ctrl and P7:PERK KO cultures. (H) Single myofibers were isolated from EDL muscle of WT mice and treated with vehicle alone or 1 µM GSK2606414 for 72h. The myofibers were then collected and stained for Pax7 and MyoD. Nuclei were identified by staining with DAPI. Representative merged images of cultured myofibers incubated with vehicle alone or GSK2606414 for 72 hr are presented here. Scale bars: 20 μm. Quantification of the percentage of (I) Pax7+/MyoD+, (J) Pax7-/MyoD+, and (K) Pax7+/MyoD- cells per myofiber in vehicle and GSK2606414-treated cultures. Analysis was done using 18–22 myofibers for each mouse. N = 3 mice in each group for D-K. Data are mean ± SD. *p<0.05, values significantly different from corresponding Ctrl or vehicle alone treated cultures by unpaired t-test.
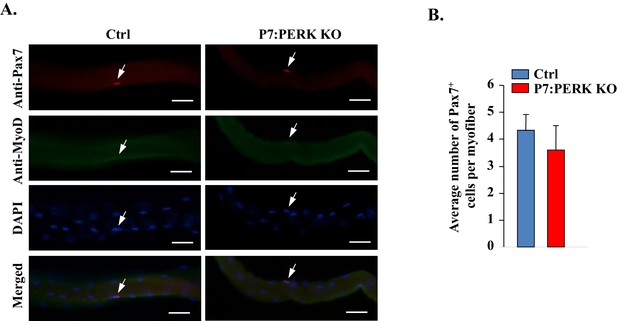
Deletion of PERK does not affect the number of Pax7+ cells on freshly isolated myofibers.
(A) Single myofibers were isolated from EDL muscle of Ctrl and P7:PERK KO mice and immediately fixed in paraformaldehyde. The myofibers were stained for Pax7 and MyoD. Nuclei were stained using DAPI. Representative individual Pax7, MyoD, and DAPI-stained and merged images of cultured myofibers. Arrows point to Pax7+ satellite cells. Scale bars: 50 μm. (B) Quantification of number of Pax7+ cells per myofiber. No MyoD+ cells were detected in the freshly isolated myofibers from EDL muscle of Ctrl or P7:PERK KO mice. N = 3 mice in each group. Analysis was done using 20–22 myofibers for each mouse. Data are mean ± SD. No significant differences were observed by unpaired Student’s t-test between Ctrl and P7:PERK KO cultures.
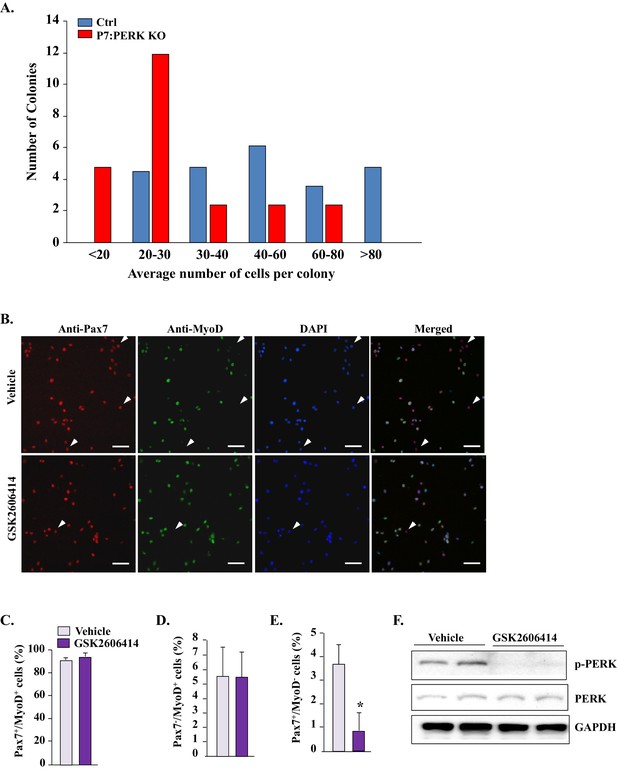
Inhibition of PERK reduces Pax7+ cells in cultures.
(A) Satellite cells were isolated from hind limb muscle of Ctrl and P7:PERK KO mice and purified by preplating. Approximately 1000 cells were then plated in tissue culture dishes and the number of colonies and number of cells per colony was measured at 12d. (B) Primary myoblasts from WT mice were treated with vehicle alone or 1 µm GSK2606414 for 24 hr. The cultures were then fixed and immunostained for Pax7 and MyoD. Nuclei were identified by staining with DAPI. Representative individual and merged images are presented here. Arrows point to Pax7+/MyoD- cells. Scale bars: 50 μm. Quantification of the percentage of (C) Pax7+/MyoD+(D) PAX7-/MyoD+ and (E) PAX7+/MyoD- cells in vehicle and GSK2606414-treated cultures. (F) Representative immunoblots presented here demonstrate that GSK2606414 reduces the levels of phosphorylated PERK in cultured myoblasts. N = 4 in each group. Data are mean ± SD. *p<0.05, values significantly different from cultures incubated with vehicle alone by unpaired t-test.
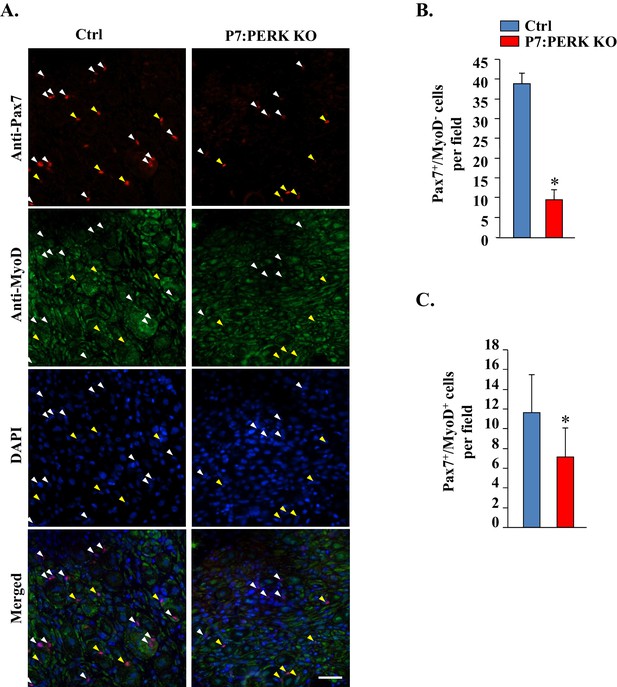
Targeted ablation of PERK reduces satellite cell count in injured muscle of mice.
(A) Transverse sections generated from 5d-injured TA muscle of Ctrl and P7:PERK KO mice were immunostained for Pax7 and MyoD. Nuclei were counterstained with DAPI. Representative individual and merged images are presented here. White color arrows point to Pax7+/MyoD- cells whereas yellow arrows point to Pax7+/MyoD+ cells. Scale bar: 50 µm. Quantification of number of (B) Pax7+/MyoD- and (C) Pax7+/MyoD+ cells per field (∼0.15 mm2) in TA muscle section of Ctrl and P7:PERK KO mice. N = 4 in each group. *p<0.05, values significantly different from corresponding TA muscle of Ctrl mice by unpaired t-test.
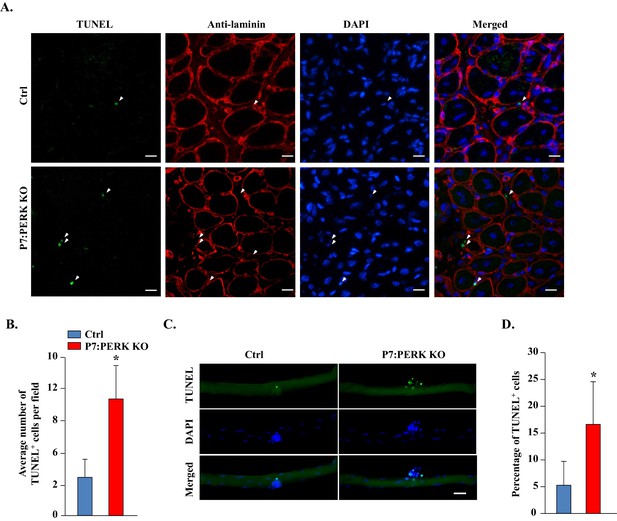
Deletion of PERK reduces survival of muscle progenitor cells during skeletal muscle regeneration and in myofiber explants.
(A) Transverse sections prepared from 5d-injured TA muscle of Ctrl and P7:PERK KO mice were processed for the detection of TUNEL+ cells. The sections were also stained for laminin. Nuclei were counterstained with DAPI. Representative merged images are presented here. Arrows point to TUNEL+ cells within laminin staining around myofibers. Scale bar: 20 µm. (B) Quantification of number of TUNEL+ cells within laminin staining per field (~0.15 mm2) in 5d-injured TA muscle of Ctrl and P7:PERK KO mice. N = 4 mice in each group. (C) Single myofibers were isolated from EDL muscle of Ctrl and P7:PERK KO mice. After 72 hr of culturing, myofibers were collected and processed for TUNEL staining. Nuclei were counterstained with DAPI. Representative merged images of cultured myofibers are presented here. Scale bar: 20 μm. (D) Quantification of the percentage of TUNEL+ cells on myofibers in Ctrl and P7:PERK KO cultures. N = 3 mice in each group. Analysis was done using 14–18 myofibers for each mouse. Data are mean ± SD. *p<0.05, values significantly different from Ctrl mice or cultures by unpaired t-test.
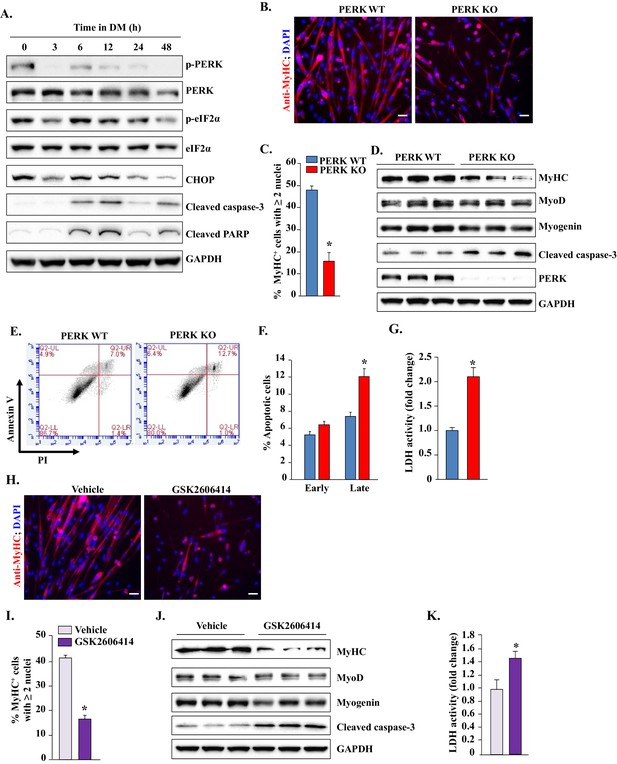
PERK is required for survival of myogenic cells during in vitro myogenesis.
(A) Primary myogenic cells isolated from WT mice were incubated in DM for the indicated time intervals and protein extracts analyzed for the levels of various proteins related to the PERK arm of the UPR. Immunoblots presented here demonstrate the levels of phosphorylated and total PERK and eIF2α and total CHOP, cleaved PARP, cleaved caspase-3 and an unrelated protein GAPDH. (B) PERK WT and PERK KO myogenic cells were incubated in DM for 48 hr and the myotube formation was monitored by staining for MyHC. Nuclei were counterstained with DAPI. Representative images presented here demonstrate that myotube formation is considerably reduced in PERK KO cultures. Scale bar: 20 µm. (C) Quantification of percentage of MyHC+ myotubes containing two or more nuclei in PERK WT and PERK KO cultures after 48 hr of incubation in DM. (D) Immunoblots presented here show levels of MyHC, MyoD, myogenin, cleaved caspase-3, and unrelated protein GAPDH in PERK WT and PERK KO cultures after 48 hr of incubation in DM. (E) PERK WT and PERK KO myogenic cells were incubated in DM for 36–48 hr. Both adherent and floating cells were collected and stained for Annexin V and propidium iodide (PI) and analyzed by FACS to detect early and late apoptotic cells. Representative dot plots are presented here. (F) Quantification of early and late apoptotic cells in PERK WT and PERK KO cultures after FACS analysis. (G) Relative amounts of lactate dehydrogenase (LDH) in supernatants of PERK WT and PERK KO cultures after 36–48 hr of incubation in DM. (H) Primary myoblasts prepared from WT mice were treated with vehicle alone or 1 µM GSK2606414 for 48 hr and the myotube formation was monitored by immunostaining for MyHC. Nuclei were identified by staining with DAPI. Representative merged images are presented here. Scale bar: 20 µm. (I) Quantification of percentage of MyHC+ myotubes containing two or more nuclei in vehicle or GSK2606414-treated cultures after 48 hr of incubation in DM. (J) Protein levels of MyHC, MyoD, myogenin, cleaved caspase-3, and GAPDH in vehicle and GSK2606414-treated WT cultures 48 hr after incubation in DM. (K) Relative amounts of LDH in supernatants of vehicle alone and GSK2606414-treated cultures. N = 4 in each group. Data are mean ± SD. *p<0.05, values significantly different from corresponding PERK WT or vehicle alone cultures by unpaired t-test.
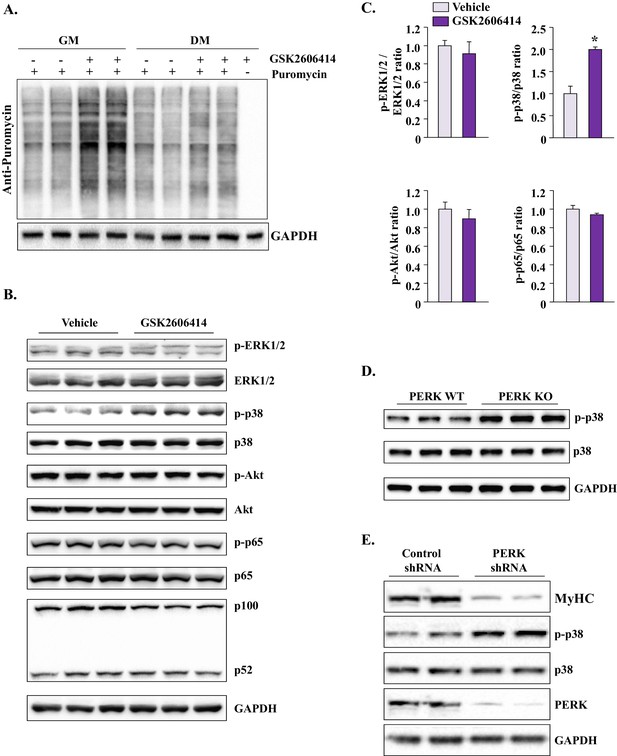
Inhibition of PERK induces protein synthesis and activates p38 MAPK in cultured myogenic cells.
(A) Primary myogenic cells prepared from hind limb muscles of WT mice were incubated with growth medium (GM) or DM with or without 1 µM GSK2606414 for 12 hr. The rate of protein synthesis was measured by performing SUnSET assay. Representative immunoblot presented here demonstrates that inhibition of PERK increases the rate of protein synthesis in myogenic cultures. (B) Primary myogenic cells were incubated in DM for 12 hr with or without 1 µM GSK2606414 and processed by performing Western blot. Immunoblots presented here demonstrate phosphorylated and total levels of ERK1/2, p38MAPK, Akt, and p65 proteins and relative amounts of p100 and p52 proteins and GAPDH. (C) Ratio of phosphorylated vs total ERK1/2, p38 MAPK, Akt, and p65 in vehicle or GSK2606414-treated cultures measured by densitometric analysis of immunoblots. (D) Immunoblots presented here demonstrate the levels of phosphorylated and total p38 and total GAPDH protein in PERK WT and PERK KO cultures at 24 hr after incubation in DM. (E) Primary myogenic cultures were transduced with adenoviral vectors expressing a scrambled (control) shRNA or PERK shRNA. The cells were incubated in DM and the levels of MyHC, phosphorylated and total p38 MAPK, and total PERK, and GAPDH were measured by performing western blot. N = 3 in each group. Data are mean ± SD. *p<0.01, values significantly different from corresponding cultures treated with vehicle alone, as determined by a Student’s unpaired t-test.
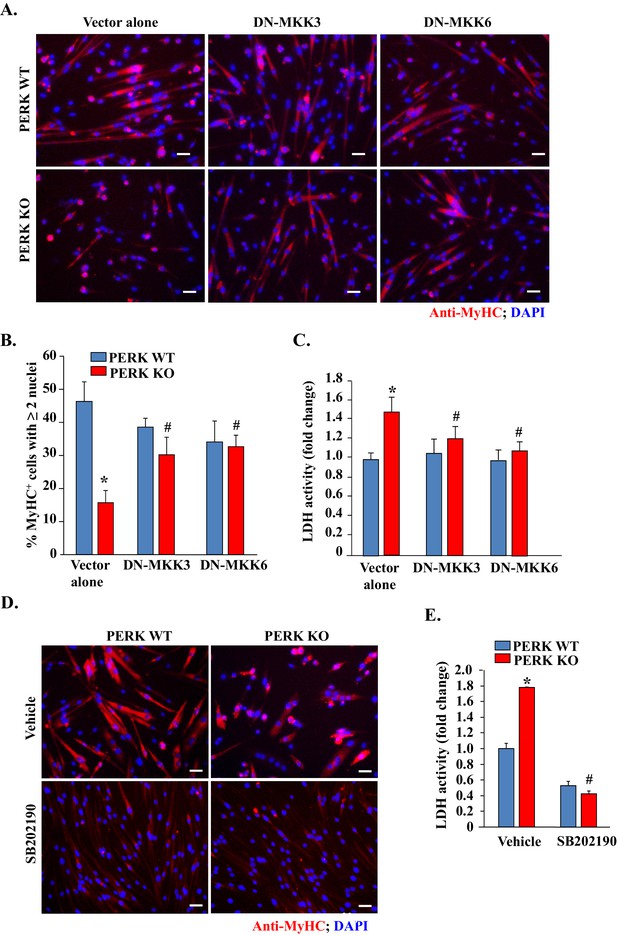
Inhibition of p38 MAPK improves myogenic cell survival and myotube formation in PERK-deficient cultures.
(A) PERK WT and PERK KO primary myogenic cells were transfected (by electroporation) with vector alone or a dominant negative (DN) mutant of MKK3 or MKK6. The cells were incubated in DM for 48 hr and myotube formation was monitored by immunostaining for MyHC. Nuclei were visualized by staining with DAPI. Representative images presented here suggest that overexpression of DN-MKK3 or DN-MKK6 improves myotube formation in PERK KO cultures. Scale bars: 20 µm. (B) Quantification of percentage of MyHC+ myotubes containing two or more nuclei in PERK WT and PERK KO cultures transfected with vector alone, DN-MKK3 or DN-MKK6 cDNA. (C) Relative amounts of LDH in supernatants of vector alone, DN-MKK3 or DN-MKK6 cDNA transfected PERK WT and PERK KO cultures incubated in DM for 48 hr. (D) PERK WT and PERK KO primary myogenic cells were incubated in DM for 48 hr with or without 20 µM SB202190. Representative images presented here suggest that treatment with SB202190 improved survival of MyHC+ cells in PERK KO cultures. Scale bars: 20 µm. (E) Relative amounts of LDH in supernatants of PERK WT and PERK KO cultures 48 hr after addition of DM and treatment with vehicle alone or SB202190. N = 4 in each group. Data are mean ± SD. *p<0.01, values significantly different from PERK WT cultures transfected with empty vector or treated with vehicle alone by unpaired t-test. #p<0.01, values significantly different from PERK KO cultures transfected with empty vector or treated with vehicle alone by unpaired t-test.
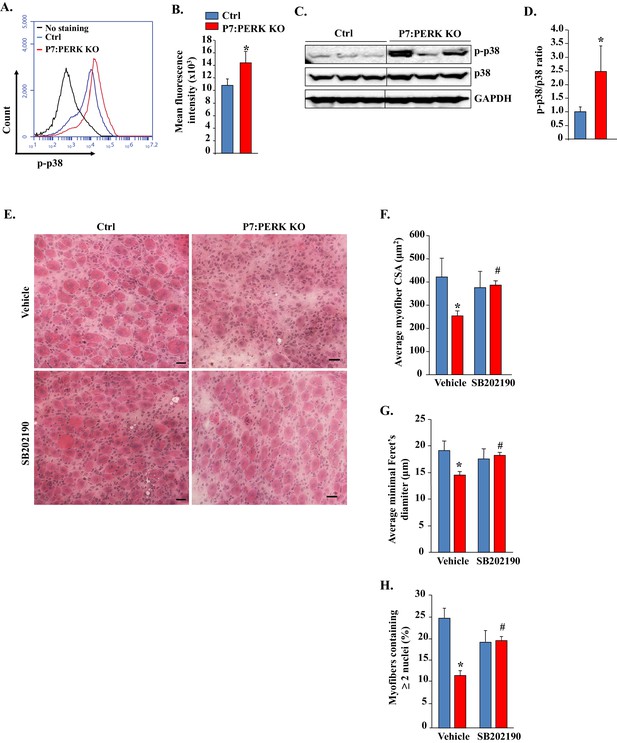
Pharmacological inhibition of p38 MAPK improves skeletal muscle regeneration in P7:PERK KO mice.
(A) TA muscle of 3-month old Ctrl and P7:PERK KO mice was injured by intramuscular injection of 1.2% BaCl2. After 5d, the muscle was collected and the single cell suspension made was analyzed for the levels of p-p38 in satellite cells. Representative histogram presented here demonstrates the levels of p-p38 in satellite cells of 5d-injured TA muscle of Ctrl and P7:PERK KO mice. (B) Quantification of mean florescence intensity in FACS analysis for p-p38 in satellite cells of 5d-injured TA muscle of Ctrl and P7:PERK KO mice. N = 4 in each group. (C) Representative immunoblots presented here demonstrate the levels of p-p38, total p38, and an unrelated protein GAPDH in 5d-injured TA muscle of Ctrl and P7:PERK KO mice. Black vertical line on immunoblots indicates that intervening lane has been spliced out. (D) Ratio of phosphorylated vs total p38 in 5d-injured TA muscle of Ctrl and P7:PERK KO mice measured by densitometric analysis of immunoblots. (E) TA muscle of 3-month old Ctrl and P7:PERK KO mice were injured by intramuscular injection of 1.2% BaCl2 solution. The mice were also treated daily with vehicle alone or with SB202190. After 5d, the injured TA muscle was isolated and processed for H&E staining and morphometric analysis. Representative photomicrographs of H&E-stained sections illustrating that treatment with SB202190 improved myofiber regeneration in P7:PERK KO mice. Scale bar: 20 µm. Quantification of (F) average cross-sectional area (CSA) and (G) average minimal Feret’s diameter of regenerating myofibers in TA muscle of Ctrl and P7:PERK KO mice. (H) Percentage of myofibers containing two or more centrally located nuclei per field at day 5 post injury in TA muscle of Ctrl and P7:PERK KO mice. N = 4 in each group. Data are mean ± SD. *p<0.05, values significantly different from corresponding Ctrl mice, as determined using unpaired Student’s t-test. #p<0.05, values significantly different from corresponding P7:PERK KO mice treated with vehicle alone, as determined using unpaired Student’s t-test.





