Angiopoietin-2 in white adipose tissue improves metabolic homeostasis through enhanced angiogenesis
Figures
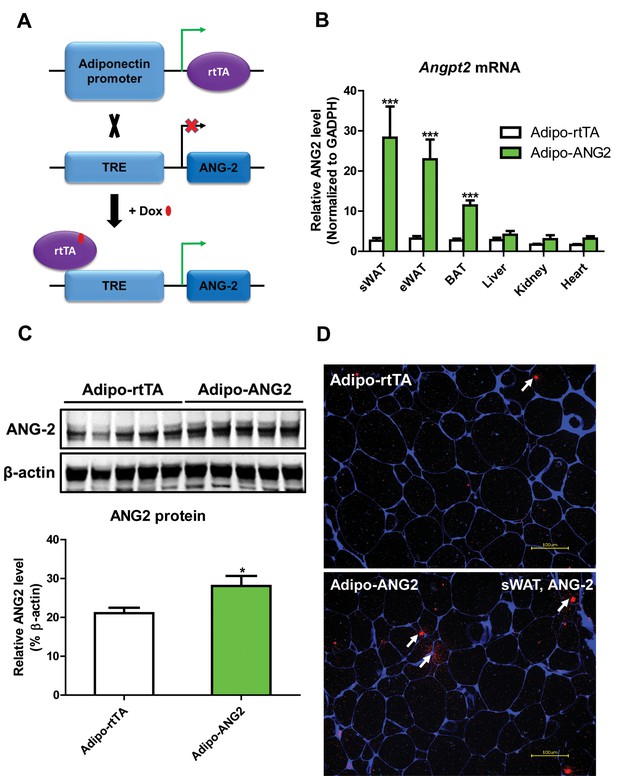
Adipocyte-specific, Dox-inducible overexpression of ANG-2.
(A) Schematic illustration of the adipocyte-specific, Dox-inducible ANG-2 overexpression mouse model. The adiponectin promoter-driven reverse tetracycline-dependent transcriptional activator (rtTA) mice were bred to tetracycline responsive element (TRE)-driven ANG-2 (TRE-ANG2) to generate the double transgenic mouse model. rtTA activity is activated to induce ANG-2 overexpression in the presence of Dox. (B) Angpt2 transcriptional changes in different tissues from control (Adipo-rtTA) mice and overexpressing (Adipo-ANG2) mice, fed with HFD/Dox 600 mg/kg for five weeks. (C) Representative Western blot image (upper panel) and quantitative analysis (lower panel) of ANG-2 and β-actin protein levels in sWAT from HFD/Dox 600 mg/kg fed Adipo-rtTA mice and Adipo-ANG2 mice. (D) Representative images of ANG-2 immunofluorescence staining (Red, Magnification = 200 × ) in both Adipo-rtTA control and Adipo-ANG2 double transgenic groups after Dox induction. Perilipin (Blue) was also stained to label adipocytes. Bar = 100 µm. For all the statistical graphs: n = 5 ~ 7 mice per group. Data are shown as mean ± s.e.m. *p<0.05.
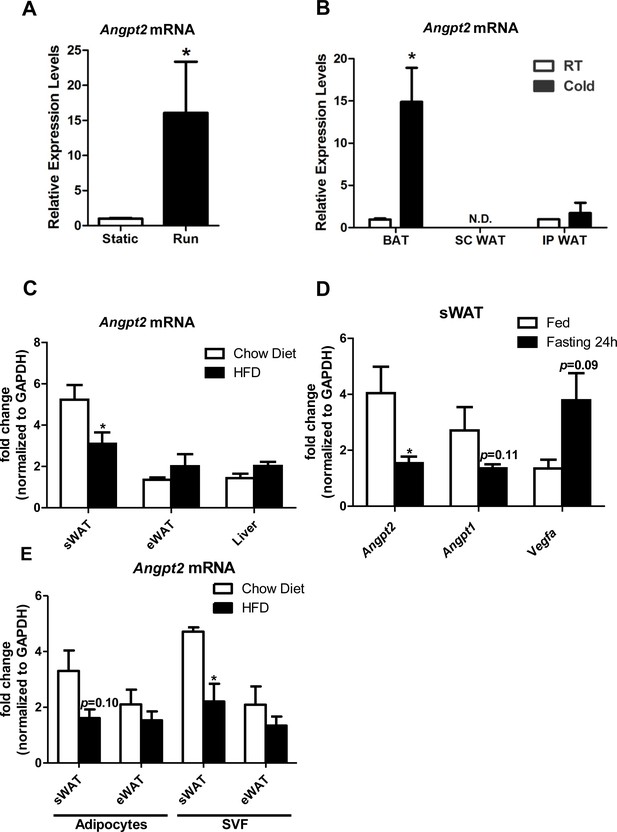
Differential regulation of Angpt2 mRNA under variety of metabolic challenges.
(A) Angpt2 mRNA levels in sWAT from mice under static conditions or subjected to a running exercise (6 hr daily) for five weeks.(B) Angpt2 transcriptional changes in BAT, subcutaneous WAT (SC WAT) and epididymal WAT (IP WAT) from mice exposed to cold (6°C) or room temperature (RT) for 72 hr.(C) Ang-2 mRNA expression in the tissues of sWAT, eWAT and liver from chow diet and HFD fed mice for 6 months.(D) Angpt2, Angpt1 and Vegfa mRNA levels under fed and fasting conditions (24 hr). (E) Angpt2 mRNA expression in adipocyte and stromal vascular fractions (SVF) of sWAT and eWAT from chow diet and HFD-fed mice for 6 months. For all figures: n = 6 mice per group. Data are shown as mean ± s.e.m. *p<0.05.
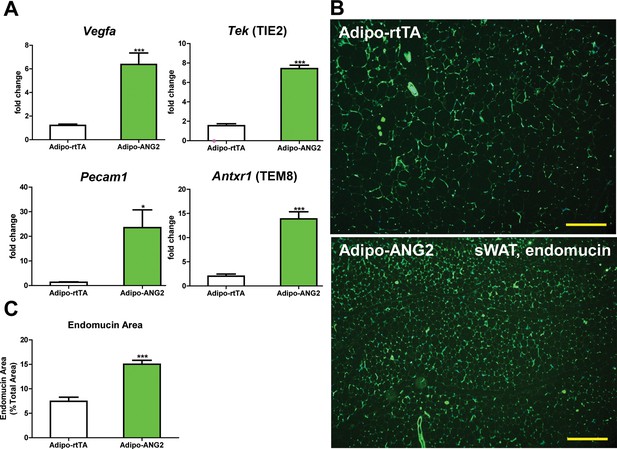
Vascular changes after overexpression of ANG-2.
(A) Angiogenic factors and endothelial cell markers including Vegfa, Tek, Pecam1 and Antxr1 mRNA levels in the harvested sWAT from 5 week HFD/Dox 600 mg/kg induced Adipo-rtTA and Adipo-ANG2 mice.(B) Representative immunofluorescence staining image of endothelial marker endomucin (Green, Magnification = 100×) and (C) the statistical result of relative endomucin area calculated by the percentage of endomucin positive signals in the total area. SWAT section staining images from both groups are shown. Bar = 160 µm. For all the statistical graphs: n = 6 mice per group. Data are shown as mean ± s.e.m. *p<0.05, ***p<0.001.
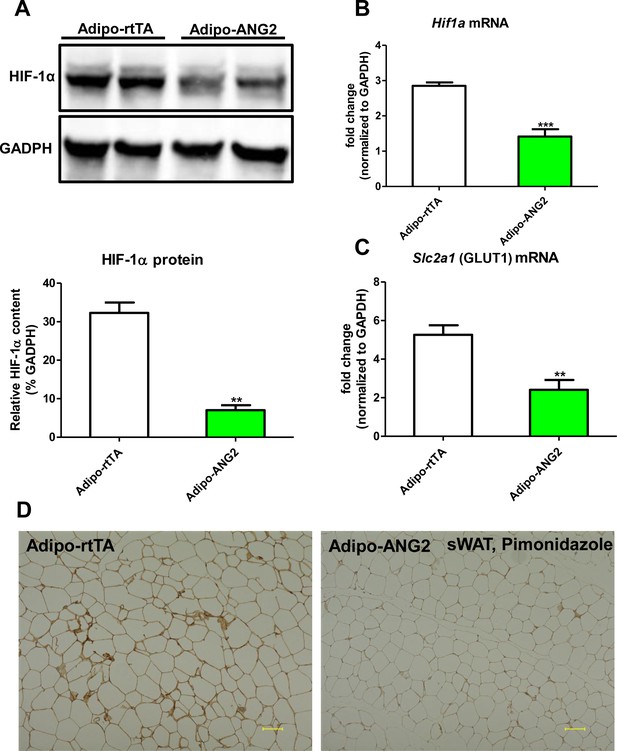
Hypoxic parameters after ANG-2 overexpression in sWAT.
(A) Representative immunoblot image (upper panel) and quantitative analysis (lower panel) of HIF-1α expression in sWAT from both control and ANG-2 transgenic mice. (B and C). Hif1a and Slc2a1 (stands for GLUT1) transcriptional changes in sWAT of both groups. (D) Representative images of pimonidazole immunohistochemistry staining (brown, Magnification = 100 × ) in both Adipo-rtTA control and Adipo-ANG2 transgenic groups after Dox induction. Bar = 100 μm. For the western blot image, n = 3 mice per group. For all other statistical figures: n = 10 mice per group. Data are shown as mean ± s.e.m. **p<0.01, ***p<0.001.
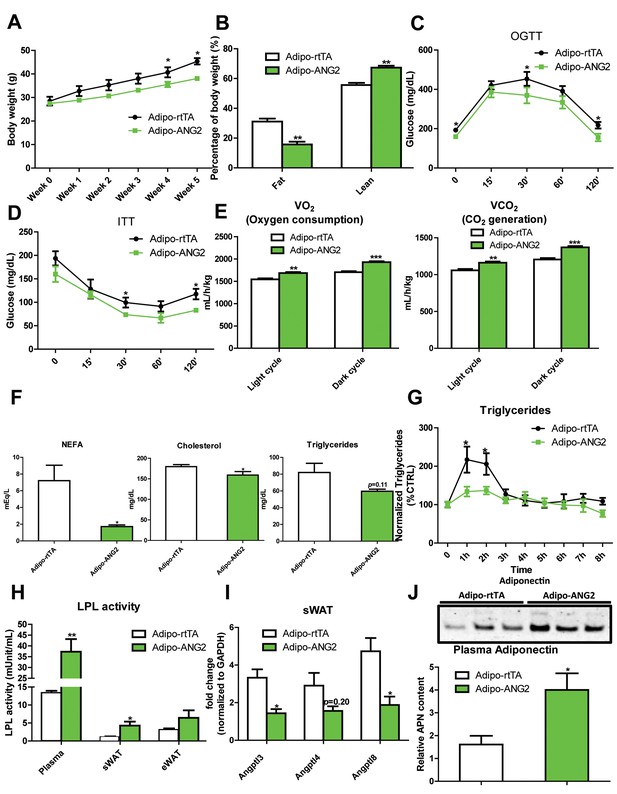
Metabolic effects in mice overexpressing ANG-2.
After HFD/Dox 600 mg/kg induction for five weeks, Adipo-rtTA control and Adipo-ANG2 transgenic mice were subjected to the following metabolic analyses: (A) Body weight was continuously monitored for five weeks.(B) Body composition data obtained via MRI. Both the percentage of fat mass and lean mass are shown. Glucose levels at different time-points from (C) oral glucose tolerance tests (OGTT) and (D) Insulin tolerance tests (ITT).(E) Oxygen consumption (VO2, left panel) and Carbon dioxide generation (VCO2, right panel) data measured by metabolic cages. The values for both light and dark cycles are shown. (F) Non-esterified free fatty acid (NEFA), cholesterol and triglyceride levels in serum samples from both groups. (G) After an oral gavage of 20% Intralipid, the clearance of serum triglycerides is shown over time for a period of 8 hr. (H) Lipoprotein lipase (LPL) activity measured through the LPL activity assay kit in the plasma, sWAT and eWAT from both groups. (I) Angiopoietin-like protein family members Angptl3, Angptl4, Angptl8 mRNA levels in the harvested sWAT from 5-week HFD/Dox 600 mg/kg fed Adipo-rtTA and Adipo-ANG2 mice. (J) Representative immunoblot image (upper panel) and quantitative analysis (lower panel) of adiponectin expression in the plasma of both groups. For the western blot image, n = 3 mice per group. For all the other statistical graphs: n = 10 mice per group. Data are shown as mean ± s.e.m. *p<0.05, **p<0.01, ***p<0.001.

Insulin signaling in the ANG-2 overexpressing mice.
(A) Representative immunoblot image of phosphorylated Akt (pAkt, Ser 473) and total Akt expression in sWAT from both control and ANG-2 overexpressing mice after saline or insulin injection (i.v.) for 5 min. For the Western blot image, n = 3 mice per group.
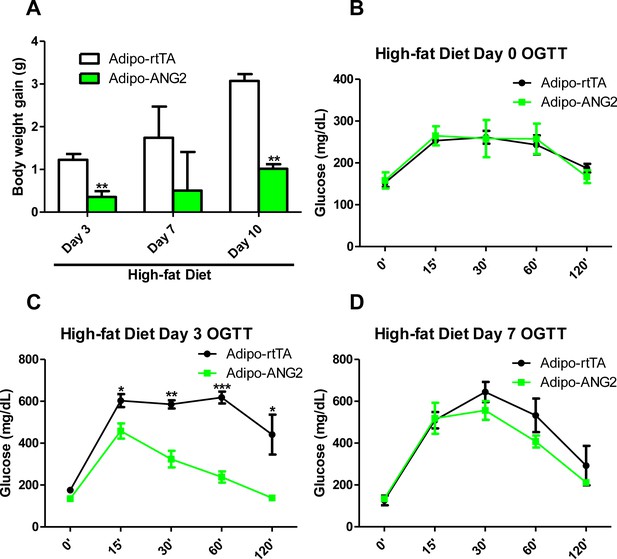
Prevention effect of ANG-2 induction against high-fat diet induced obesity.
Mice in both control and ANG-2 overexpression groups were pre-induced with Dox 600 mg/kg for five weeks, and then Dox containing food was replaced with high-fat diet. After subjecting the mice onto high-fat diet, (A) body weight gains on Day 3, Day 7 and Day 10 are recorded; (B, C and D). Oral glucose tolerance test (OGTT) results on high-fat diet Day 0, Day 3 and Day 7 are shown, respectively. For all statistical figures: n = 6 mice per group. Data are shown as mean ± s.e.m. *p<0.05, **p<0.01, ***p<0.001.
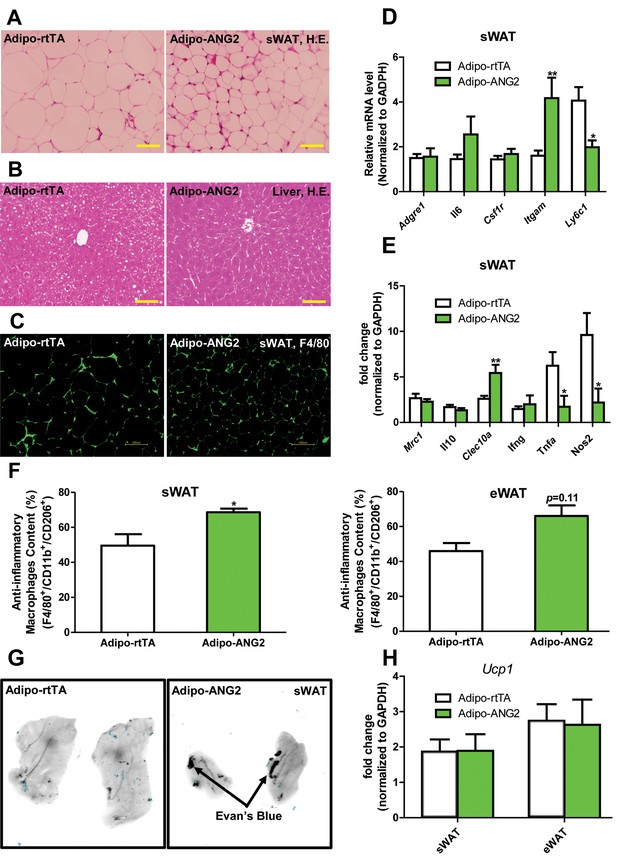
Inflammatory and histological changes after overexpression of ANG-2.
(A) Representative H&E staining of sWAT sections (Magnification = 200×) from 5 week HFD/Dox 600 mg/kg fed Adipo-rtTA and Adipo-ANG2 mice. Bar = 60 µm. (B) Representative H&E staining of liver tissues (magnification = 200×) from both groups. Lipid droplets are evident in the liver sections. Bar = 60 µm.(C) Representative immunofluorescence staining picture of the macrophage marker F4/80 (Green, Magnification = 200×) in sWAT sections from both groups. Bar = 100 µm. (D and E). Inflammatory marker mRNA levels assessed by qPCR in sWAT of control and ANG-2 transgenic mice. General inflammatory markers: Adgre1 (F4/80), Il6 (IL-6), Csf1r (CD115), Itgam (CD11b); pro-inflammatory macrophage markers: Ly6c, Ifng, Tnfa, Nos2; anti-inflammatory macrophage markers: Mrc1 (gene symbol for CD206), Il10, Clec10a (gene symbol for CD301). (F) The relative content of anti-inflammatory macrophages (M2 type macrophages) in both sWAT (left panel) and eWAT (right panel) from control and ANG-2 overexpressing mice. For the flow cytometry analysis, anti-inflammatory macrophages are considered as the F4/80+/CD11b+/CD206+ population, and the relative content in the total macrophage population is shown. (G) Representative Evan’s Blue staining of sWAT from Adipo-rtTA and Adipo-ANG2 mice. Black arrow indicates positive staining of Evan’s Blue dye, reflecting enhanced leakage from blood vessels in sWAT. (H) Beiging adipose tissue marker Ucp1 analyzed through qPCR in sWAT and eWAT of control and ANG-2 transgenic mice. For the flow cytometry study, n = 3 mice per group. For all the other statistical graphs: n = 10 mice per group. Data are shown as mean ± s.e.m. *p<0.05, **p<0.01.
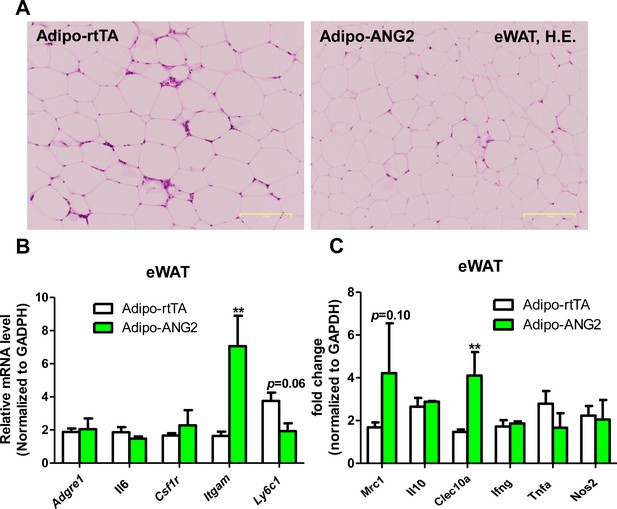
Inflammation in epididymal WAT (eWAT) from ANG-2 overexpressing mice.
(A) Representative H and E stain of eWAT sections (Magnification = 100 × ) from 5-week HFD/Dox 600 mg/kg fed Adipo-rtTA and Adipo-ANG2 mice. Bar = 160 µm. (B and C). Inflammatory marker mRNA levels measured through qPCR in eWAT of control and ANG-2 transgenic mice. General inflammatory markers: Adgre1, Il6, Csf1r, Itgam; pro-inflammatory macrophage markers: Ly6c1, Ifng, Tnfa, Nos2; anti-inflammatory macrophage markers: Mrc1, Il10, Clec10a. For all statistical figures: n = 10 mice per group. Data are shown as mean ± s.e.m. **p<0.01.
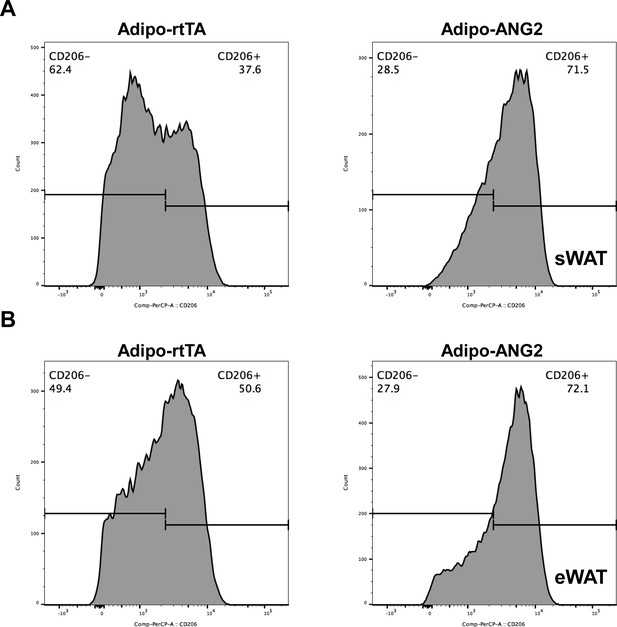
Representative histogram of flow cytometry analysis for anti-inflammatory (M2) macrophages in ANG-2 transgenic mice.
(A) Representative flow cytometry histogram demonstrating the relative percentage of anti-inflammatory macrophages in the total macrophage population, specific anti-inflammatory macrophage marker CD206 is labeled with PerCP-A, and CD206-positive (CD206+) as well as CD206-negative (CD206-) cells are shown in sWAT stromal vascular fractions from both Adipo-rtTA (left panel) and Adipo-ANG2 (right panel) mice. (B) Representative flow cytometry histogram demonstrating the relative percentage of anti-inflammatory macrophages in the total macrophage population for eWAT stromal vascular fractions from both Adipo-rtTA (left panel) and Adipo-ANG2 (right panel) mice.
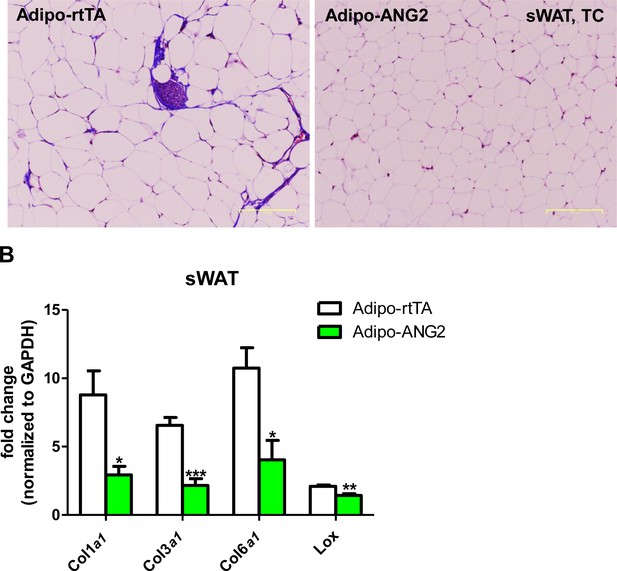
Fibrotic changes after ANG-2 overexpression.
(A) Representative Trichrome (TC) staining pictures of sWAT sections (Magnification = 100 × ) from both Adipo-rtTA and Adipo-ANG2 groups. Fibrotic changes are demonstrated as the blue staining. Bar = 160 µm. (B) Transcriptional levels of key pro-fibrotic molecules analyzed through qPCR in sWAT from both groups. Fibrotic molecules: Collagen 1α1 (Col1a1), Col3a1, Col6a1 and Lox. For all statistical figures: n = 10 mice per group. Data are shown as mean ± s.e.m. *p<0.05, **p<0.01, ***p<0.001.
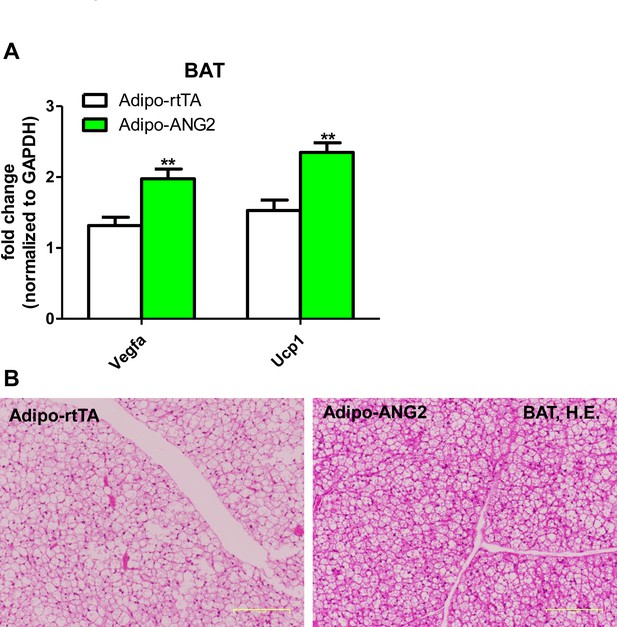
The maintenance of brown adipose tissue (BAT) in the ANG-2 overexpressing mice.
(A) Transcriptional levels of angiogenic gene Vegfa and brown adipose tissue marker Ucp1 assessed via qPCR in the BAT from control and ANG-2 overexpressing mice. (B) Representative H.E. staining images of BAT sections (Magnification = 100 × ) from both Adipo-rtTA control and Adipo-ANG2 transgenic groups. Bar = 160 µm. For all figures: n = 10 mice per group. Data are shown as mean ± s.e.m. **p<0.01.
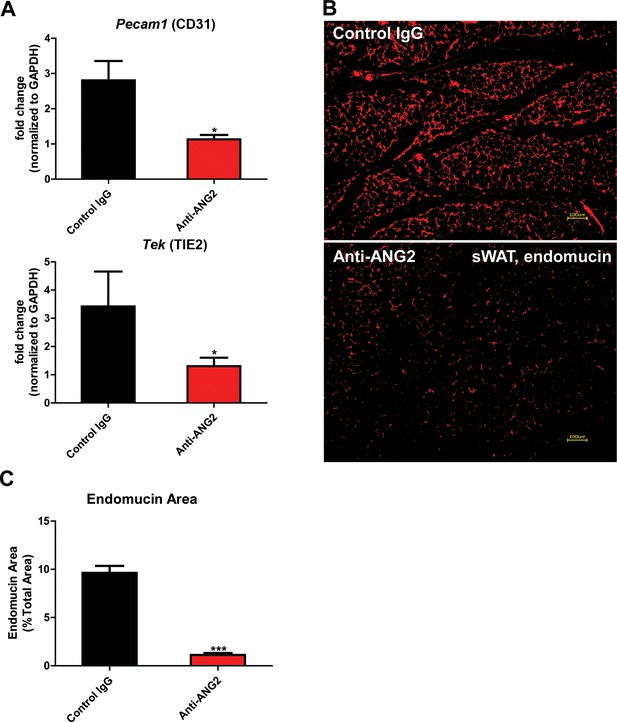
Endothelial markers and vascular staining in ANG-2 antibodies administrated mice.
(A) Endothelial cell markers Pecam1 and Tek mRNA changes in the collected sWAT from 5 week HFD stimulated wild-type mice, with administration of either control IgG or Anti-ANG2 antibody twice/week at the dosage of 4 µg/g body weight. (B) Representative immunofluorescence staining image of endothelial marker endomucin (red, magnification = 100×) and (C) the statistical result of relative endomucin area in sWAT sections from both groups. Bar = 100 µm. For all the statistical graphs: n = 5 ~ 6 mice per group. Data are shown as mean ± s.e.m. *p<0.05, ***p<0.001.
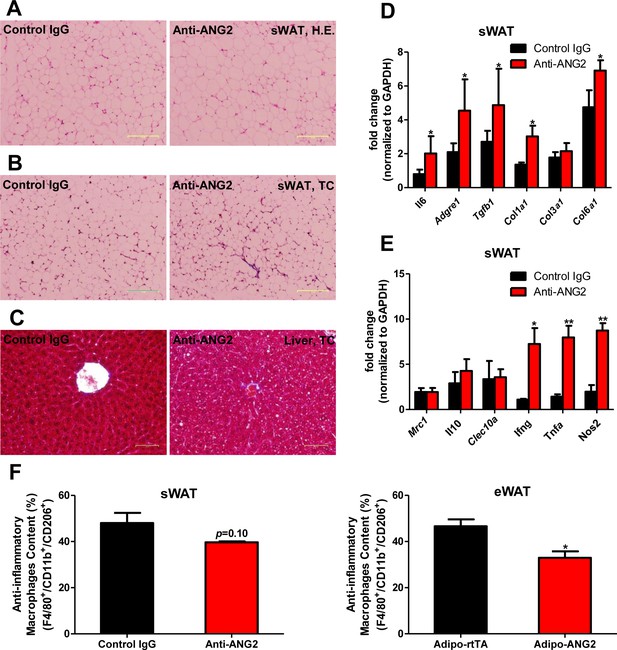
Inflammatory and fibrotic changes as well as histological features after ANG-2 neutralization.
(A) Representative H.E. staining image of sWAT sections (Magnification = 100×) from 5 week HFD fed mice with control IgG or Anti-ANG2 antibody injection. Bar = 160 µm. (B) Representative Trichrome (TC) staining pictures of sWAT sections (Magnification = 100×) from both groups. Fibrotic changes are demonstrated as the blue staining. Bar = 160 µm. (C) Representative TC staining image of liver tissues (Magnification = 200×) from both groups. Lipid droplets are shown the liver sections. Bar = 60 µm. (D and E) Transcriptional levels of key inflammatory markers and crucial pro-fibrotic molecules measured through qPCR in sWAT from control IgG and Anti-ANG2 mice. General inflammatory markers: Adgre1, Il6, Csf1r, Itgam; pro-inflammatory macrophage markers: Ly6c, Ifng, Tnfa, Nos2; anti-inflammatory macrophage markers: Mrc1, Il10, Clec10a; fibrotic molecules: TGF-β1, Collagen 1α1 (Col1a1), Col3a1, Col6a1. (F) The relative content of anti-inflammatory macrophages (M2 type macrophages) in both sWAT (left panel) and eWAT (right panel) from control IgG and ANG-2 antibody-injected mice. Through the flow cytometry analysis, anti-inflammatory macrophages are considered as the F4/80+/CD11b+/CD206+ population, and the relative percentage in the total macrophage population is shown. For the flow cytometry study, n = 3 mice per group. For all the statistical graphs: n = 5 mice per group. Data are shown as mean ± s.e.m. *p<0.05, **p<0.01.
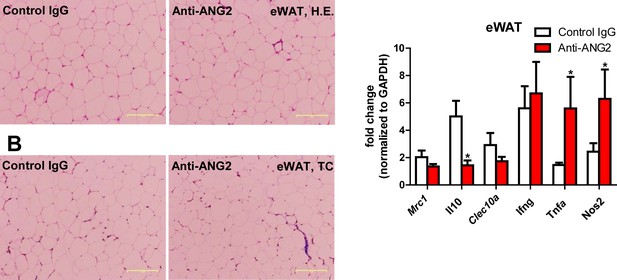
Histological and inflammatory features in eWAT after ANG-2 neutralization.
(A) Representative H and E image of eWAT sections (Magnification = 100 × ) from 5 week HFD fed mice with control IgG or anti-ANG2 antibody administration. Bar = 160 µm. (B) Representative Trichrome (TC) stains of eWAT sections (Magnification = 100 × ) from both groups. Fibrotic changes are shown as blue staining. Bar = 160 µm. (C) Transcriptional levels of inflammatory markers tested through qPCR in eWAT from control IgG and Anti-ANG2 mice. Pro-inflammatory macrophage markers: Ifng, Tnfa, Nos2; anti-inflammatory macrophage markers: Mrc1, Il10, Clec10a. For all the statistical graphs: n = 5 mice per group. Data are shown as mean ± s.e.m. *p<0.05.

Representative histogram of flow cytometry analysis for anti-inflammatory (M2) macrophages in the ANG-2 neutralizing mice.
(A) Representative flow cytometry histogram demonstrating the relative percentage of anti-inflammatory macrophages in the total macrophage population, specific anti-inflammatory macrophage marker CD206 is labeled with PerCP-A, and CD206-positive (PerCP-A+) as well as CD206-negative (PerCP-A-) cells are shown in sWAT stromal vascular fractions from both control IgG (left panel) and anti-ANG2 (right panel) antibody administrated mice. (B) Representative flow cytometry histogram demonstrating the relative percentage of anti-inflammatory macrophages in the total macrophage population for eWAT stromal vascular fractions from both control (left panel) and antibody neutralizing (eight panel) mice.
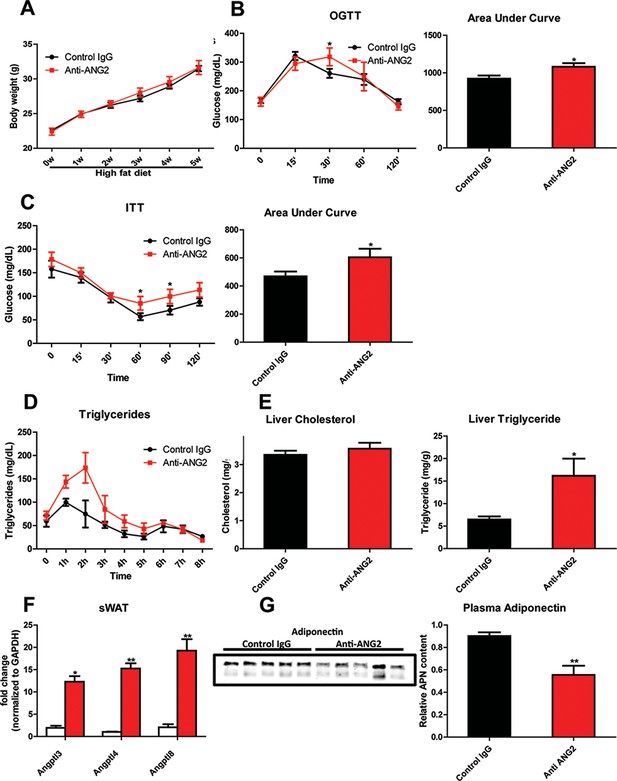
Systemic metabolic effects after ANG-2 antibody injections.
During HFD challenges for five weeks in the wild-type mice, which were administrated with either 4 µg/g body weight control IgG or anti-ANG2 antibody and the mice then underwent the following metabolic analyses: (A) Body weight was monitored for five weeks.(B) Glucose levels over different time-points during an OGTT (left panel) and quantification of areas under curve (right panel) presented as the mean area under each glucose level curve. (C) The insulin sensitivity demonstrated by ITT (left panel) and quantification of area under the curve (right panel) from both groups.(D) After an oral gavage of 20% Intralipid, the clearance of serum triglycerides is shown over time for a period of 8 hr. (E) Liver cholesterol (left panel) and triglycerides levels (right panel) from both groups. (F) Angiopoietin-like protein family members Angptl3, Angptl4 and Angptl8 mRNA levels in sWAT from 5-week HFD fed control IgG and Anti-ANG2-treated mice.(G) Representative immunoblot images (left panel) and quantitation (right panel) of adiponectin expression in the plasma of both groups. For all the statistical graphs: n = 5 mice per group. Data are shown as mean ± s.e.m. *p<0.05, **p<0.01.
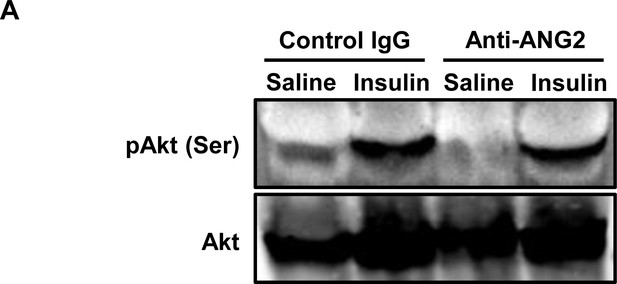
Insulin signaling in sWAT after ANG-2 antibody administration.
(A) Representative immunoblot image of phosphorylated Akt (pAkt, Ser 473) and total Akt expression in sWAT from both control IgG and ANG-2 neutralizing antibody administrated mice after saline or insulin injection (i.v.) for 5 min. For the Western blot image, n = 3 mice per group.
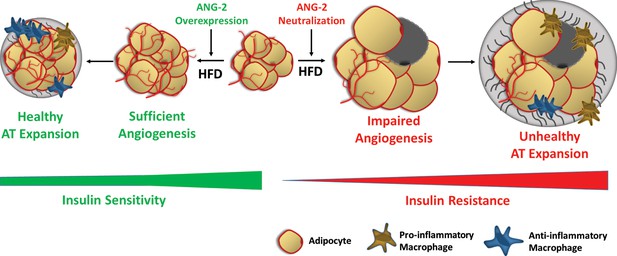
Schematic illustration of the role of ANG-2 during AT expansion and modulation of insulin sensitivity.
When ANG-2 is genetically overexpressed in the WAT upon a HFD challenge, it results in sufficient angiogenesis and leads to a further healthy AT expansion with signature improvements of vasculature and anti-inflammatory macrophage infiltration, associated with maintenance of insulin sensitivity. Conversely, during HFD feeding, if the endogenous ANG-2 is neutralized by antibodies, the AT demonstrates an unhealthy expansion pattern with features of impaired vascularization and enhanced pro-inflammatory as well as increased pro-fibrotic changes, eventually giving rise to insulin resistance.
Additional files
-
Supplementary file 1
Primer sequences for qPCR.
- https://doi.org/10.7554/eLife.24071.021
-
Supplementary file 2
Statistical information.
- https://doi.org/10.7554/eLife.24071.022





