Analysis of the NK2 homeobox gene ceh-24 reveals sublateral motor neuron control of left-right turning during sleep
Figures
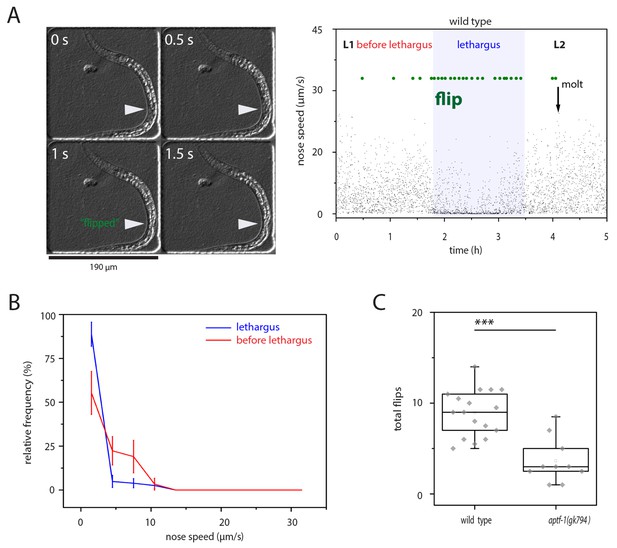
left-right turning is associated with sleep and appears to be facilitated by this behavior.
(A) Flipping during sleep: Images showing flipping in an example time lapse movie of an L1 larva cultured in a hydrogel microcompartment. Shown are DIC images. At time point 0s the developing gonad is located on the left side (arrowhead), at time point 0.5 s the larva turns and at time points 1s and 1.5s the developing gonad can be seen on the right side. The right plot shows the behavior over time during the L1 to L2 stage for one animal. Lethargus is defined here as a lack of feeding as seen as a lack of pharyngeal pumping and it is the phase in which sleep occurs, i.e. movement is strongly reduced and quiescence bouts occur. Nose speed measurements show the reduction of mobility during sleep. Flips are displayed in green. (B) Most flips occur during phases of low mobility. Shown is a frequency distribution of flips as a function of movement five seconds before the flip. Compare also images in A). (C) Flipping is strongly reduced in aptf-1(-), which is lacking sleep behavior, consistent with the view that sleep behavior facilitates flipping.
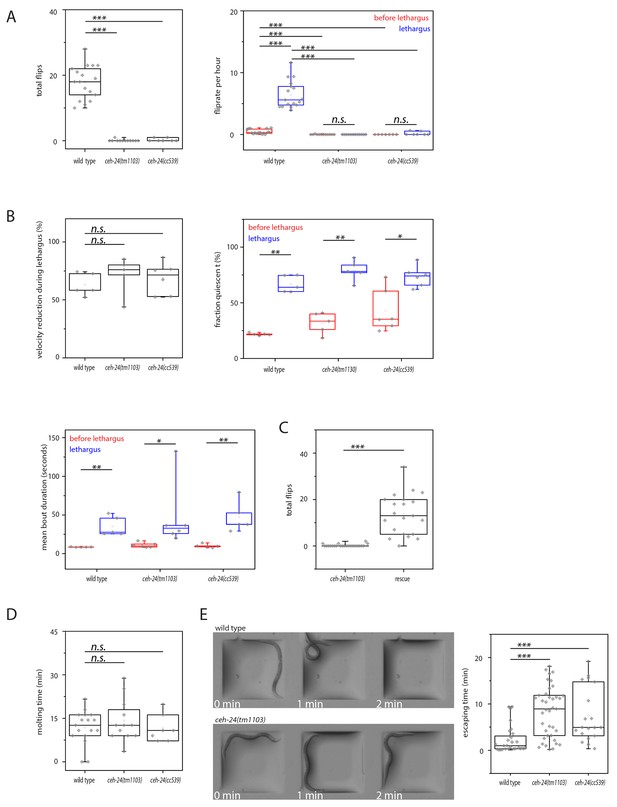
ceh-24 is required for left-right turning and escape from an indentation.
(A) Flipping is strongly reduced in ceh-24(-): The left plot shows the reduction of total number of flips scored in a movie that lasted from two hours before sleep onset until two hours after sleep end (Kolmogorov Smirnov test, wild type [N = 17], ceh-24(tm1103) [N = 12], ceh-24(cc539) [N = 7]). The right plot shows the flip rate per hour during and outside of lethargus (paired Wilcoxon Signed Ranks test for comparison of sleep versus wake, and Kolmogorov Smirnov test for comparisons between genotypes, wild type [N = 17], ceh-24(tm1103) [N = 12], ceh-24(cc539) [N = 7]). (B) Sleep behavior appears normal in ceh-24 mutants: Shown is the reduction of nose speed during lethargus compared with the speed outside of lethargus in percent (Kolmogorov Smirnov test, wild type [N = 5], ceh-24(tm1103) [N = 6], ceh-24(cc539) [N = 6]). Also shown are quiescence bouts displayed as the fraction of worms that was quiescent and the mean quiescence bout duration. (C) A ceh-24 transgene array rescues the flipping defect in ceh-24(-): a rescue transgene containing of the ceh-24 promoter and the ceh-24 coding region was transformed into ceh-24(-) mutant worms and the total number of flips was scored (Kolmogorov Smirnov test, animals were selected that expressed the array and had normal-looking sublateral neurons with proper sublateral processes, ceh-24(tm1103) [N = 24], rescue [N = 21]). (D) Cuticle shedding appears normal in ceh-24 mutant worms as judged by the time from resumption of pumping until completion of cuticle shedding (Kolmogorov Smirnov test, wild type [N = 17], ceh-24(tm1103) [N = 12], ceh-24(cc539) [N = 7]). (E) Escape from an indentation is impaired in ceh-24(-): Young adult worms were placed into shallow indentations that were printed into agarose and the time the worms needed to crawl out was scored (two-sample t-test, wild type [N = 29], ceh-24(tm1103) [N = 39], ceh-24(cc539) [N = 19]). Chamber dimension was 700 μm × 700 μm, 65 μm deep. *** denotes p<0.001, n.s. denotes p>0.05.
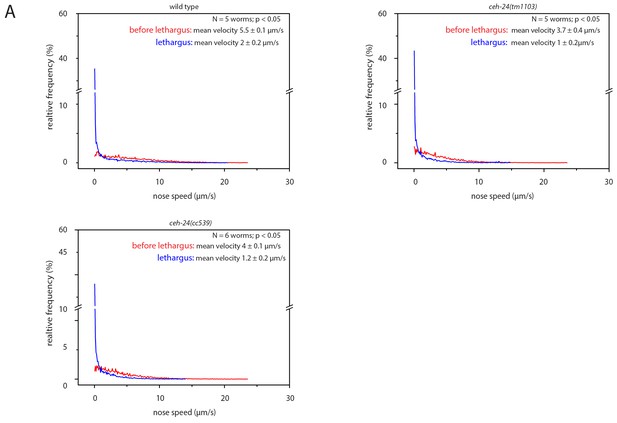
Sleep behavior appears normal in ceh-24(-).
Frequency distribution of nose speeds during lethargus and outside of lethargus in wild type, ceh-24(tm1103), and ceh-24(cc539). Relates to Figure 2.
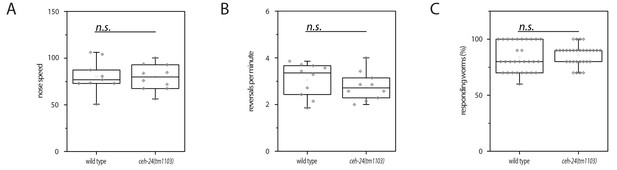
Locomotion and response to mechanical stimulation of adults on an agar surface appears normal in ceh-24(-).
(A) Crawling speed of wild type and ceh-24(tm1103). (B) The number of spontaneous reversals of wild type and ceh-24(tm1103). (C) The fraction of worms responding to gentle mechanical stimulation in wild type and ceh-24(tm1103). Relates to Figure 2.
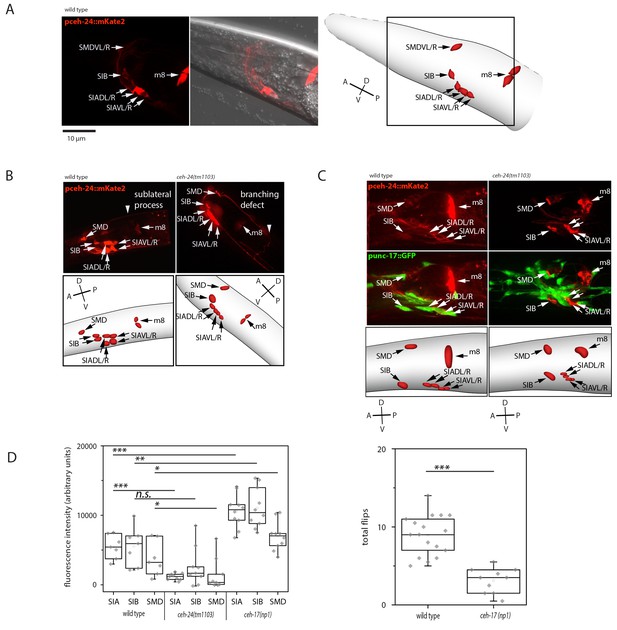
ceh-24 is required for sublateral process formation and cholinergic function.
(A) ceh-24 is expressed in the sublateral SMB, SIB, and SIA neurons as well as in the m8 muscle cells: An adult animal that is expressing mKate2 from the ceh-24 promoter is shown in DIC and in fluorescence imaging. Cartoons show worm and cell body outlines. (B) Sublateral processes are not formed properly in ceh-24(-): processes ended prematurely and branched in ceh-24(-) in all animals tested (Kolmogorov Smirnov test, p<0.001) (N = 10, 10/10 wt animals had no branching and 10/10 mutant animals had branching defects). (C) Expression of the synaptic vesicle acetylcholine transporter gene unc-17 is abolished or strongly reduced in the SIA neurons in ceh-24(-). The plot shows the quantification of unc-17-expressing neurons (Kolmogorov Smirnov test, wild type [N = 7], ceh-24(tm1103) [N = 9]). (D) ceh-17 mutants, which have a branching defect in the SIAs, have normal unc-17 expression and have a flip defect (Kolmogorov Smirnov test, wild type [N = 7], ceh-17(np1) [N = 14]). *** denotes p<0.001, ** denotes p<0.01, * denotes p<0.05, n.s. denotes p>0.05.
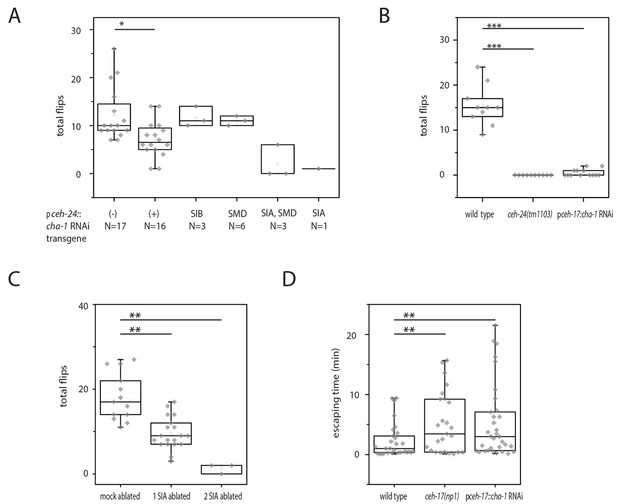
Cholinergic function in the SIA neurons is required for flipping.
(A) Mosaic analysis of cha-1 RNAi in the sublateral neurons suggests a crucial role for cholinergic transmission from the SIAs: A total of 370 individuals carrying an array expressing double-stranded RNA corresponding to cha-1 were filmed to score flipping. After filming, individuals with restricted expression in a subset of neurons were selected. (B) cha-1 RNAi in SIA only (driven by the ceh-17 promoter) confirms a crucial role for cholinergic transmission from these neurons (wild type [N = 10], ceh-14(tm1103) [N = 10], pceh-17::cha-1 RNAi [N = 13]). (C) Ablation of some of the SIAs leads to a flipping defect (mock ablated [N = 13], one SIA neuron ablated [N = 19], two SIA neurons ablated [N = 3]). (D) cha-1 RNAi in SIA leads to a defect in escaping from an indentation (wild type [N = 29], ceh-17(np1) [N = 25], pceh-17::cha-1RNAi [N = 33]). The Kolmogorov Smirnov test was used for all experiments, *** denotes p<0.001, ** denotes p<0.01, * denotes p<0.05.
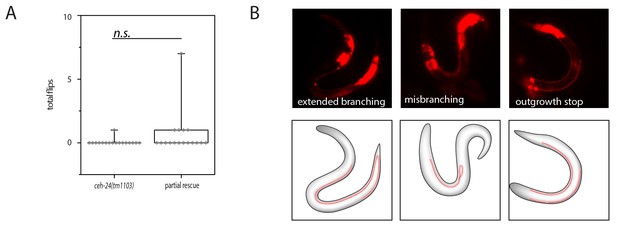
Lack of rescue of ceh-24(-) using ceh-24(+) driven by the ceh-17 promoter.
(A) Quantification of flipping in ceh-24(tm1103) and ceh-24(tm1103); Ex[pceh-17::ceh-24::SL2mKate2]. (B) Fluorescence images of outgrowth defects in ceh-24(tm1103); Ex[pceh-17::ceh-24::SL2mKate2].
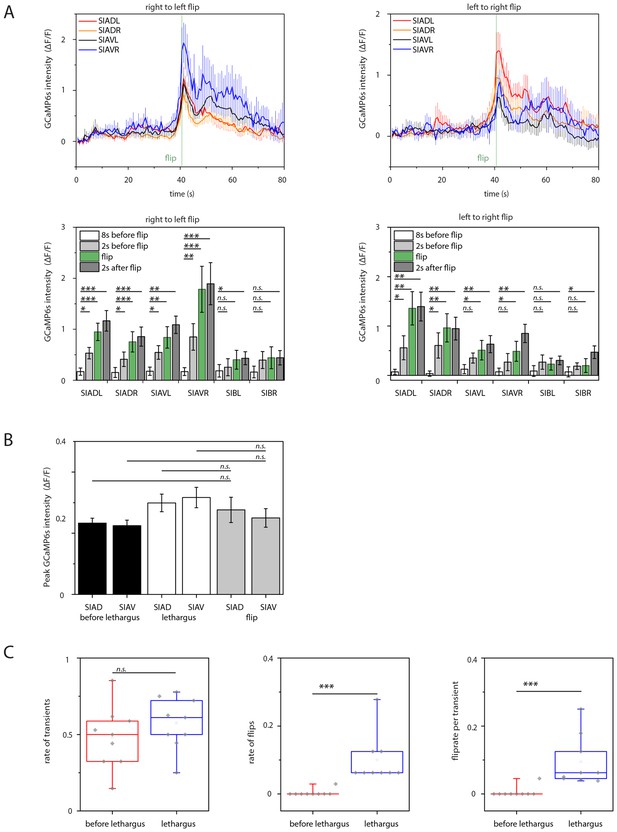
Sublateral SIA neurons are active during flipping.
(A) The SIA neurons activate during flipping: calcium measurements were aligned to the flip. SIA activation and flipping coincided. SIA activation was seen both for flips from the right side to the left side and for flips from the left side to the right side. Histograms show statistical tests for calcium transient maxima (Wilcoxon signed ranks test, right to left flip [N = 14], left to right flip [N = 12]). (B) SIA neurons show calcium transients both outside and during lethargus: SIA calcium peaks were compared in three conditions, outside lethargus, during lethargus without coincidental flipping, and during flipping (Mann-Whiney U test, nine individual worms were analyzed, numbers of activation transients analyzed were: 17 during flip, 63 during lethargus without flipping, 148 outside lethargus without flipping). (C) The probability that an activation of the SIAs coincides with a flip is increased during lethargus. (Mann-Whiney U test, N = 9). *** denotes p<0.001, ** denotes p<0.01, * denotes p<0.05, n.s. denotes p>0.05.
-
Figure 5—source data 1
SIA activation transients are highly variable outside of lethargus.
The SIAs activate with different transient strengths relative to each other and variably during and outside of lethargus: Individual SIA activation patterns during wake were unequal regarding their transient strengths and highly variable between activation transients. Each row (A–H) contains three example traces from the same individual worm, one example is shown for a flip, one for a trace containing transients during lethargus without flipping and one for a trace outside of lethargus.
- https://doi.org/10.7554/eLife.24846.011
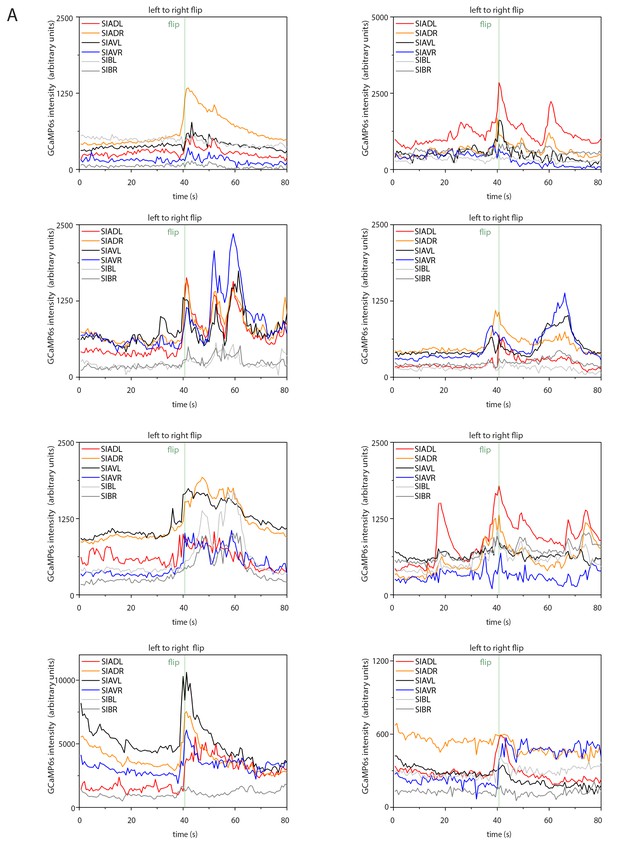
Sample calcium transients during left-to-right flipping.
Individual calcium traces during left-to-right flipping before normalization.
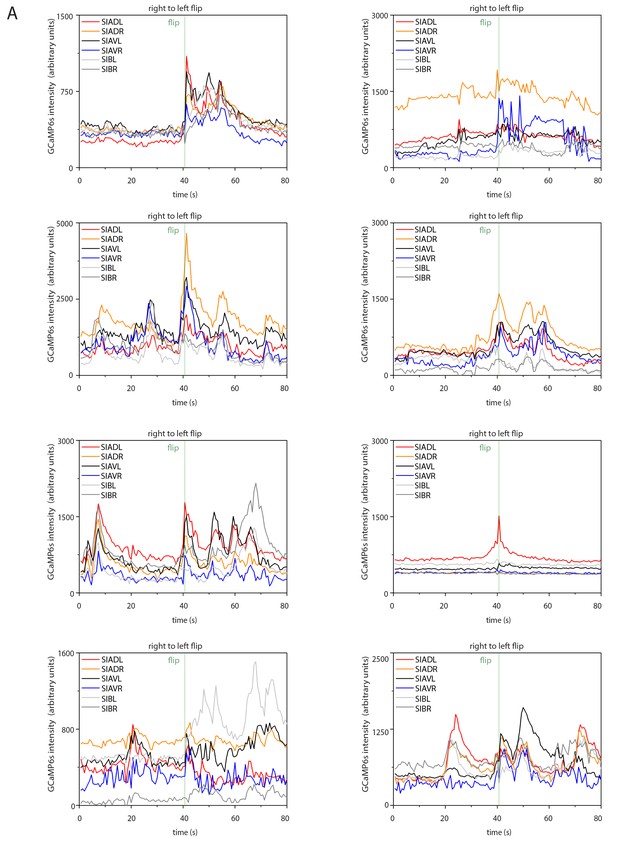
Sample calcium transients during right-to-left flipping.
Individual calcium traces during right-to-left flipping before normalization.
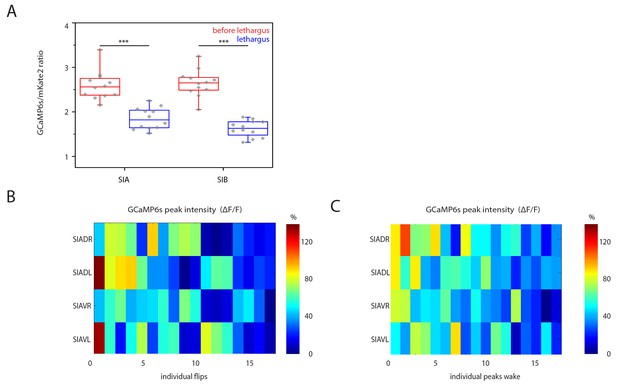
SIA baseline calcium sensor signals are reduced during lethargus and heatmap of calcium signal maxima.
(A) Baseline calcium signal levels are significantly reduced during lethargus. (B) Heatmap of the calcium transient during flipping and during outside of flipping for the SIA neurons for 17 individual animals.
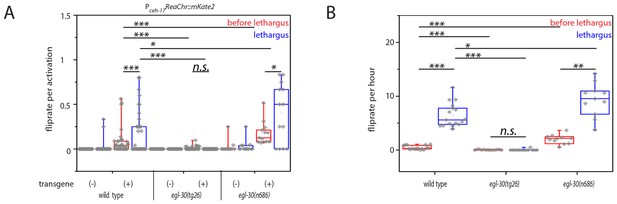
Optogenetic activation of SIA neurons can induce flipping with a higher chance during lethargus.
(A) ReaChR-induced depolarization of the SIAs triggers flipping with a higher chance during lethargus. The SIAs were filmed and were activated every 30 min with yellow light and we scored whether worms flipped during these short movies. SIA activation could induce flipping in and outside of lethargus, but the likelihood of triggering a flip was higher during lethargus. Consistent with the calcium imaging data, the majority of SIA activations did not coincide with flipping. Flipping was impaired in hyperactive egl-30 mutant worms and increased in hypoactive worms. As controls we used worms of the same strain that did not visibly express the SIA::ReaChr array. Wild type (no transgene N = 25, transgene N = 38), egl-30(tg26) (no transgene N = 20, transgene N = 40), egl-30(n686) no transgene N = 15, transgene N = 14). (B) Endogenous flipping is impaired in hyperactive worms and increased in hypoactive worms. Wild type (N = 17), egl-30(tg26) (N = 13), egl-30(n686) (N = 11). Kolmogorov-Smirnov test was used for comparisons between genotypes, paired Wilcoxon Signed Ranks test for sleep-wake comparisons, *** denotes p<0.001, ** denotes p<0.01, * denotes p<0.05, n.s. denotes p>0.05.
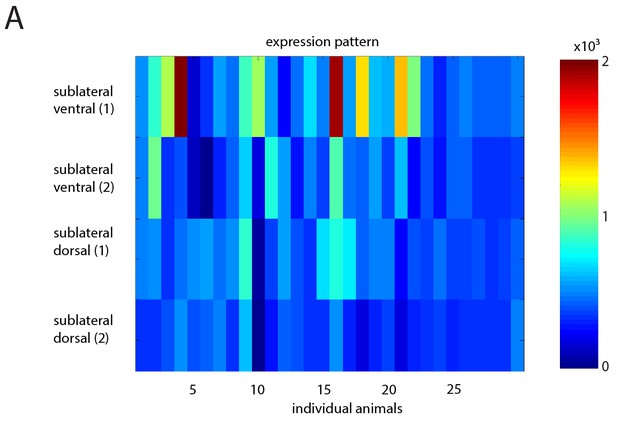
Heatmap showing variability in extrachromosomal array expression of ReaChr in the SIA neurons.
(A) Heatmap of mKate2 expression (fused to ReaChr) in individual SIAs for multiple individual animals. Compare with Figure 6.
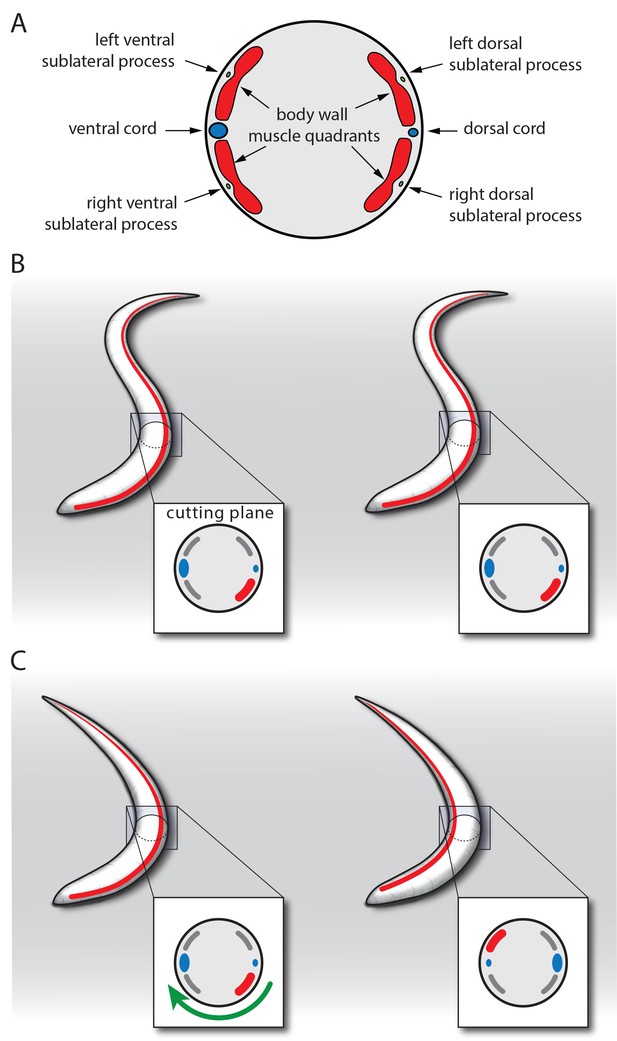
Model for SIA neuron-controlled flipping.
(A) Anatomy of the innervation of body wall muscles by sublateral neurons. Cross-section through C. elegans showing the ventral and dorsal cords that control undulating 2D movement. Body wall muscle is organized into four quadrants that run along the long axis of the worm. Along the four body wall muscle quadrants run the sublateral processes, which each emerge from one sublateral SIA neuron (SIAVL, SIAVR, SIADL, and SIADR). Thus, the separate innervation of the four body wall muscle quadrants by the four sublateral SIA processes provides an ideal anatomical basis for controlling three-dimensional movements. (B–C) Model for SIA-induced flipping: B) In a worm that is outside of lethargus, the body wall muscles are under tension and the SIA neurons may act to control specific movements. Flipping is prohibited because the worm is confined to the plane by the undulating body posture. (C) During lethargus, sleep behavior occurs and the overall activity of neurons and muscles is reduced and the worm assumes a less-bent posture. Activation of the SIAs may trigger flipping in some cases in which the musculature on the convex side contracts. This favors a bent posture with the side of the contraction occurring on the concave side. To transition to this posture, the worm rotates 180 degrees around its longitudinal axis. Sublateral SIA neurons likely act as motor neurons that control this three-dimensional motion through separate innervation of the four body wall muscle quadrants.





