Chronic lithium treatment elicits its antimanic effects via BDNF-TrkB dependent synaptic downscaling
Figures
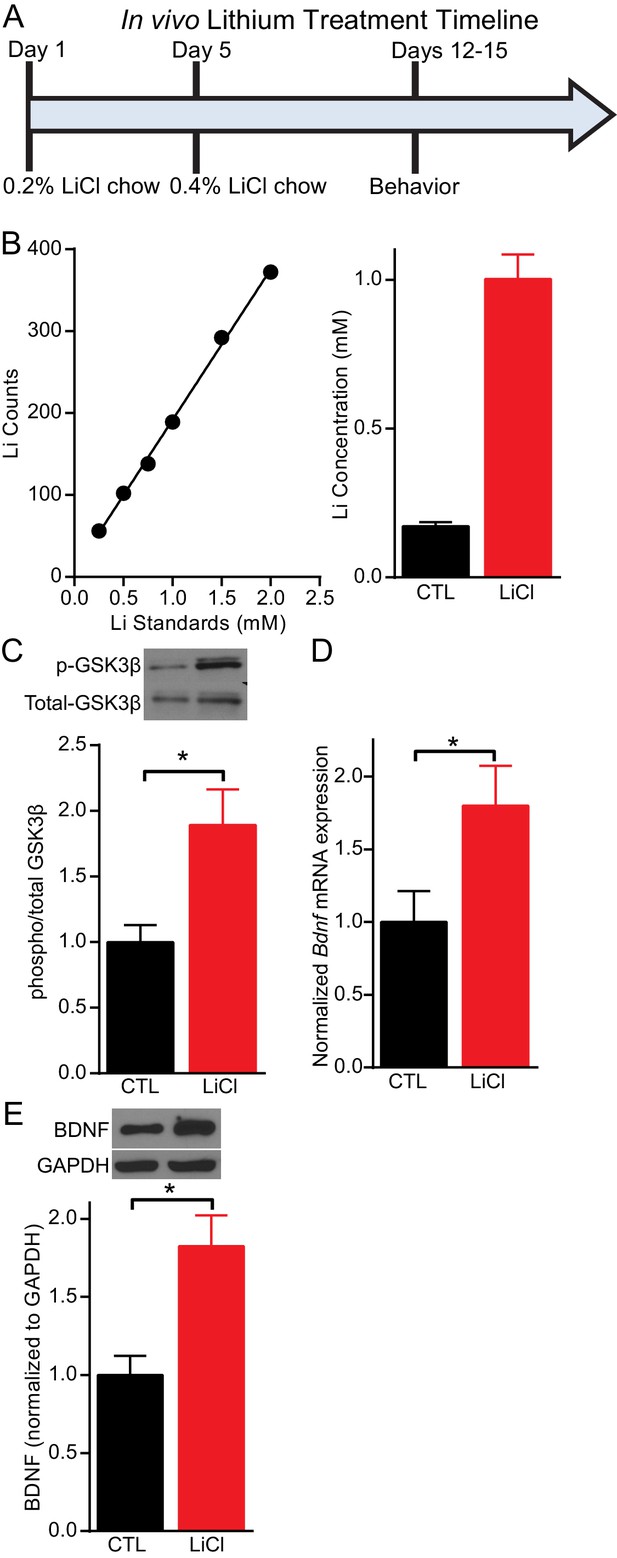
Lithium serum concentration within the therapeutic range increases BDNF expression in mice.
(A) Timeline of chronic lithium chloride (LiCl) treatment and behavioral testing. (B) (Left) Example standard curve of lithium counts used to calculate lithium concentration in blood serum. (Right). Average lithium concentration in blood serum in control and treated mice (n= 9–12 per group). (C) Chronic lithium treatment in mice produces a significant decrease in immobility in the FST compared to the control group (Student’s unpaired t test *p<0.0001, n = 10 mice per group). (D) Chronic lithium treatment caused a significant increase in Bdnf mRNA expression in the hippocampus (Student’s unpaired t test *p=0.04, n = 8–9 mice per group). (E) Chronic lithium treatment caused a significant increase in BDNF protein expression in the hippocampus (Student’s unpaired t test *p=0.002, n = 10 mice per group).
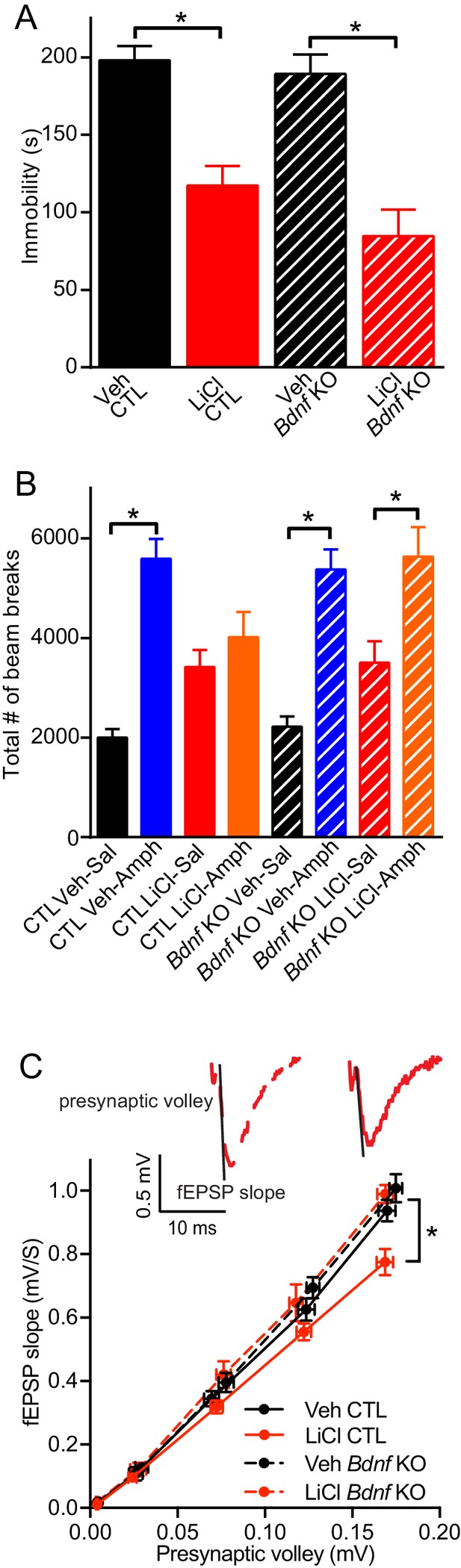
BDNF expression is required for the antimanic-like effect of lithium.
(A) Chronic lithium treatment caused a significant decrease in the immobility time in the FST in littermate CTL and inducible Bdnf KO mice in comparison to vehicle chow treated mice (ANOVA F3,90 = 18.1 *p<0.0001, Dunnett’s multiple comparisons Veh CTL vs LiCl CTL *p<0.0001, Veh Bdnf KO vs LiCl Bdnf KO *p<0.0001, n = 19–28 mice per group). (B) Acute amphetamine injection caused a significant increase in locomotor activity in control and Bdnf KO mice in comparison to the saline injected mice. Lithium treatment resulted in a nonsignificant increase in locomotor activity of CTL and Bdnf KO mice compared to vehicle treated mice. Chronic lithium treatment blunted the increased locomotor activity following acute amphetamine injection in the CTL littermate mice, but not in the Bdnf KO mice (ANOVA F7,98 = 12.75 p<0.0001, Tukey’s multiple comparisons CTL Veh-Sal vs CTL Veh-Amph *p<0.0001, CTL LiCl-Sal vs CTL LiCl-Amph p=0.97, CTL Veh-Sal vs CTL LiCl-Sal p=0.23, Bdnf KO Veh-Sal vs Bdnf KO Veh-Amph *p<0.0001, Bdnf KO LiCl-Sal vs Bdnf KO LiCl-Amph *p=0.01, Bdnf KO Veh-Sal vs Bdnf KO LiCl-Sal p=0.44, n = 11–15 mice per group). (C) Chronic lithium treatment resulted in a 20% reduction in the I/O curve in the CTL mice chronically treated with LiCl chow in comparison to Veh CTL mice, which is plotted as the slope of the fEPSP is plotted as a function of the presynaptic fiber volley. Linear fit slopes were calculated for Veh CTL(5.53 ± 0.2) vs CTL LiCl (4.656 ± 0.03825). There were no significant differences in the fEPSP slope between Veh Bdnf KO slices (5.85 ± 0.178) and LiCl treated Bdnf KO (5.899 ± 0.1703). (Two-way ANOVA Interaction F1,29 = 3.24 p=0.08, Row (Genotype) F1,29 = 5.09 *p=0.032, Column (Diet) F1,29 = 12.61 *p=0.001. Sidak’s multiple comparisons Veh CTL vs LiCl CTL *p=0.029, Veh Bdnf KO vs LiCl Bdnf KO p=0.999, Veh CTL vs Veh Bdnf KO p=0.739, LiCl CTL vs LiCl Bdnf KO *p=0.007 n = 6–10 recordings per group).
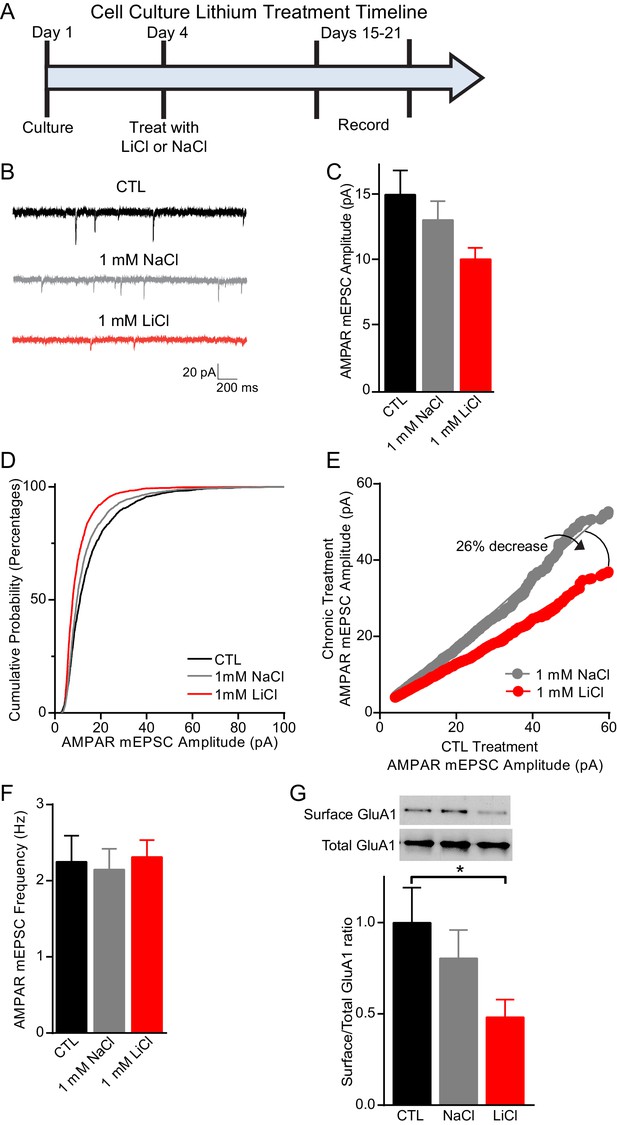
Chronic lithium treatment causes a significant decrease in synaptic scaling and surface GluA1 expression.
(A) Timeline for treatment of dissociated hippocampal neurons with LiCl and NaCl. (B) Example traces of AMPAR mEPSCs from CTL untreated (top), 1 mM NaCl treated (middle) and 1 mM LiCl treated (bottom) dissociated hippocampal neurons. (C) Chronic LiCl treatment of cultured hippocampal neurons caused a trend towards decreased AMPA mEPSC average amplitudes in comparison to CTL neurons. Chronic NaCl treatment did not cause a change in average AMPA mEPSC amplitude in comparison to CTL neurons (ANOVA F2,39 = 2.597 p=0.0873, Bonferroni’s multiple comparisons CTL vs NaCl p>0.999, CTL vs LiCl p=0.091). (D) Cumulative probability histogram showing a significant leftward shift (decrease) in the amplitudes of AMPAR-mEPSCs from cells chronically treated with 1 mM LiCl in comparison to CTL untreated and 1 mM NaCl treated neurons (Kolmogorov-Smirnov test: CTL vs 1 mM >LiCl *p=1.75×10−43, D = 0.234, 1 mM NaCl vs 1 mM LiCl *p=8.14×10−27, D = 0.167, n= 12–18 recordings per condition). (E) Rank order plot of CTL untreated AMPAR mEPSC amplitudes vs 1 mM LiCl AMPAR mEPSC amplitudes revealed a 41% decrease. Rank order plot of 1 mM NaCl AMPAR mEPSC amplitudes vs 1 mM LiCl AMPAR mEPSC amplitudes showed a 26% decrease. (CTL vs LiCl line of best fit y = 0.59x, CTL vs NaCl line of best fit y = 0.85x, Difference between NaCl and LiCl. 26, n= 12–18 recordings per condition). (F) AMPAR-mEPSC frequency is indistinguishable between CTL untreated, NaCl treated, and LiCl treated neurons (ANOVA F4,57 = 0.129 p=0.97, n = 12–18 recordings). (G) Surface biotinylation experiments revealed that chronic lithium treatment of hippocampal neurons results in a significant decrease in the surface/total GluA1 ratio (ANOVA F2,21 = 2.911 p=0.0766, Dunnett’s multiple comparisons CTL vs NaCl p=0.58, CTL vs LiCl *p=0.04, n= 3 separate experiments).
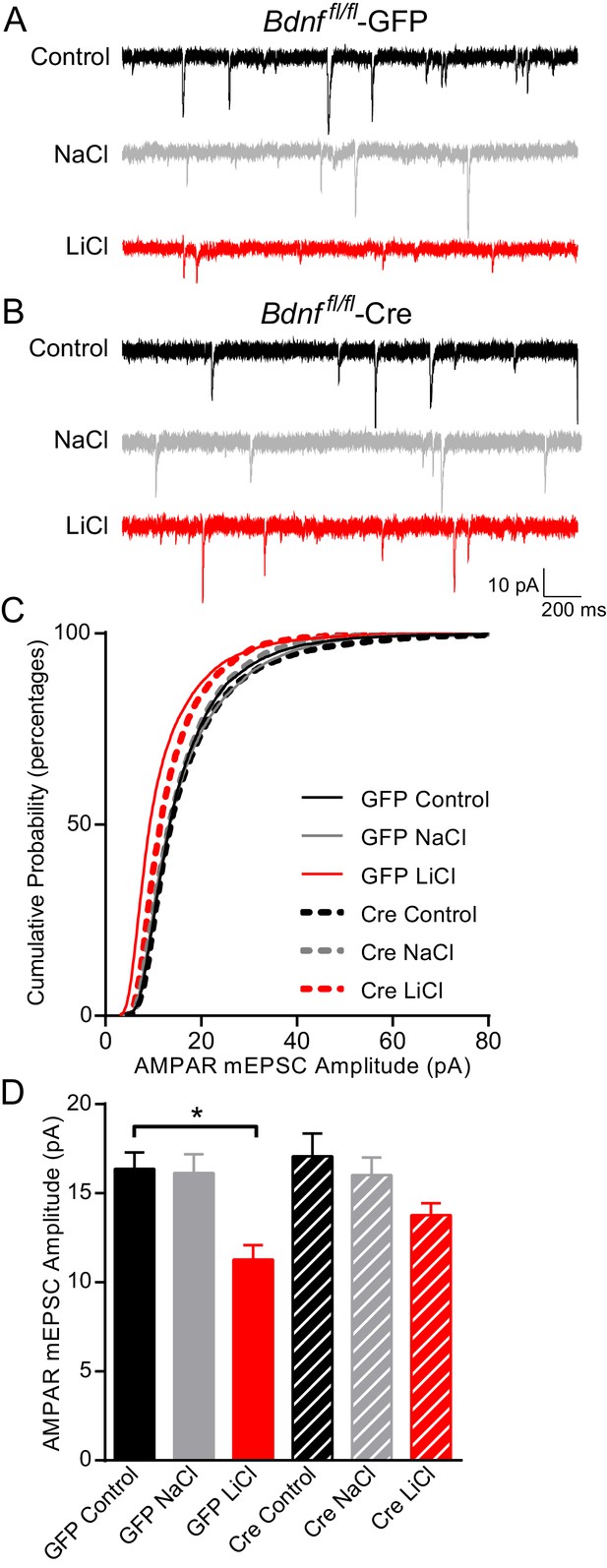
BDNF is required for the lithium-mediated decrease in AMPAR mEPSC amplitude.
(A) Example traces from GFP infected Bdnf fl/fl neurons with no treatment (CTL, top) or 11–17 day incubation with 1 mM NaCl (middle) or 1 mM LiCl (bottom). (B) Example traces from Cre infected Bdnf fl/fl neurons with no treatment (control, top) or 11–17 day incubation with 1 mM NaCl (middle) or 1 mM LiCl (bottom). (C) Cumulative probability histogram showing a significant leftward shift (decrease) in AMPAR mEPSC amplitudes between Bdnf fl/fl -GFP control untreated and Bdnf fl/fl -GFP LiCl conditions. There is also a significant leftward shift (decrease) between Bdnf fl/fl -GFP LiCl and Bdnf fl/fl -Cre LiCl conditions (Kolmogorov-Smirnov test: GFP Control vs GFP LiCl p<0.0001 D = 0.309, GFP LiCl vs Cre LiCl *p<0.0001 D = 0.19, n = 10–13 recordings per condition). (D) Lithium caused a significant decrease in AMPAR mEPSC amplitudes in Bdnf fl/fl -GFP neurons compared to control Bdnf fl/fl -GFP neurons. However, lithium did not impact AMPAR mEPSC amplitudes between Bdnf fl/fl -Cre neurons in comparison to untreated control Bdnf fl/fl -Cre neurons (ANOVA F5,59 = 5.694 *p=0.0002, Tukey’s multiple comparisons GFP Control vs GFP LiCl *p=0.003, Cre Control vs Cre LiCl p=0.197, n = 10–13 recordings per condition).
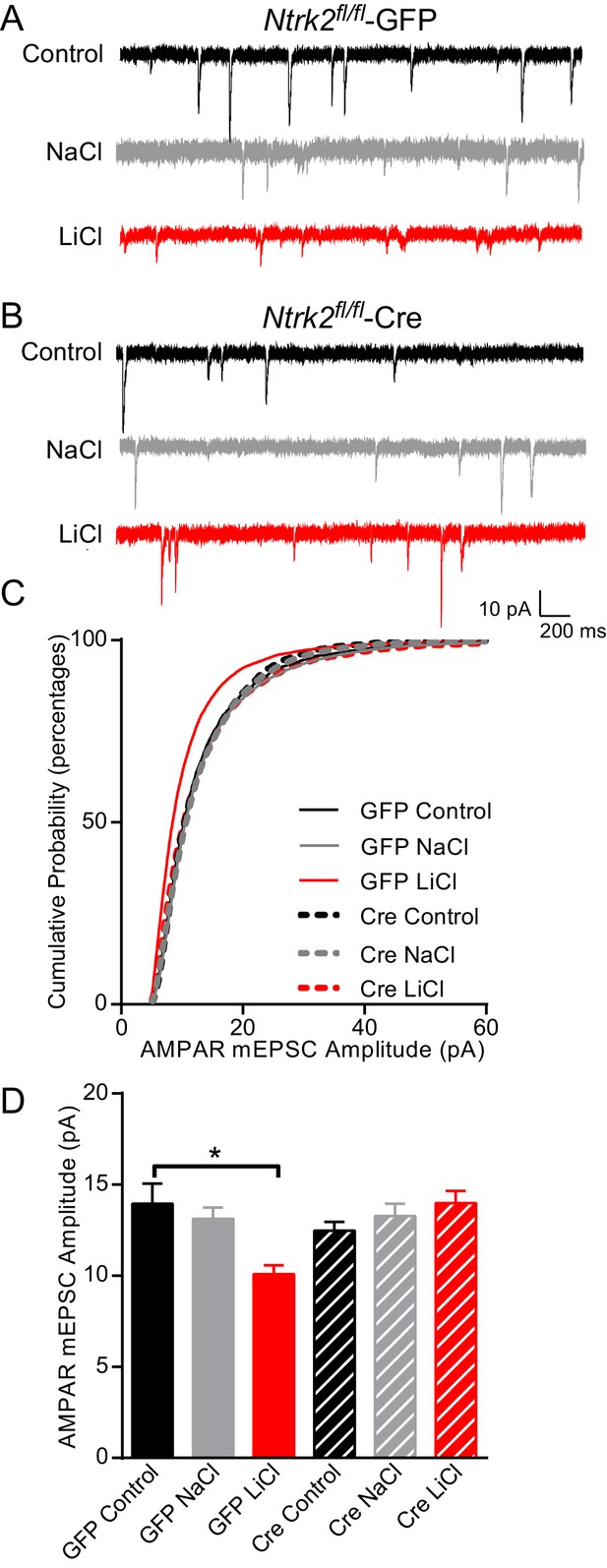
Lithium-mediated decrease in AMPAR mEPSC amplitude is dependent on TrkB.
(A) Example traces from GFP infected Ntrk2 fl/fl neurons, untreated Control (top), 1 mM NaCl treatment (middle), and 1 mM LiCl treatment (bottom). (B) Examples from Cre infected Ntrk2 fl/fl neurons, untreated Control (top), 1 mM NaCl treatment (middle) and 1 mM LiCl treatment (bottom). (C) Cumulative probability histogram showing a significant leftward shift (decrease) in AMPAR-mEPSC amplitudes from Ntrk2 fl/fl -GFP neurons treated with 1 mM LiCl. (Kolmogorov-Smirnov test: GFP control vs GFP LiCl *p<0.0001, D = 0.181, GFP LiCl vs Cre LiCl *p<0.0001, D = 0.153, n = 9–17 recordings per condition). (D) Lithium caused a significant decrease in AMPAR-mEPSC amplitudes in Ntrk2 fl/fl -GFP neurons compared to untreated control Ntrk2 fl/fl -GFP neurons. However, lithium was unable to cause any significant changes in AMPAR-mEPSCs in Ntrk2 fl/fl -Cre neurons compared to untreated Ntrk2 fl/fl -Cre control (ANOVA F5,76 = 5.107 *p=0.0004, Bonferonni multiple comparison test: GFP control vs GFP LiCl *p=0.002, Cre Control vs Cre LiCl p>0.999, n = 9–17 recordings per condition).
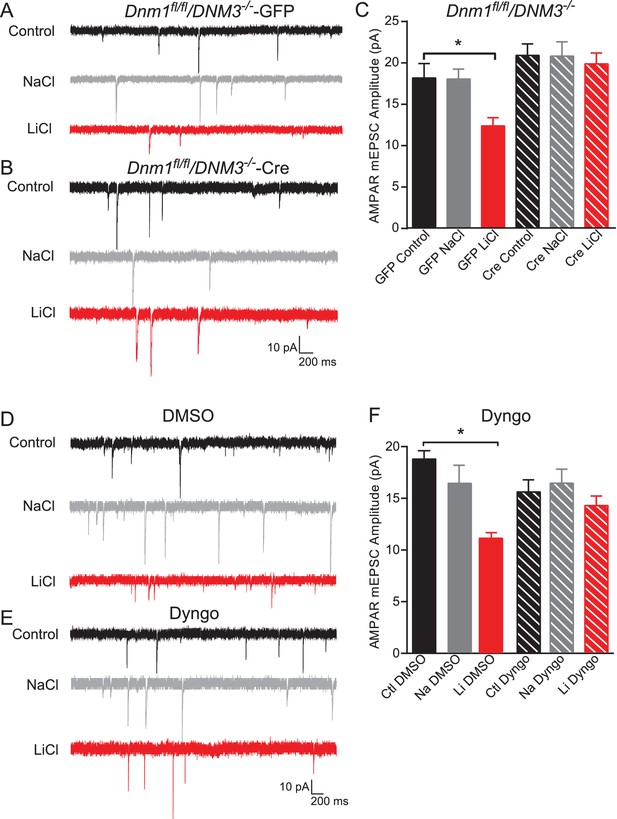
Dynamin-dependent endocytosis is required for lithium-mediated decrease in AMPAR-mEPSC amplitudes.
(A) Example traces from GFP infected Dnm1 fl/fl/Dnm3−/− neurons, control (top), chronic NaCl treatment (1 mM, middle) and chronic LiCl treatment (1 mM, bottom). (B) Example traces from Cre infected Dnm1 fl/fl/Dnm3−/− neurons, control (top), chronic NaCl treatment (1 mM, middle), and chronic LiCl treatment (1 mM, bottom). (C) Chronic lithium treatment caused a significant decrease in AMPAR mEPSC amplitude in Dnm1 fl/fl/Dnm3−/− neurons infected with lenti-GFP virus compared to untreated Dnm1 fl/fl/Dnm3−/− neurons. However, there was no significant difference in AMPAR-mEPSC amplitudes between untreated and lithium treated Dnm1 fl/fl/Dnm3−/− neurons infected with lenti-CreGFP virus (ANOVA F5,68 = 5.048 *p=0.0006, Bonferonni multiple comparison test: GFP control vs GFP LiCl *p=0.02, Cre Control vs Cre LiCl p=0.455, n = 8–12 recordings per condition). (D) Example traces from wildtype neurons recorded with DMSO-internal solution, control (top), chronic NaCl treatment (1 mM, middle), and chronic LiCl treatment (1 mM, bottom). (E) Example traces from wildtype neurons recorded with Dyngo-internal solution, control (top), chronic NaCl treatment (1 mM, middle), and chronic LiCl treatment (1 mM, bottom). (F) In comparison to control untreated neurons, chronic lithium treatment caused a significant decrease in AMPAR-mEPSC amplitudes. In contrast, lithium did not cause a significant change in AMPAR-mEPSC amplitudes in comparison to the untreated control when Dyngo is included in the internal pipette solution (ANOVA F5,69 = 6.985, *p<0.0001, Bonferonni multiple comparison test: DMSO control vs DMSO LiCl *p<0.0001, Dyngo Control vs Dyngo LiCl p>0.999, n = 11–17 recordings per condition).





