H+- and Na+- elicited rapid changes of the microtubule cytoskeleton in the biflagellated green alga Chlamydomonas
Figures
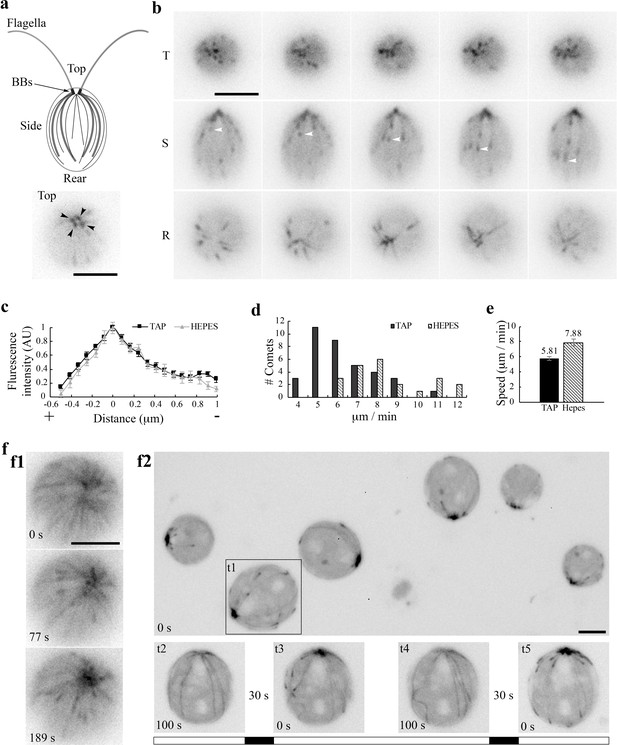
EB1 in Chlamydomonas.
(a) A schematic picture depicting flagella and the MT network in the cell body (top panel). Black dots, basal bodies (BB). Thick lines, four stable rootlet microtubule bundles. Thin lines in the cell body, the dynamic cortical MTs. A top view of EB1-NG transgenic cells reveals a pattern that resembles 4 BBs (bottom panel). (b) Time-lapse fluorescent images taken 10 s apart from the top (T), side (S) and rear (R) of cells resuspended in the TAP culture medium. EB1-NG appeared like typical comets (arrowheads), emerging from the BB area, coursing along the contour of the cell body and then vanishing as approaching the rear end. The frame rate was 1 frame/s. (c) Normalized line scans along the length of MT plus ends showed a similar EB1 intensity profile in the TAP medium (n = 18 comets from 6 cells) and the Na+/HEPES buffer (n = 11 comets from 3 cells). The position with peak intensity was designated as 0. The value was negative toward plus end; positive toward BBs. AU, arbitrary unit of fluorescence intensity. (d) The distribution and (e) the mean and the SEM of EB1 comet speed in the TAP medium (n = 36 comets from 6 cells in 6 recordings) and 5 mM Na+/HEPES buffer (n = 22 comets from 3 cells in 3 recordings) are significant different (Mann-Whitney U test, p<0.001). (f) Altered MT patterns during fluorescence microscopy. The EB1 comet pattern occasionally switched to a bird cage pattern (f1). Comets returned while the bird cage pattern receded in ~ 1 min. In flattened cells that were compressed by the cover slip gradually, both MTs and comets became explicit (f2, top panel). Comets disappeared after ~100 s (bottom panel, t2), but returned after illumination was switched off for 30 s (t3). The process was repeatable after another 100 s illumination and then another light off period (t4 and t5). The alternate white and black bars illustrate the scheme of alternate illumination and dark periods. Scale bars, 5 μm.
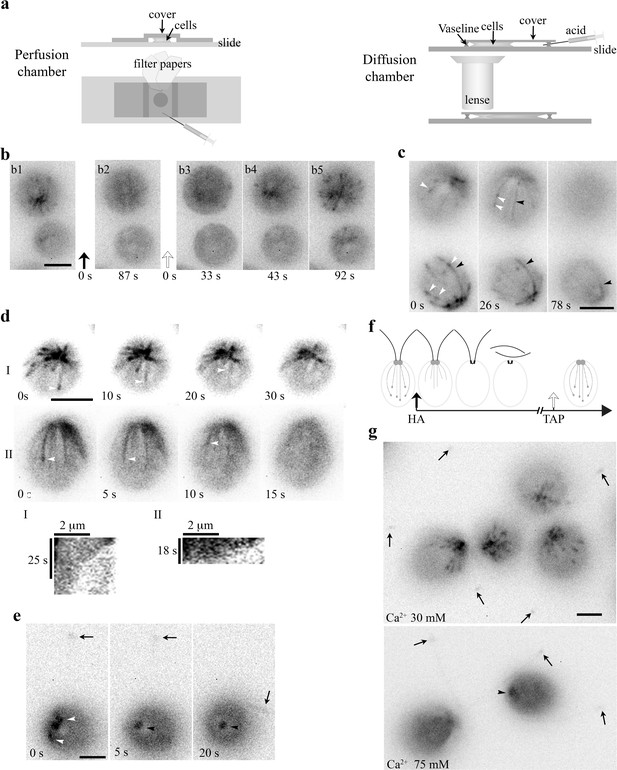
An HA pulse elicited swift sequential changes in the MT system.
(a) Schematics depicting an open-ended perfusion chamber (left panels) and a diffusion chamber (right panels) for capturing the HA-induced rapid changes. (b) A 10 μl aliquot of cells resuspended in the TAP medium was placed in a perfusion chamber. The images (b1, 2) were captured before and after perfusion with 20 mM HA/TAP (pH4.5, t = 0, black arrow). The following recordings (b3-5) captured the events right after the TAP medium (pH7) was injected to wash away HA (t = 0, clear arrow). B3 is the first clear image after fluid and cells stopped flowing. Comets already disappeared within 87 s after HA perfusion. They started emerging 43 s after wash. (c) The process preceding HA-induced disappearance of EB1 comets in diffusion chambers. A 40 μl aliquot of cells resuspended in HEPES was placed in a diffusion chamber encircled by Vaseline, under the coverslip and an objective lens. HA was injected to the other side of the chamber and diffused toward cells that were being imaged. During the gradual acidification process, both comets (white arrowheads) and shank binding MTs (black arrowheads) were evident first and then both patterns vanished. (d) Time-lapse images and kymographs revealed endwise resorption of EB1-decorated MTs (white arrowheads). (e) Comets (white arrowheads) in the cell body vanished first before the excision of flagella (arrows). Following deflagellation, EB1 diffused away from the tip. EB1 signals remained at BBs but was static (black arrowhead). (f) A schematic depicting sequential changes in MTs upon exposure to HA and a subsequent wash with the TAP medium. Dotted lines with a comet, growing MTs. Dotted lines alone, shrinking MTs. Solid lines, shrinking MTs with EB1 shank binding. (g) Effects of [Ca2+]ex on MTs. In cells resuspended in 30 mM [Ca2+]ex, flagella remained attached (arrows in left panel), while comets were vibrant. In the 75 mM [Ca2+]ex group, comets disappeared and flagella were amputated (arrows in right panel). Static EB1 signals remained at BBs (arrowhead). Scale bars, 5 μm.
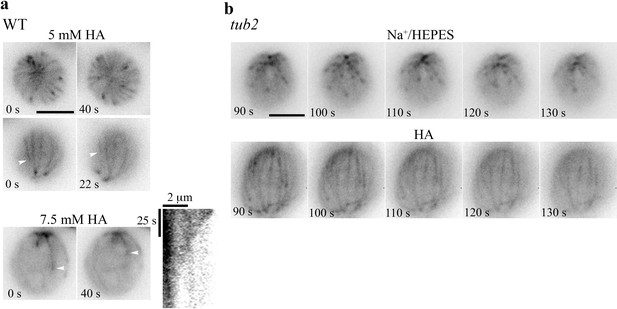
Abatements of HA-induced changes.
(a) Concentration-dependent HA effects on MTs in WT cells. WT cells resuspended in 5 mM HA exhibited a bird cage pattern with dynamic comets (top and middle panels). Shrinking MTs were visible in the two side view images taken 22 s apart (white arrowhead). Most patterns were absent in cells resuspended in 7.5 mM HA, except a few shrinking MTs (bottom panels, white arrowheads). A kymograph revealed the endwise resorption. (b) EB1-NG in tub2 cells appeared as normal comets in pH7.4 Na+/HEPES (top panels), but as a bird cage with fine MT fibers in 10 mM pH3 HA (bottom panels). Most MTs froze, but some appeared in and out of focus. Time-lapse images were shown at 10 s intervals. Scale bars, 5 μm.
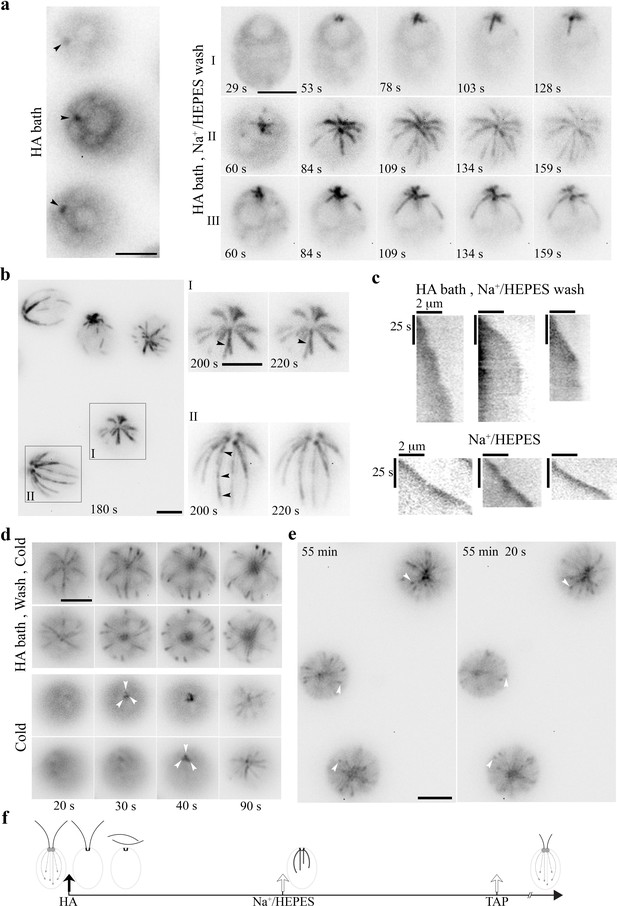
HA bath and a subsequent wash induced long-lived yet reversible changes to the MT system.
(a) Static EB1 signals remained at BBs after cells were resuspended in 10 mM pH3 HA for 5 min (left panel). After replacing HA with the HEPES buffer, EB1 signal at the BB area intensified within 1 min (black arrowheads). But newly formed MTs were thick and prominent, lacking the typical comet (right panel, cell I). In cells recorded 60 s after wash (cell II and III), MT elongation slowed down gradually. (b) In cells imaged ~180 s after wash, EB1-decorated MTs in all cells stopped growing (top panel), as highlighted in two additional images of two representative cells captured 20 s apart. In addition, a MT fiber in cell I split into two (arrowhead), while a fiber in cell II had multiple comets aligned in tandem (arrowheads), as if new MTs nucleated or grew on older ones. (c) Kymographs comparing the growth of three representative MTs in cells pretreated with 5 min HA bath and then the wash buffer (top panels) and in control cells in the HEPES wash buffer (lower panels). Comets in the latter manifested as an intense spot at the plus end. The sharper slopes in the former indicated slower growth. Unchanged slopes indicated paused growth. (d) Long-lived MTs formed after HA bath and wash were cold resistant (top panels). As shown in two representative cells, frozen MTs remained in the image captured 20 s after 3 min on ice. The changes in subsequent images were due to focal plane drifts as cells were floating gradually as warming up. In control cells without previous HA exposure (bottom panels), EB1 signals were absent initially (20 s), consistent with cold lability. Dynamic comets (white arrowheads) gradually emerged at the BB area afterwards. (e) Although the MT system froze within minutes after HA bath and HEPES wash (t = 0 min), dynamic comets resumed after 55 mins in cells recovered in TAP media as shown in two images captured 20 s apart. Scale bars, 5 μm. (f) A schematic summarizing the sequence of MT changes induced by HA bath and wash.
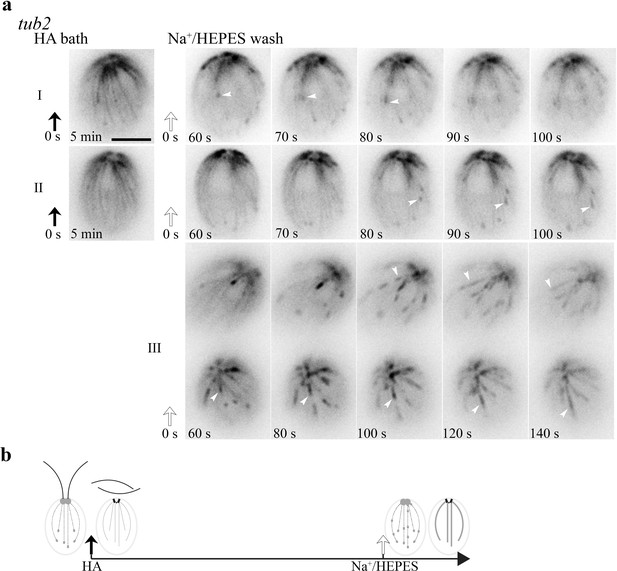
The MT pattern in tub2 cells recovered from the HA bath.
(a) The frozen bird cage pattern of fine MTs remains after 5 min HA bath as shown in two representative cells (left panel). After wash, dynamics resumes within 60 s. Notably, comets (panel I and II, white arrowheads) reemerged at the plus end of existing fine MTs, instead of the BB area. Meanwhile the bird cage pattern was fading. MTs were growing, but comets were lengthening and the pace was very slow. In videos recorded only after wash, the recovery was faster. Some comets already emerged from the BB area. Some moved along old MTs and lengthened (panel III, white arrowheads). (b) A schematic summary of HA-induced changes in tub2. Scale bars, 5 μm.
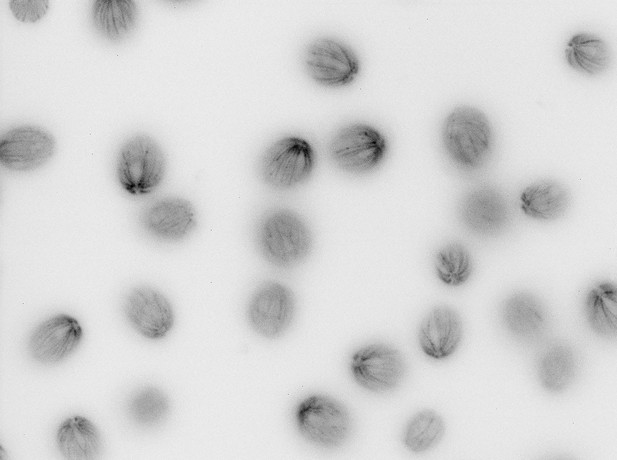
(for Figure 5a) tub2 cells in 5 min HA bath.
https://doi.org/10.7554/eLife.26002.013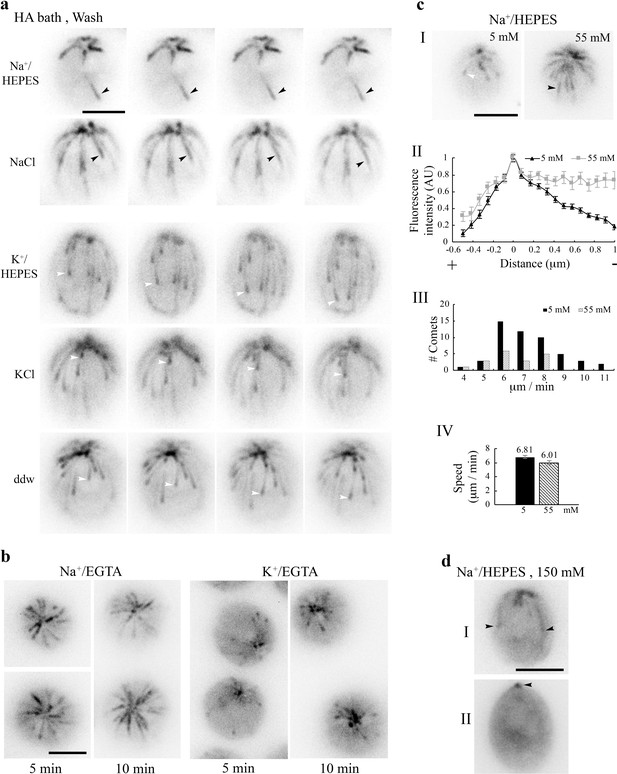
Na+-dependent changes of the MT system.
(a) MTs in cells were largely frozen after 5 min 10 mM pH3 HA bath and 3 min in the wash solution, such as 5 mM pH7.4 Na+/HEPES buffer or 5 mM NaCl solution (black arrowheads). In contrast, growing MTs with a comet (white arrowheads) returned if the wash buffer lacked Na+, such as 5 mM K+/HEPES buffer, 5 mM KCl solution, or ddw. (b) Thick MTs in cells resuspended in 21 mM Na+/EGTA for 5 min or 10 min (left panel), contrary to comets in cells in 21 mM K+/EGTA (right panel). Thick MTs were still growing after 5 min incubation but static after 10 min incubation. (c) High [Na+]ex, without pre-exposure to HA, was sufficient to alter comet patterns. Contrary to typical comets in cells resuspended in the HEPES buffer with 5 mM Na+, long comets were thick in cells resuspended in 55 mM Na+ for 5 min (panel I). Normalized linescans confirmed little tapered intensity (panel II, n = 36 comets from 11 cells in 5 mM Na+; n = 13 comets from 4 cells in 55 mM Na+). As shown in the range of speed (panel III), long comets were moving, and the mean speeds of short and long comets were significantly different (panel IV, n = 51 from 11 cells in 5 mM Na+; n = 18 from 4 cells in 55 mM Na+) (p<0.05). (d) Two representative cells after 5 min in 150 mM Na+/HEPES. Some cells still retained a few thick MTs (cell I). Some only had static EB1 signals at the BB area (cell II, arrowhead). Scale bars, 5 μm.
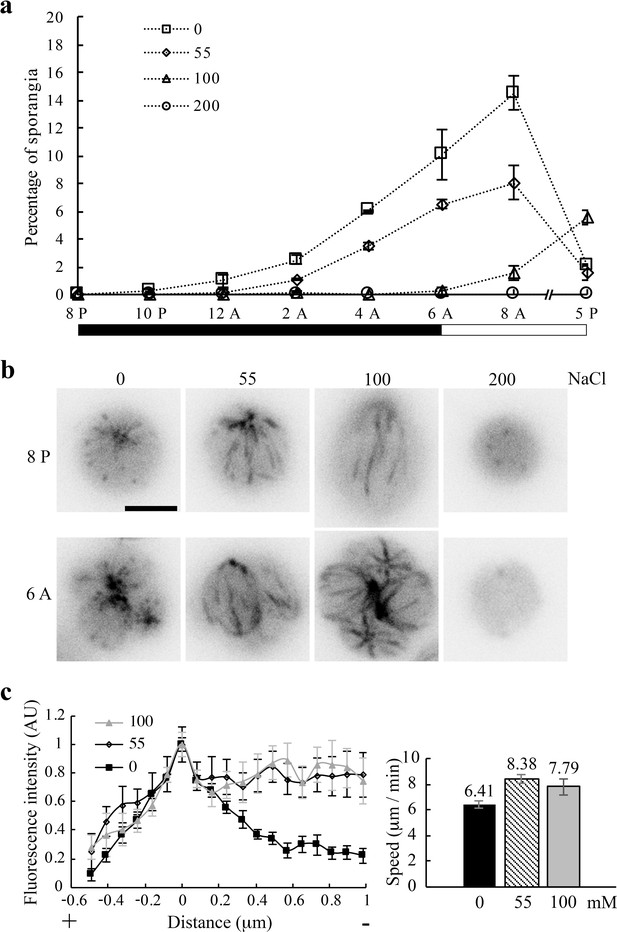
Na+ concentration-dependent inhibition of cell division.
(a) Reduced abundance and delayed appearance of sporangia with newly divided daughter cells as a function of NaCl concentration in TAP media. Triplicate cell pellets from a typical log-phase culture were suspended in each indicated solution immediately before the dark period. One aliquot from each sample was fixed with 2% glutaraldehyde periodically for 12 hr and then at 5 PM. (n > 500 single cells and sporangia). Black bar, dark period; clear bar, light period. (b) Representative single cells and sporangia for each [NaCl] group immediately before and after the dark period respectively. Comets lengthened in 55 mM and 100 mM Na+/TAP, while MTs remained dynamic in cells at both states. No comet or sporangium was evident in 200 mM samples. Scale bar, 5 μm. (c) Quantifications of comets. A linescan plot of comets in 8 PM samples (left panel) showed longer comets in cells in the 55 mM (n = 8 comets from 3 cells at 55 mM) and 100 mM group (n = 7 comets from 2 cells) than that in the the 0 mM group (n = 13 comets from 8 cells). Mean comet speeds (right panel) in the 55 mM (n = 24 from 5 cells) and 100 mM group (n = 10 from 2 cells) taken at 10 PM were significantly faster than that in the 0 mM group (n = 42 from 7 cells) (two-tailed t test, p<0.001 for the 0 mM and 55 mM group; Mann-Whitney U test, p=0.05 for the 0 mM and 100 mM group). The difference of the 55 mM and 100 mM group was not statistically significant (two-tailed t test, p=0.385).
Videos
(for Figure 1b) EB1-NG comets in WT cells.
https://doi.org/10.7554/eLife.26002.003(for Figure 1f1) Transient bird-cage pattern in WT cells that occurred sporadically during imaging.
https://doi.org/10.7554/eLife.26002.004(for Figure 1f2) Disappearance and return of comets in compressed cells following alternate periods of illumination and darkness.
https://doi.org/10.7554/eLife.26002.005(for Figure 3a) WT cells treated with 5 mM HA.
https://doi.org/10.7554/eLife.26002.009(for Figure 4a) WT cells in Na+/HEPES after HA bath.
https://doi.org/10.7554/eLife.26002.011(for Figure 5a) tub2 cells in Na+/HEPES after HA bath.
https://doi.org/10.7554/eLife.26002.014Tables
Summary of the treatments and corresponding patterns of microtubules (MTs) and EB1-NeonGreen (NG) in wild type and tub2 mutant cells.
Identical treatments were highlighted with a same gray shade for easy comparison. HA, acetic acid; TAP, Tris-Acetate-Phosphate culture medium.
| Treatments | MT (revealed by EB1-NG) | EB1-NG |
|---|---|---|
| WT | ||
| TAP or 5 mM Na+/HEPES (Control) | Dynamic; cold labile | Comets |
| 20 mM pH4.5 HA/TAP pulse (perfusion as pH shock) | Invisible | No comets |
| 100 mM HA pulse (diffusion) | Shrink | Fibers; long comets |
| 10 mM pH3.0 HA | Invisible | |
| 7.5 mM pH3.4 HA | Shrink | Fibers; no comets |
| 5.0 mM pH3.5 HA | Dynamic | Fibers; comets |
| 10 mM pH3.0 HA bath; 5 mM [Na+]ex wash | Freeze; bundle/branch; cold resistant | Fibers; no comets |
| 10 mM pH3.0 HA bath; 5 mM [K+]ex wash | Dynamic | Comets |
| 21 mM Na+/EGTA | Freeze; bundle/branch; cold resistant | Fibers; no comets |
| 21 mM K+/EGTA | Dynamic | Comets |
| 55 mM [Na+]ex | Dynamic | Long comets |
| 150 mM [Na+]ex | Absence or a few low dynamic MTs | Fibers; no comets |
| TAP + 55 mM [Na+]ex | Dynamic | Fibers; long comets |
| TAP + 100 mM [Na+]ex | Dynamic | Fibers; long comets |
| TAP + 200 mM [Na+]ex | Absence or a few low dynamic MTs | Fibers; no comets |
| tub2 (colchicine-resistant) | ||
| TAP or 5 mM Na+/HEPES | Dynamic | Comets |
| 10 mM pH3.0 HA | Freeze | Fibers; no comets |
| 10 mM pH3.0 HA bath; 5 mM [Na]ex wash | Freeze; bundle/branch | Fibers; no comets |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.26002.017





