Analysis of SUMO1-conjugation at synapses
Figures
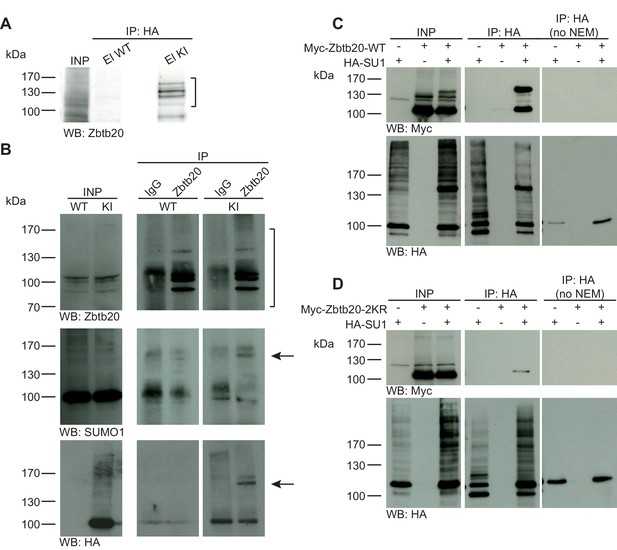
Zbtb20 is a SUMO1 substrate in vivo and in vitro.
(A) SDS-PAGE (4–12%) followed by anti-Zbtb20 Western blot analysis of input and HA peptide eluate fractions from anti-HA immunoprecipitation in the presence of 20 mM NEM from WT and His6-HA-SUMO1 KI brains. Zbtb20 is specifically enriched in KI eluate (bracket), indicating that Zbtb20 is a SUMO1 target in vivo. (B) Representative SDS-PAGE (8%) followed by anti-Zbtb20, SUMO1, and HA Western blot analysis of input and eluate fractions of anti-Zbtb20 and anti-IgG immunopurifications from WT and His6-HA-SUMO1 KI brains in the presence of 20 mM NEM. Anti-Zbtb20 Western blot confirm the enrichment of Zbtb20 in both WT and KI brain samples solely when anti-Zbtb20 antibody is used (upper panel, brackets). Anti-SUMO1 Western blot analysis revealed a shifted band corresponding to SUMO1-Zbtb20 in both WT and KI (middle panel, black arrow). Anti-HA Western blot revealed a shifted band corresponding to His6-HA-SUMO1-Zbtb20 solely in KI eluate (lower panel, black arrow). (C) SDS-PAGE (10%) followed by Western blot analysis of input and eluate fractions of anti-HA immunoprecipitation in the presence or absence of 20 mM NEM from HEK cells overexpressing HA-SUMO1 and Myc-Zbtb20-WT, alone or in combination. Anti-HA Western blot confirms enrichment of HA-SUMO1 conjugates in the presence of NEM while all SUMO1 conjugates (with the exception of RanGAP1) are lost when NEM is omitted (lower panel). Anti-Myc Western blot analysis confirms the co-enrichment of Myc-Zbtb20-WT in the presence of NEM and when co-expressed with HA-SUMO1. (D) SDS-PAGE (10%) followed by Western blot analysis of input and eluate fractions of anti-HA immunoprecipitation in the presence or absence of 20 mM NEM from HEK cells overexpressing HA-SUMO1 and Myc-Zbtb20-2KR, alone or in combination. Anti-HA Western blot confirms enrichment of HA-SUMO1 conjugates in the presence of NEM while all SUMO1 conjugates (with the exception of RanGAP1) are lost when NEM is omitted (lower panel). Anti-Myc Western blot analysis shows that Myc-Zbtb20-2KR is not co-enriched in the presence of NEM and when co-expressed with HA-SUMO1 (upper panel). Images are representatives of at least three independent experiments.
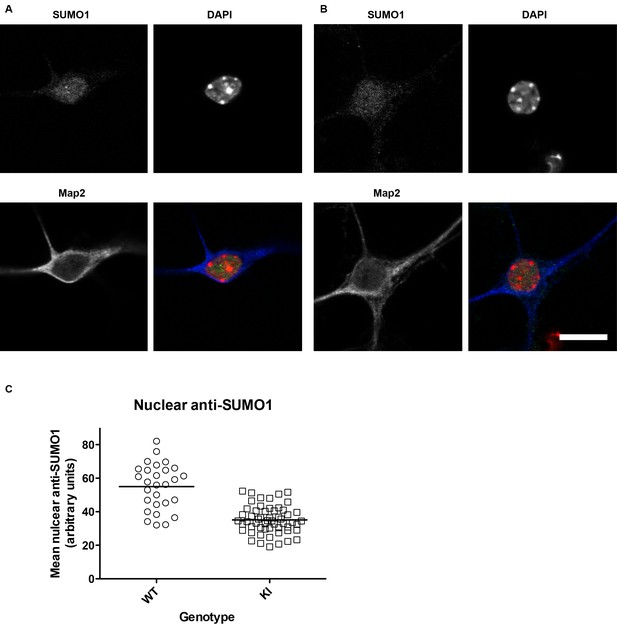
SUMO1 levels in the nuclei of His6-HA-SUMO1 KI neurons (also relates to Figures 2–8, which include results obtained with the His6-HA-SUMO1 KI).
Primary hippocampal neurons from WT (12 days in vitro) and His6-HA-SUMO1 KI mice (11 days in vitro) were fixed, permeabilised with Triton X-100, and immunolabelled with antibodies against SUMO1 and Map2, as well as DAPI to label nuclei. Representative images are shown of a WT neuron (A) and a His6-HA-SUMO1 KI neuron (B). (C) Graph showing the nuclear anti-SUMO1 labelling intensity of neuronal nuclei from WT and His6-HA-SUMO1 KI mice (WT, N = 2 mice, 28 neurons; His6-HA-SUMO1 KI, N = 4 mice, 56 neurons). The scatter plots show the data from individual cells while the horizontal line shows the mean nuclear anti-SUMO1 intensity from each genotype (WT, 54.8 AU, His6-HA-SUMO1 KI, 35 AU). Scale bar = 10 µm.

Characterisation of the anti-HA immunoprecipitation and of the anti-Zbtb20 antibody.
(A) Characterisation of the anti-HA immunoprecipitation in the His6-HA-SUMO1 KI line. SDS-PAGE (4–12%) followed by Western blot analysis of input and HA peptide eluate fraction using anti-HA antibody from anti-HA IP from WT and His6-HA-SUMO1 KI brains in the presence of 20 mM NEM. Anti-HA signal is observed solely in the KI eluate, indicating the specific and strong enrichment of His6-HA-SUMO1 species. (B) Characterization of the specificity of anti-Zbtb20 antibody. Total brain homogenate from embryonic day E18 mice being (WT, +/+; heterozygous Zbtb20 KO, +/−, and homozygous Zbtb20 KO) analysed by SDS-PAGE (10%) and Western blotting with anti-Zbtb20 antibodies. Western blot analysis using anti-Actin antibody ensured equal loading of lanes.
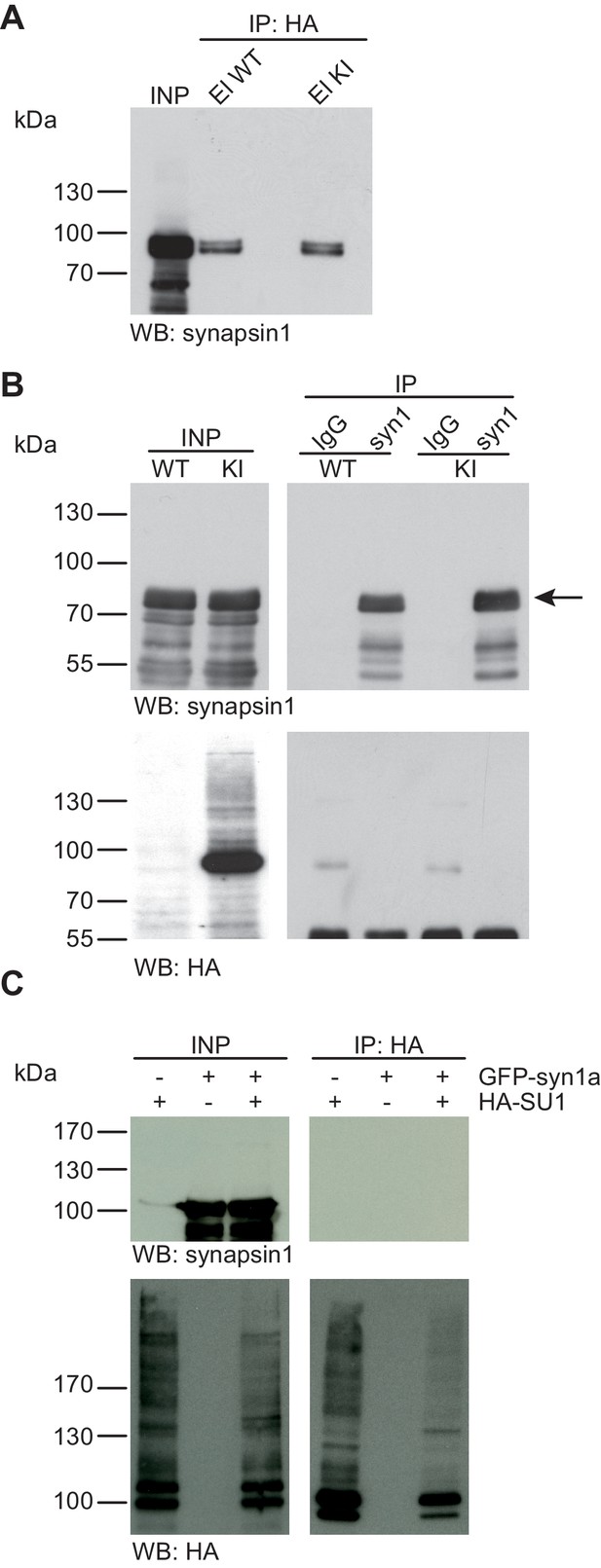
Synapsin1a is not SUMO1-conjugated in vivo and in vitro.
(A) SDS-PAGE (4–12%) followed by Western blot analysis using anti-synapsin1 antibody of input and HA peptide eluate fractions from anti-HA immunoprecipitation in the presence of 20 mM NEM from WT and His6-HA-SUMO1 KI brains. The presence of synapsin1 in both WT and KI eluates indicates non-specific binding of synapsin1a to the affinity matrix. (B) Representative SDS-PAGE (10%) followed by Western blot analysis of input and eluate fractions of anti-synapsin1 and anti-IgG immunopurifications in the presence of 20 mM NEM from WT and His6-HA-SUMO1 KI brains. Anti-synapsin1 Western blot confirms enrichment of synapsin1 after affinity purification using synapsin1 antibody and not using nonrelated IgG (black arrow). However, anti-HA Western blot does not detect any SUMO1-synapsin1 shifted band. (C) SDS-PAGE (10%) followed by Western blot analysis of input and eluate fractions of anti-HA immunoprecipitation in the presence of 20 mM NEM from HEK cells overexpressing HA-SUMO1 and GFP-synapsin1a, alone or in combination. While anti-HA Western blot confirms enrichment of HA-SUMO1 species (lower panel), synapsin1a is not detected in the eluate samples (upper panel). Images are representatives of at least three independent experiments.
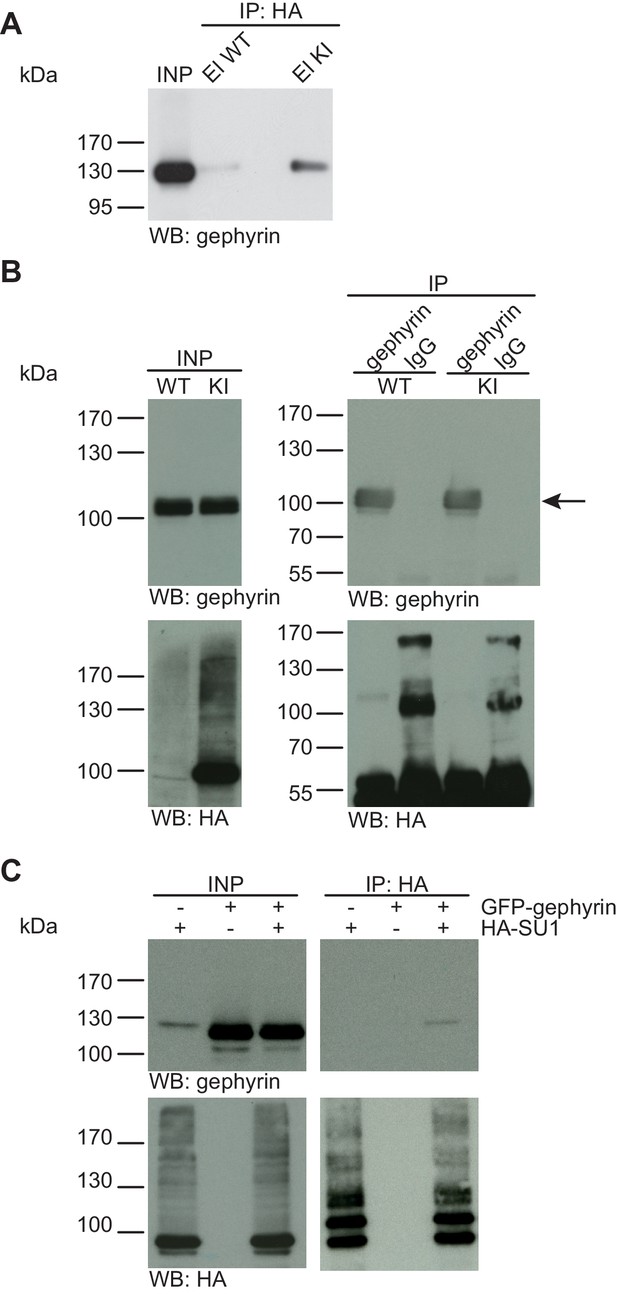
Gephyrin is not SUMO1-conjugated in vivo and in vitro.
(A) SDS-PAGE (4–12%) followed by anti-gephyrin Western blot analysis of input and HA peptide eluate fractions from anti-HA immunoprecipitation in the presence of 20 mM NEM from WT and His6-HA-SUMO1 KI brains. The presence of gephyrin in both WT and KI eluates indicates non-specific binding of gephyrin to the affinity matrix. (B) Representative SDS-PAGE (10%) followed by Western blot analysis of input and eluate fractions of anti-gephyrin and anti-IgG immunopurifications in the presence of 20 mM NEM from WT and His6-HA-SUMO1 KI brains. Anti-gepyhrin Western blot confirms the enrichment of gepyhrin in both WT and KI brains after affinity purification using anti-gephyrin antibody but not when using mouse IgG (black arrow, upper panel). Importantly, anti-HA Western blot analysis does not reveal SUMO1-gephyrin bands. (C) SDS-PAGE (10%) followed by Western blot analysis of input and eluate fractions of anti-HA immunoprecipitation in the presence of 20 mM NEM from HEK cells overexpressing HA-SUMO1 and GFP-gephyrin, alone or in combination. Anti-HA Western blot analysis confirms the enrichment of HA-SUMO1 conjugates (lower panel) but no SUMO1-gephyrin signal is observed in the eluate fractions (upper panel). Images are representatives of at least three independent experiments.
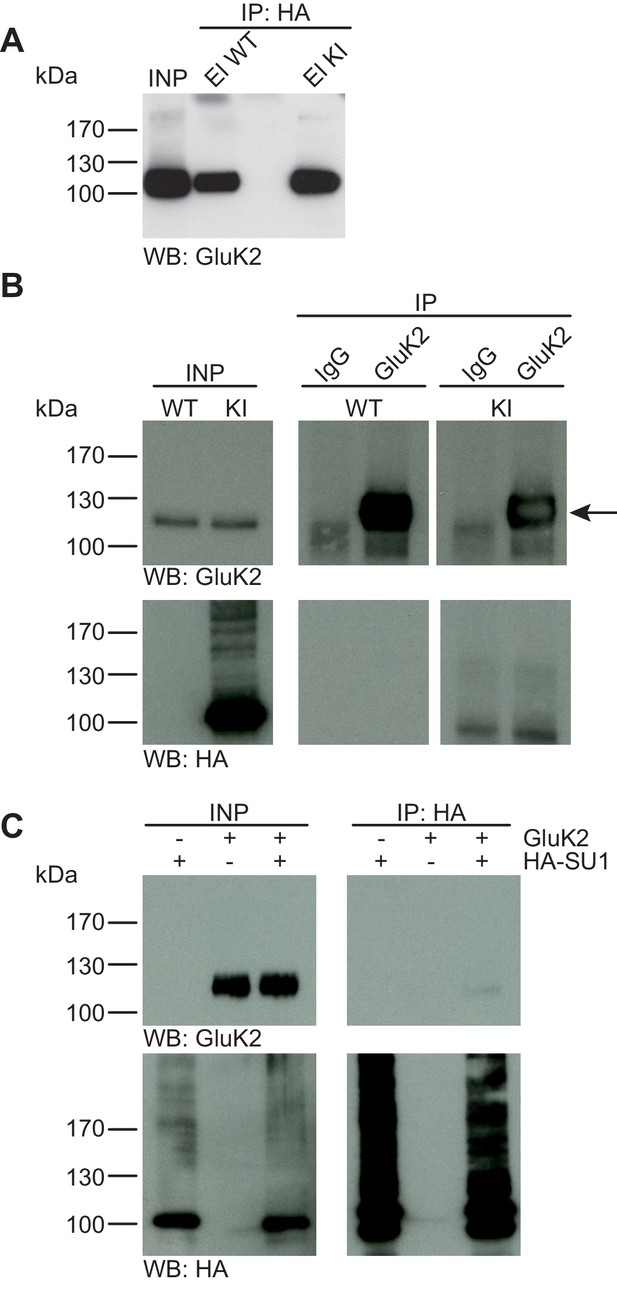
GluK2 is not SUMO1-conjugated in vivo and in vitro.
(A) SDS-PAGE (4–12%) followed by Anti-GluK2 Western blot analysis of input and HA peptide eluate fractions from anti-HA immunoprecipitation in the presence of 20 mM NEM from WT and His6-HA-SUMO1 KI brain. The presence of GluK2 in both WT and KI eluates indicates non-specific binding of GluK2 to the affinity matrix. (B) Representative SDS-PAGE (10%) followed by anti-HA and anti-GluK2 Western blot analysis of input and eluate fractions from anti-GluK2 and anti-IgG immunoprecipitations in the presence of 20 mM NEM from WT and His6-HA-SUMO1 KI brains. GluK2 is specifically enriched in both WT and KI samples after affinity purification using anti-GluK2 but not in control experiment conducted with rabbit IgG (upper panel). However, anti-HA Western blot does not reveal any band that could have corresponded to SUMO1-GluK2 (lower panel). (C) Representative SDS-PAGE (10%) followed by anti-HA and anti-GluK2 Western blot of input and eluate fractions from anti-HA immunopreciptation in the presence of 20 mM NEM from HEK cells overexpressing HA-SUMO1 and GluK2, alone or in combination. Anti-HA Western blot analysis confirms the enrichment of HA-SUMO1 conjugates (lower panel) but no SUMO1-GluK2 band is observed in the eluate fractions (upper panel). Images are representatives of at least three independent experiments.
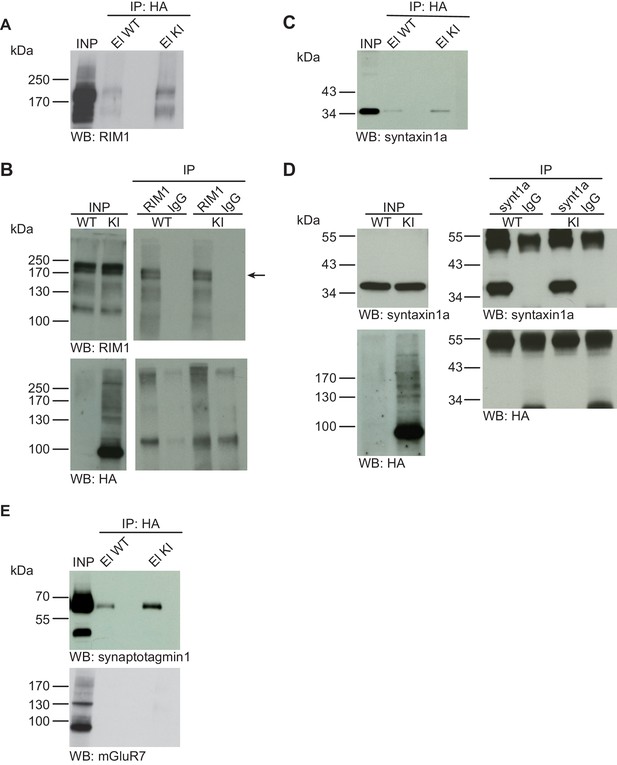
Syntaxin1, RIM1, SynaptotagminI, and mGluR7 are not SUMO1-conjugated in vivo.
(A) SDS-PAGE (4–12%) followed by Western blot analysis using anti-RIM1 antibody of input and HA peptide eluate fractions from anti-HA immunoprecipitation in the presence of 20 mM NEM from WT and His6-HA-SUMO1 KI brains. The presence of RIM1 in both WT and KI eluates indicates non-specific binding of RIM1 to the affinity matrix (B) Representative SDS-PAGE (8%) followed by Western blot analysis of input and eluate fractions of anti-RIM1 and anti-IgG immunopurifications in the presence of 20 mM NEM from WT and His6-HA-SUMO1 KI brains. Western blot analysis using anti-RIM1 confirms the enrichment of RIM1 in both WT and KI samples solely when anti-RIM1 antibody is used (upper panel). However, no SUMO1-RIM1 band is apparent (lower panel). (C) SDS-PAGE (4–12%) followed by anti-syntaxin1α Western blot analysis of input and HA peptide eluate fractions from anti-HA immunoprecipitation in the presence of 20 mM NEM from WT and His6-HA-SUMO1 KI brains. The presence of syntaxin1a in both WT and KI eluates indicates non-specific binding of syntaxin1a to the affinity matrix (D) Representative SDS-PAGE (12%) followed by Western blot analysis of input and eluate fractions of anti-syntaxin1α and anti-IgG immunopurifications in the presence or absence of 20 mM NEM from WT and His6-HA-SUMO1 KI brains. Western blot analysis using anti-syntaxin1a confirms the enrichment of syntaxin1a in both WT and KI samples solely when anti-syntaxin1a antibody is used (upper panel). However, no SUMO1-syntaxin1a band is apparent (lower panel). (E) SDS-PAGE (4–12%) followed by anti-synaptotagmin1 and mGluR7 Western blot of input and anti-HA peptide eluate fractions from anti-HA immunoprecipitation in the presence of 20 mM NEM from WT and His6-HA-SUMO1 KI brains. The presence of synaptotagmin1 in both WT and KI eluates indicates non-specific binding of synaptotagmin1 to the affinity matrix, while mGluR7 is not enriched in either case. Images are representatives of at least three independent experiments.
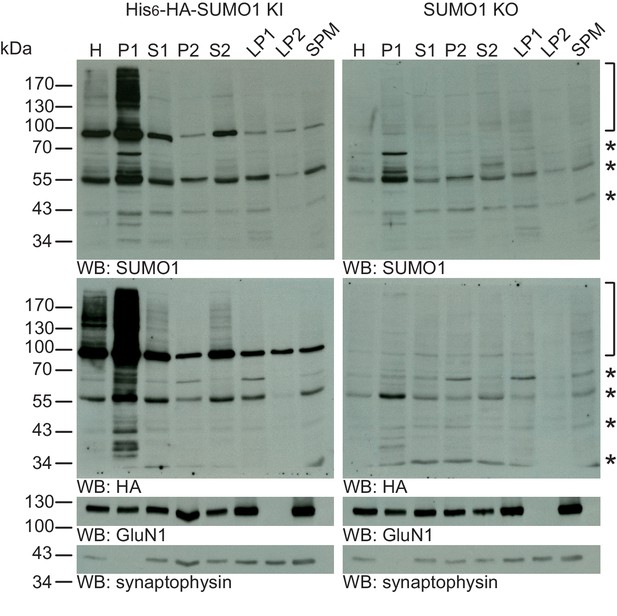
SUMO1 conjugates are mainly in nuclear fractions.
SDS-PAGE (4–12%) followed by Western blot analysis of subcellular fractions performed in the presence of 20 mM NEM of His6-HA-SUMO1 KI and WT mouse brain using anti-SUMO1 (upper two panels) and anti-HA antibodies (below), and antibodies to GluN1 and synaptophysin to validate the fractionation procedure (lower two panels). Bracket indicates specific SUMO1 substrates while stars indicate non-specific SUMO1 bands present in both His6-HA-SUMO1 KI and SUMO1 KO. H, homogenate; P1, nuclear pellet; S1, supernatant after P1 sedimentation; P2, crude synaptosomal pellet; S2, supernatant after P2 sedimentation; LP1, lysed synaptosomal membranes; LS1, supernatant after LP1 sedimentation; SPM, synaptic plasma membranes. Images are representatives of at least three independent experiments.
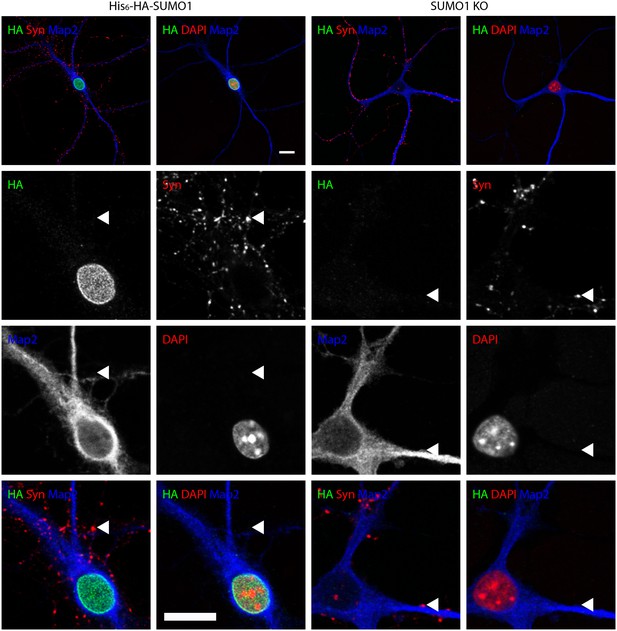
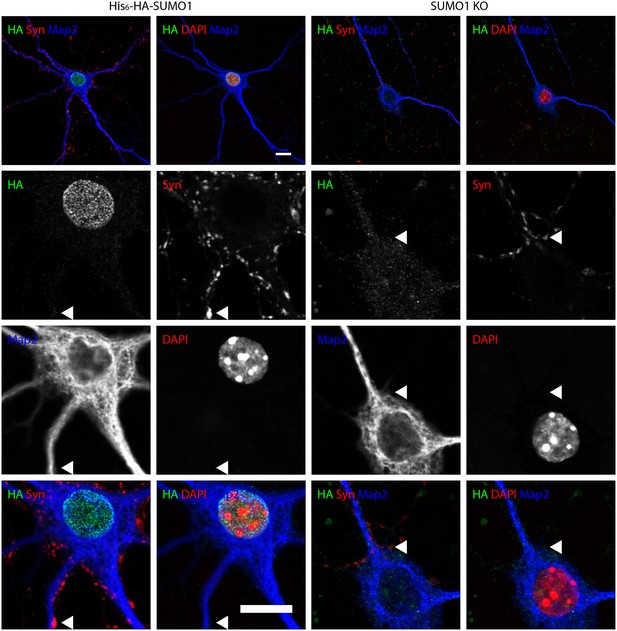
Anti-HA immunolabelling in His6-HA-SUMO1 KI neurons permeabilised with Triton X-100 is mainly nuclear.
Hippocampal neurons from either His6-HA-SUMO1 KI mice or SUMO1 KO mice were fixed in 4% PFA and permeabilised using 0.3% Triton X-100, 10% goat serum, and 0.1% fish skin gelatin for 20 min. Primary antibodies were 1:1000 mouse anti-HA (green), 1:2000 rabbit anti-synapsin 1/2 (red), and 1:500 chicken anti-Map2 (blue). In His6-HA-SUMO1 KI cells, anti-HA labelling was primarily observed in nuclei. Faint anti-HA labelling and occasional puncta were also observed, which did not generally correspond with synapsin-positive puncta (arrowheads). Data representative of 2 independent experiments. Scale bars, 10 µm.
Anti-HA immunolabelling in His6-HA-SUMO1 KI neurons permeabilised with 20 μg/ml digitonin is mainly nuclear.
Hippocampal neurons from either His6-HA-SUMO1 KI mice or SUMO1 KO mice were fixed in 4% PFA and permeabilised using 20 μg/ml digitonin for 20 min, then blocked with 10% horse serum for 20 min. Primary antibodies were 1:1000 mouse anti-HA (green), 1:2000 rabbit anti-synapsin 1/2 (red), and 1:500 chicken anti-Map2 (blue). In His6-HA-SUMO1 KI cells, anti-HA labelling was primarily observed in nuclei. Faint anti-HA labelling and occasional puncta were also observed, which did not generally correspond with synapsin-positive puncta (arrowheads). Data representative of 2 independent experiments. Scale bars, 10 µm.
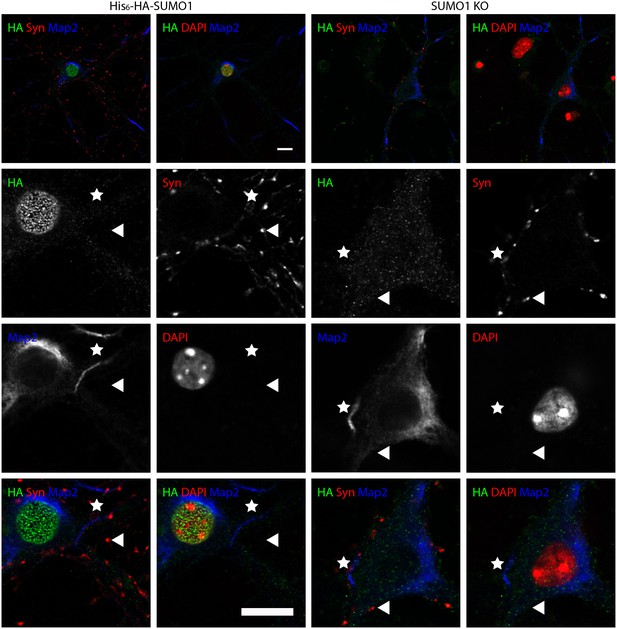
Anti-HA immunolabelling in His6-HA-SUMO1 KI neurons permeabilised with 10 μg/ml digitonin is mainly nuclear.
Hippocampal neurons from either His6-HA-SUMO1 KI mice or SUMO1 KO mice were fixed in 4% PFA and permeabilised using 10 μg/ml digitonin, then blocked with 10% horse serum for 10 min. Primary antibodies were 1:1000 mouse anti-HA (green), 1:2000 rabbit anti-synapsin 1/2 (red), and 1:500 chicken anti-Map2 (blue). In His6-HA-SUMO1 KI cells, anti-HA labelling was primarily observed in nuclei. Faint anti-HA labelling and occasional puncta were also observed, which did not generally correspond with synapsin-positive puncta (arrowheads). Permeabilisation with 10 μg/ml digitonin resulted in incomplete immunolabelling with Map2, with dendrites appearing 'fragmented' (indicated by stars) as compared to samples permeabilised with Triton X-100 or 20 μg/ml digitonin. Data representative of 2 independent experiments. Scale bars, 10 µm.
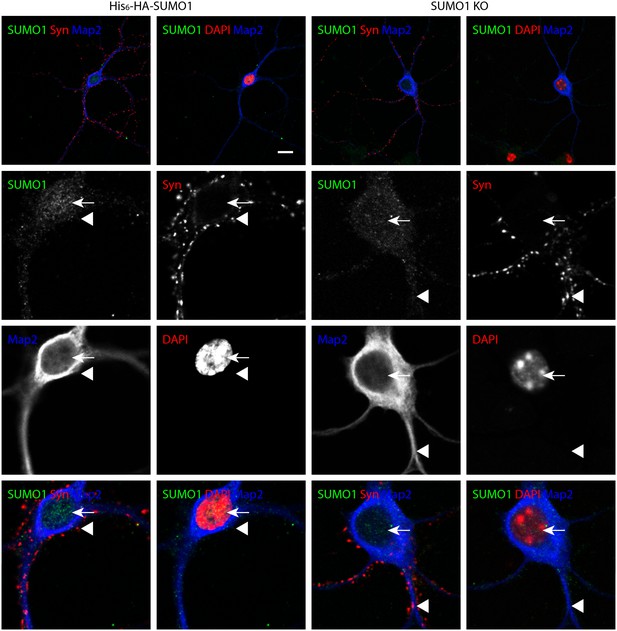
Specific anti-SUMO1 immunolabelling in His6-HA-SUMO1 KI neurons permeabilised with Triton X-100 is mainly nuclear and not in synaptic boutons.
Hippocampal neurons from either His6-HA-SUMO1 KI mice or SUMO1 KO mice were fixed in 4% PFA and permeabilised using 0.3% Triton X-100. Primary antibodies were 1:50 mouse anti-SUMO1 (green), 1:2000 rabbit anti-synapsin 1/2 (red), and 1:500 chicken anti-Map2 (blue). In His6-HA-SUMO1 KI neurons but not in SUMO1 KO neurons, anti-SUMO1 labelling was observed in nuclei (arrows). Additional anti-SUMO1 labelling was observed similarly in both His6-HA-SUMO1 KI and SUMO1 KO samples, which did not appear to correspond with synapsin-positive puncta (arrowheads). Data are representative of 5 independent experiments. Scale bars, 10 μm.
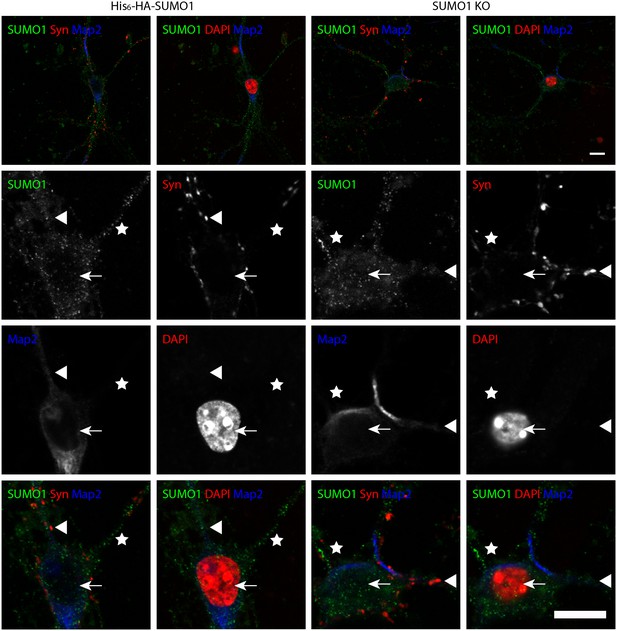
Specific anti-SUMO1 immunolabelling in His6-HA-SUMO1 KI neurons permeabilised with 10 μg/ml digitonin does not correspond with synaptic boutons.
Hippocampal neurons from either His6-HA-SUMO1 KI mice or SUMO1 KO mice were fixed in 4% PFA and permeabilised using 10 μg/ml digitonin. Primary antibodies were 1:50 mouse anti-SUMO1 (green), 1:2000 rabbit anti-synapsin 1/2 (red), and 1:500 chicken anti-Map2 (blue). In His6-HA-SUMO1 KI neurons anti-SUMO1 labelling was generally absent in nuclei (arrows) as well as in SUMO1 KO neurons. Additional anti-SUMO1 labelling was observed similarly in both His6-HA-SUMO1 KI and SUMO1 KO samples, which did not appear to correspond with synapsin-positive puncta (arrowheads). Permeabilisation with 10 μg/ml digitonin resulted in incomplete immunolabelling with Map2, with dendrites appearing 'fragmented' (indicated by stars). Data are representative of 5 independent experiments. Scale bars, 10 μm.
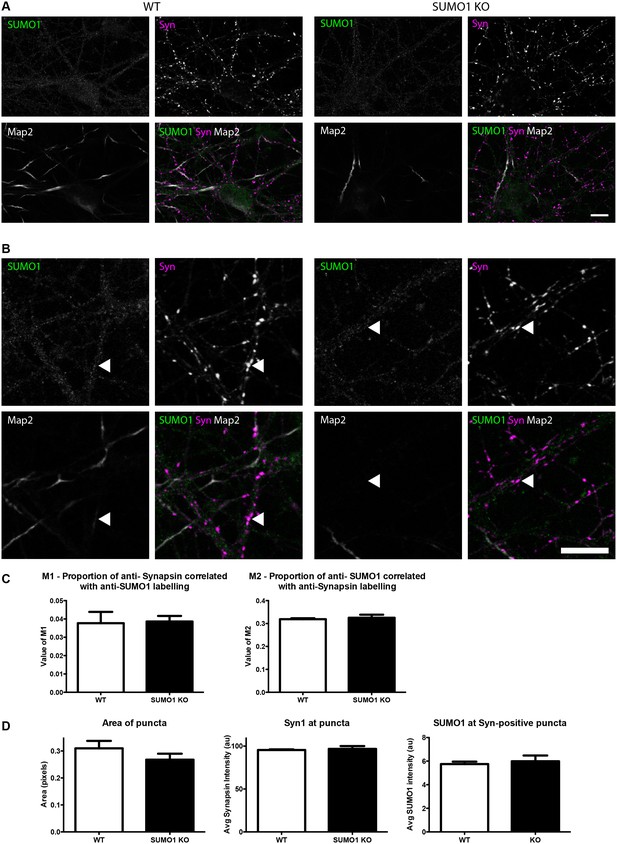
Quantification of anti-SUMO1 immunolabelling shows that SUMO1 is not specifically detected at presynaptic sites in digitonin-permeabilised neurons.
(A) Primary hippocampal neurons from WT and SUMO1 KO littermates were fixed, then digitonin-permeabilised (10 μg/ml), and immunolabelled with antibodies against SUMO1 (green), synapsin (purple), and Map2 (white). A region of interest (neuron) was identified, automatically divided into a grid composed of 6–12 fields of view, and z-stacks were taken for each field. Fields of view were 'stitched' back together to form overview images. (B) Inset regions from (A) are shown in greater detail. (C) Manders’ correlation coefficients between anti-SUMO1 and anti-synapsin signals were determined. The degree of colocalisation between anti-synapsin and anti-SUMO1 was not different between WT and SUMO1 KO neurons. (D) In a separate analysis, synapsin-positive puncta were automatically segmented and the area (left panel) and fluorescence intensity (centre panel) was calculated in synapsin-positive regions of interest (puncta). The intensity of anti-SUMO1 immunolabelling was also quantified at synapsin-positive puncta (right panel). No significant difference between WT and SUMO1 KO neurons was observed for anti-SUMO1 intensity at synapsin-positive puncta. These data indicate that anti-SUMO1 staining at synaptic sites is not specific for SUMO1. Images represent immunostaining of cultures from 3 mice of each genotype. Scale bar, 10 μm. Error bars represent SEM.
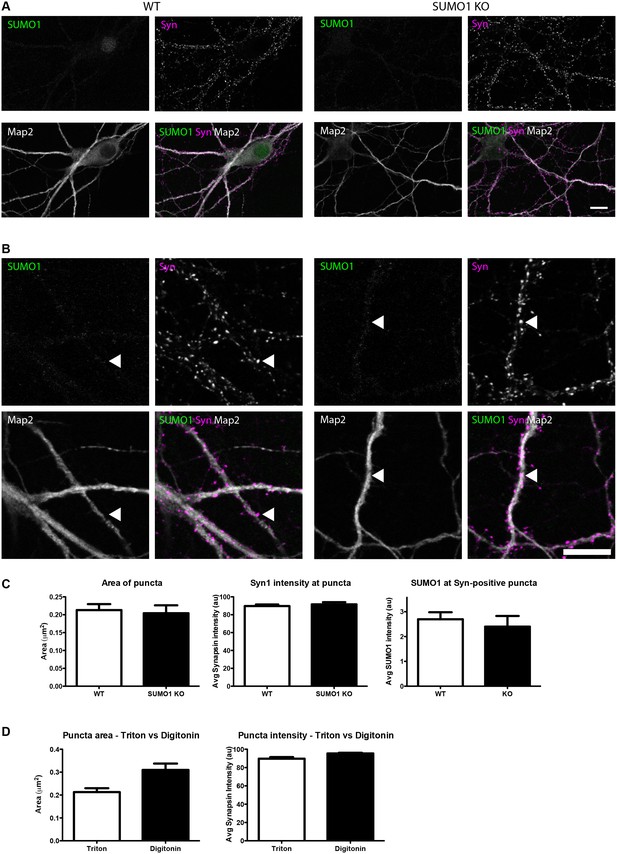
Quantification of anti-SUMO1 immunolabelling shows that SUMO1 is not specifically detected at presynaptic sites in Triton X-100-permeabilised neurons.
(A) Primary hippocampal neurons from WT and SUMO1 KO littermates were fixed, then Triton X-100-permeabilised, and immunolabelled with antibodies against SUMO1 (green), synapsin (purlple), and Map2 (white). A region of interest (neuron) was identified, automatically divided into a grid composed of 6–12 fields of view, and z-stacks were taken for each field. Fields of view were 'stitched' back together to form overview images. (B) Inset regions from (A) are shown in greater detail. (C) Synapsin-positive puncta were automatically segmented and the area (left panel) and fluorescence intensity (centre panel) were calculated in synapsin-positive regions of interest (puncta). The intensity of anti-SUMO1 immunolabelling was also quantified at synapsin-positive puncta (right panel). No significant difference between WT and SUMO1 KO neurons was observed for anti-SUMO1 intensity at synapsin-positive puncta. These data indicate that anti-SUMO1 staining at synaptic sites is not specific for SUMO1. (D) Graphs comparing the area and intensity of synapsin-positive puncta from WT neurons permeabilised with either Triton X-100 or digitonin. Although the mean area of synapsin puncta was somewhat higher when digitonin was used for permeabilisation, this difference was not quite significant (p=0.057). Images represent immunostaining of cultures from 3 mice of each genotype. Scale bar, 10 μm. Error bars represent SEM.
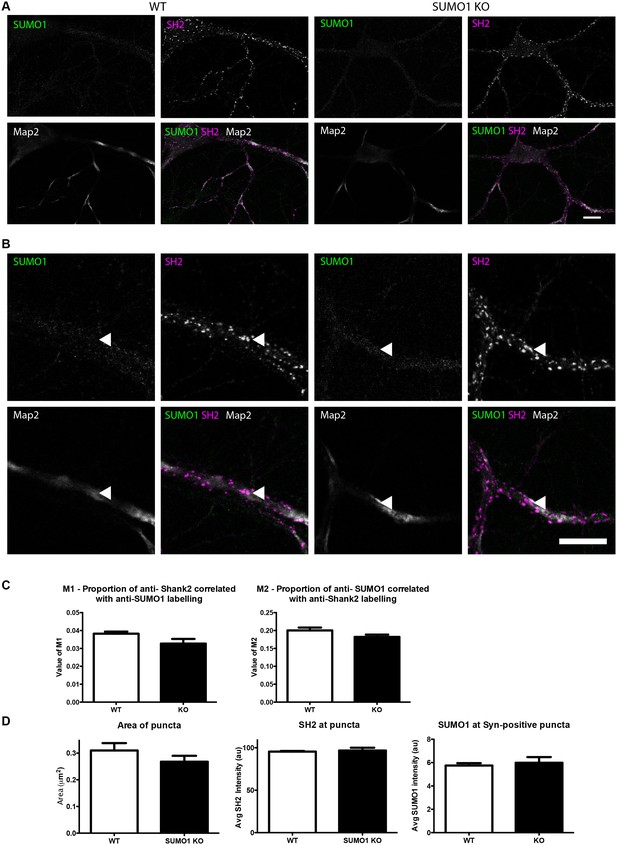
Quantification of anti-SUMO1 immunolabelling shows that SUMO1 is not specifically detected at postsynaptic sites in digitonin-permeabilised neurons.
(A) Primary hippocampal neurons from WT and SUMO1 KO littermates were fixed, then digitonin-permeabilised (10 μg/ml), and immunolabelled with antibodies against SUMO1 (green), shank2 (purple), and Map2 (white). A region of interest (neuron) was identified, automatically divided into a grid composed of 6–12 fields of view, and z-stacks were taken for each field. Fields of view were 'stitched' back together to form overview images. (B) Inset regions from (A) are shown in greater detail. (C) Manders’ correlation coefficients between anti-SUMO1 and anti-shank2 were determined. The degree of colocalisation between anti-shank2 and anti-SUMO1 was not different between WT and SUMO1 KO neurons. (D) In a separate analysis, shank2-positive puncta were automatically segmented and the area (left panel) and fluorescence intensity (centre panel) were calculated in shank2-positive regions of interest (puncta). The intensity of anti-SUMO1 immunolabelling was also quantified at shank2-positive puncta (right panel). No significant difference between WT and SUMO1 KO neurons was observed for anti-SUMO1 intensity at shank2-positive puncta. These data indicate that anti-SUMO1 staining at shank2-positive postsynaptic sites is not specific for SUMO1. Images represent immunostaining of cultures from 3 mice of each genotype. Scale bar, 10 μm. Error bars represent SEM.
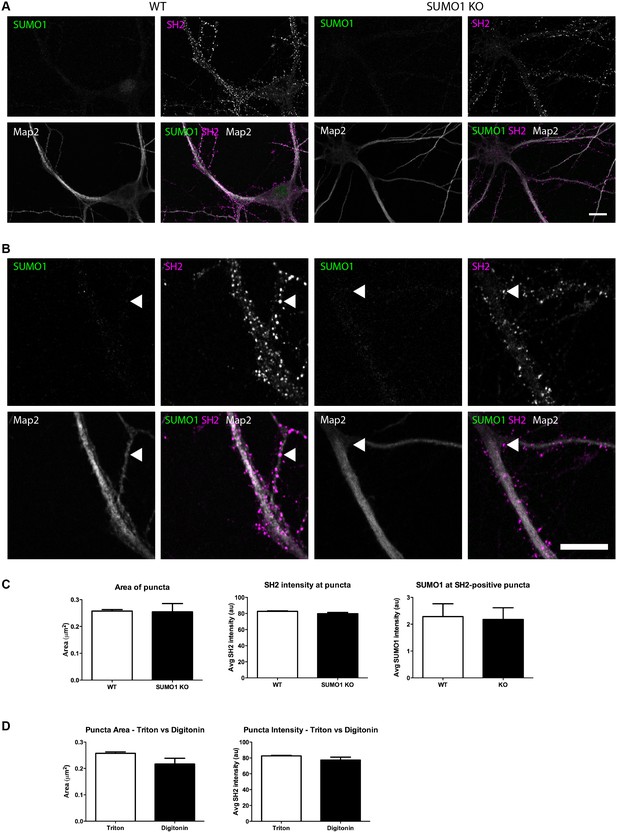
Quantification of anti-SUMO1 immunolabelling shows that SUMO1 is not specifically detected at postsynaptic sites in Triton X-100-permeabilised neurons.
(A) Primary hippocampal neurons from WT and SUMO1 KO littermates were fixed, then triton X-100-permeabilised, and immunolabelled with antibodies against SUMO1 (green), shank2 (purple), and Map2 (white). A region of interest (neuron) was identified, automatically divided into a grid composed of 6–12 fields of view, and z-stacks were taken for each field. Fields of view were 'stitched' back together to form overview images. (B) Inset regions from (A) are shown in greater detail. (C) Shank2-positive puncta were automatically segmented and the area (left panel) and fluorescence intensity (centre panel) were calculated in shank2-positive regions of interest (puncta). The intensity of anti-SUMO1 immunolabelling was also quantified at shank2-positive puncta (right panel). No significant difference between WT and SUMO1 KO neurons was observed for anti-SUMO1 intensity at shank2-positive puncta. These data indicate that anti-SUMO1 staining at synaptic sites is not specific for SUMO1. (D) Graphs comparing the area and intensity of shank2-positive puncta from WT neurons permeabilised with either Triton X-100 or digitonin. The method of permeabilisation did not change the area or intensity of the shank2-positive puncta. Images represent immunostaining of cultures from 3 mice of each genotype. Scale bar, 10 μm. Error bars represent SEM.
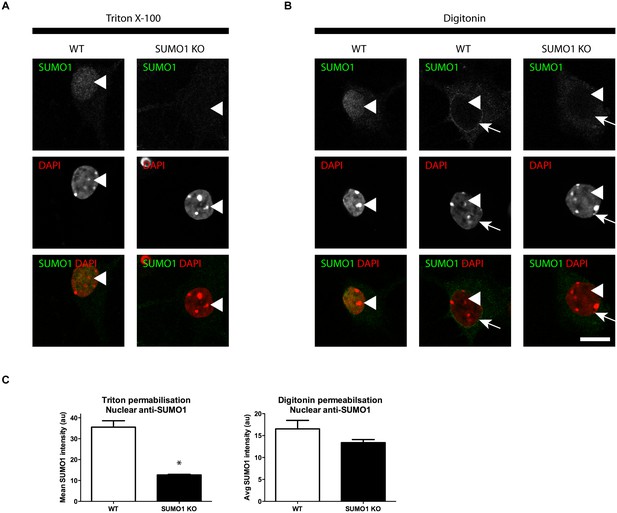
Anti-SUMO1 immunolabelling is reduced in the nuclei of SUMO1 KO neurons permeabilised with Triton X-100.
Representative immunolabelling against SUMO1 (green) and DAPI (red) in neurons from WT or SUMO1 KO mice is shown. (A) In Triton X-100-permeabilised neurons, anti-SUMO1 specifically labelled the nucleus of WT neurons but not of SUMO1 KO neurons (arrowheads). (B) In digitonin-permeabilised (10 μg/ml) neurons, neurons occasionally exhibited nuclear anti-SUMO1 immunolabelling (arrowheads, left panels). However, most WT neurons exhibited no nuclear anti-SUMO1 immunolabelling when permeabilised with digitonin (arrowheads, centre panel), though a ring of anti-SUMO1 immunolabelling was often evident around the nucleus (arrows). SUMO1 KO neurons permeabilised with digitonin exhibited no anti-SUMO1 immunolabelling within or around the nucleus (right panels, arrows and arrowheads). (C) Graphs showing the average intensity of anti-SUMO1 immunolabelling within the nucleus of Triton X-100- and digitonin-permeabilised neurons. Overall, SUMO1 KO neurons exhibited a significant reduction in nuclear anti-SUMO1 immunolabelling intensity when permeabilised with Triton X-100 but not with digitonin. Images represent immunostaining of cultures from 3 mice of each genotype. Scale bar, 10 μm. Error bars represent SEM.





