The complex of TRIP-Br1 and XIAP ubiquitinates and degrades multiple adenylyl cyclase isoforms
Figures
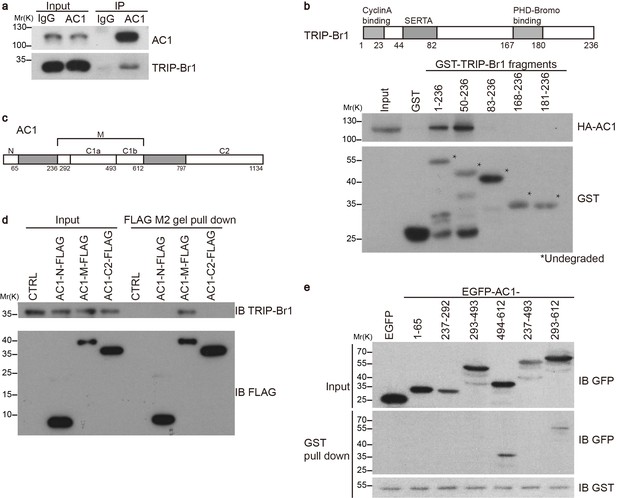
TRIP-Br1 interacts with AC1.
(a) Endogenous AC1 in HeLa cells was immunoprecipitated (IP) with anti-AC1 antibody or control IgG and immunoblotted with anti-AC1 (top) and anti-TRIP-Br1 antibodies (bottom). (b) GST-TRIP-Br1 truncation mutants (bottom) were used to pull down HA-AC1 (middle) expressed in HEK293T cells. The domains included in the GST-TRIP-Br1 truncation mutants are illustrated in the schematic of TRIP-Br1 (top). *undegraded GST-TRIP-Br1 fragments. (c) Schematic of AC1. N, N-terminus; C1 and C2, catalytic domains 1 and 2, respectively; filled areas, transmembrane domains; M, cytosolic region between the 2 transmembrane domains. (d) Three purified FLAG-His-tagged AC1 fragments, AC1-N, AC1-M, and AC1-C2 (bottom), were used to pull down purified TRIP-Br1 (His-tagged at both N and C termini, top). CTRL, control: bovine serum albumin used instead of FLAG-His-tagged AC1 fragments. (e) GST-TRIP-Br1 was used to pull down GFP-tagged AC1 N-terminus and truncation fragments of C1 domain expressed in HEK293T cells. All experiments shown here are representative of 3–5 independent experiments.
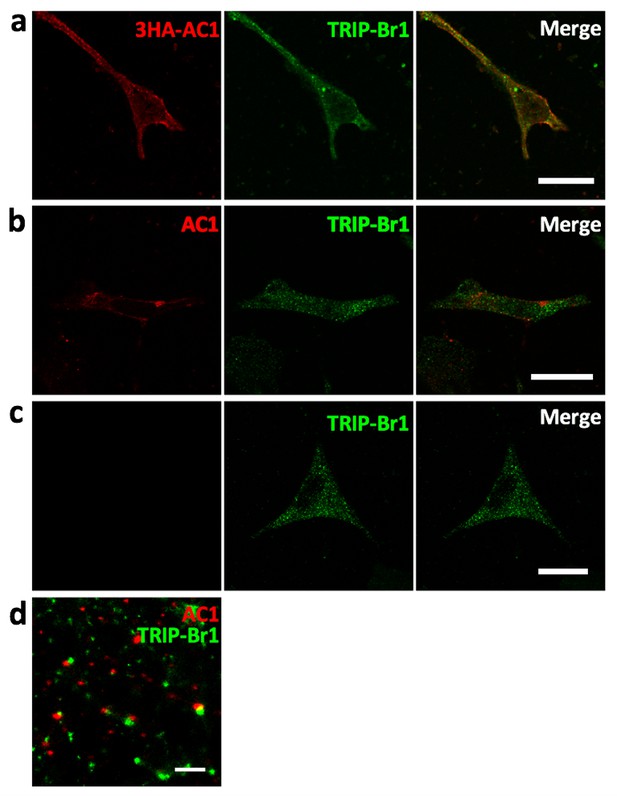
Colocalization of AC1 with TRIP-Br1.
(a) Confocal images of HeLa cells transfected with 3HA-AC1. The cells were immunostained with anti-HA and anti-TRIP-Br1 antibodies. (b–c) Confocal images of untransfected HeLa cells. Anti-AC1 antibody was delivered into live cells by Lipofectamine2000, and the signal of anti-AC antibody was amplified by Atto 488-conjugated biotin because endogenous AC1 was barely detectable by conventional immunostaining (b). (c) is a control without anti-AC1 primary antibody for b. (d) STORM (stochastic optical reconstruction microscopy) image of non-transfected HeLa cells immuno-stained with anti-AC1 and anti-TRIP-Br1 antibodies. Because the optical depth of the image is 200 nm, endogenous AC1 and TRIP-Br1 probably interacted directly in or near the plasmalemma. Scale bars: 20 µm in a–c, and 500 nm in d.
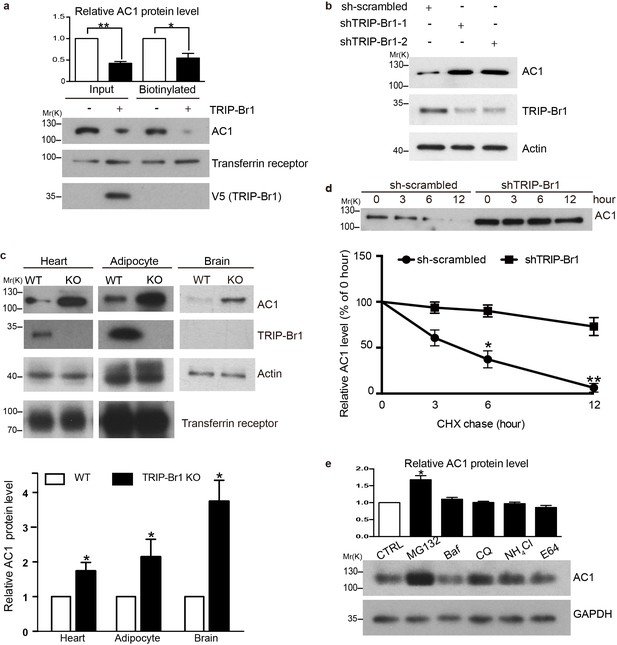
TRIP-Br1 promotes AC1 degradation.
(a) Stable expression of TRIP-Br1-V5 markedly reduced total and cell-surface protein levels of endogenous AC1 in HEK293T cells. Cell-surface AC1 was biotinylated and isolated. Transferrin receptor: loading control. Quantification of the western blots is shown at the top: **, different from the control cell (CTRL), p=0.0053; *p=0.048; n = 3 independent experiments. (b) Knocking down TRIP-Br1 with two different shRNAs (shTRIP-Br1-1 and shTRIP-Br1-2) increased AC1 protein expression in HEK293T cells. Actin: loading control. (c) Knocking out TRIP-Br1 in mice elevated AC1 expression in heart tissue (n = 8, p=0.01), and adipocytes (n = 4, p=0.049), and the brain (n = 3, p=0.011). (d) Changes in the expression of endogenous AC1 with or without shTRIP-Br1-1 treatment were examined for 12 hr after CHX treatment in HEK293T cells (upper). The results are quantified in the lower panel; *, different from control (CTRL), p=0.013 (6 h); **p=0.004 (12 h); n = 3. (e) Effect of proteasomal and lysosomal inhibitors on endogenous AC1 in HEK293T cells. Baf, bafilomycin A1; CQ, chloroquine diphosphate. Quantification of the western blots is shown at the top: *, different from the control (CTRL), p=0.029; n = 3 independent experiments.
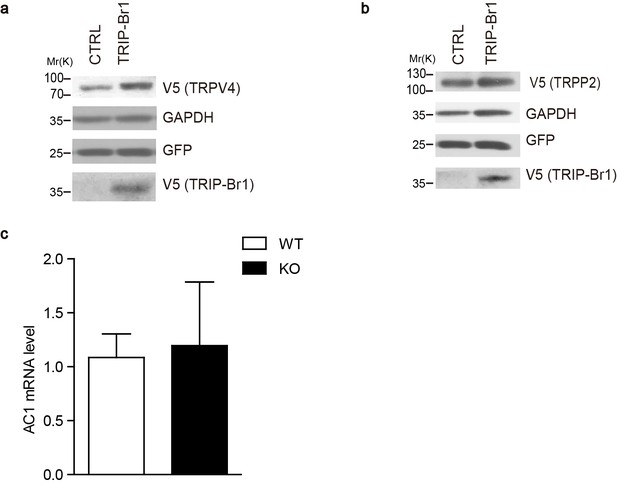
Effect of TRIP-Br1 on the expression of other membrane proteins and AC1 mRNA levels.
(a–b) Stable expression of TRIP-Br1-V5 did not affect the protein levels of HA-tagged TRPV4 (transient receptor potential vanilloid 4) (a) and TRPP2 (transient receptor potential polycystin 2) (b) transiently expressed in HEK293T cells. GAPDH, loading control; GFP, transfection efficiency control. (c) AC1 mRNA levels showed no change (compared to control) in the heart tissue of TRIP-Br1 knockout mice in a real-time PCR assay (WT n = 5, KO n = 4; p=0.853). The mRNA level of AC1 was normalized relative to that of GAPDH.
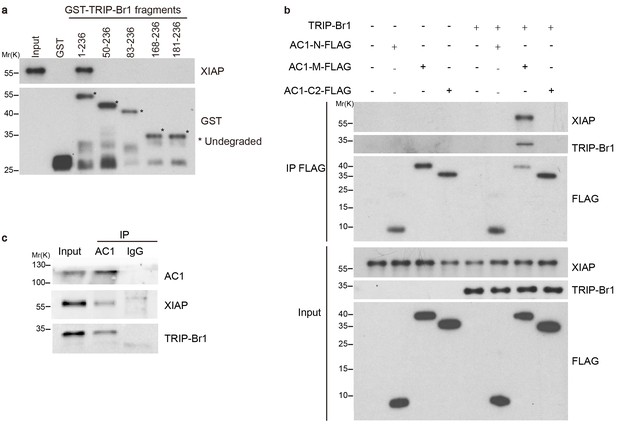
TRIP-Br1 bridges the interaction of AC1 with XIAP E3 ligase.
(a) Full-length TRIP-Br1 fused with GST—but not GST-TRIP-Br1 truncation mutants or GST alone—captured His-XIAP purified from E. coli. (b) Three purified FLAG-His-tagged AC1 fragments, AC1-N, AC1-M, and AC1-C2, were used to pull down purified XIAP in the presence or absence of purified TRIP-Br1 (His-tagged at both N and C termini). (c) Endogenous AC1 in HeLa cells was IPed with anti-AC1 antibody or control IgG and immunoblotted with anti-AC1, anti-XIAP, and anti-TRIP-Br1 antibodies.
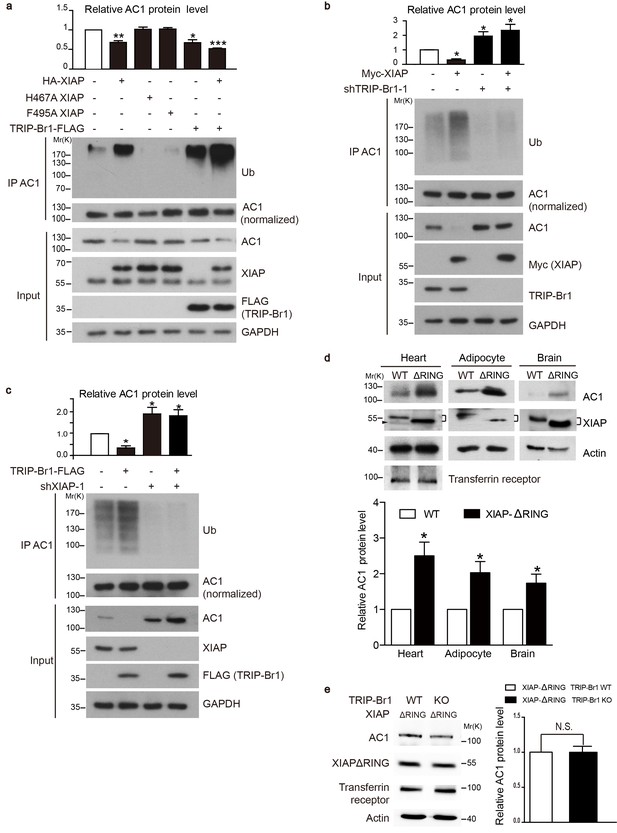
XIAP binding results in AC1 ubiquitination and degradation in a TRIP-Br1 dependent manner.
(a–c) Ubiquitinated and total endogenous AC1 were examined following overexpression of HA-XIAP or its 2 mutants (H467A and F495A) and TRIP-Br1-FLAG in control HeLa cells (a) and in HeLa cells in which we knocked down TRIP-Br1 (b) or XIAP (c). shTRIP-Br1-1 and shXIAP-1, small hairpin RNA; Ub, ubiquitin; GAPDH, loading control. Loading of immunoprecipitated AC1 (or other AC isoforms) was normalized (adjusted to equal amounts) to clearly reveal the differences in ubiquitination of AC1 (or other AC isoforms) in this and other figures. Total endogenous AC1 levels in panels a–c) are quantified in their respective top bar graphs: *, different from control, p≤0.041; **p=0.0019; ***p<0.0001; n = 3 for all three panels. (d) Deleting the RING domain in XIAP in mice elevated AC1expression in heart tissue, adipocytes, and the brain. *, different from control, p≤0.045, n = 4 for all 3 tissues. Connected dashes, XIAP; arrowhead, nonspecific band. (e) Deleting the RING domain in XIAP in mice eliminated TRIP-Br1’s effect on AC1 in the brain. N.S., not different from TRIP-Br1, p=0.943, n = 4. In panels d and e, actin, loading control; transferrin receptor, membrane-protein negative control.
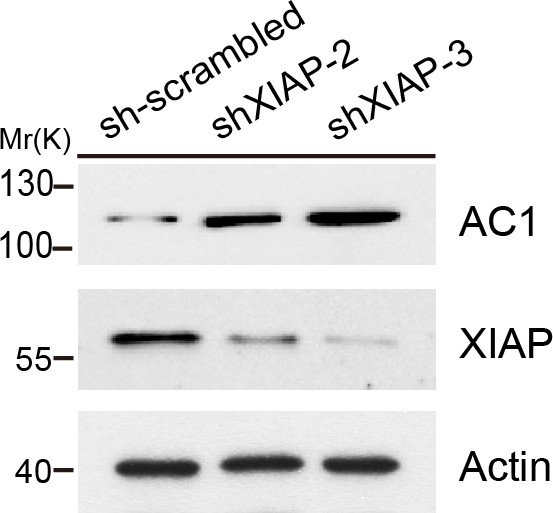
Effect of two other XIAP shRNAs on AC1 protein expression.
In addition to the XIAP shRNA sequence (shXIAP-1) used in Figure 4c, two other different XIAP shRNAs (shXIAP-2 and shXIAP-3) were used to knock down endogenous XIAP in HeLa cells to exclude off-target effects of XIAP shRNAs. Actin, loading control.
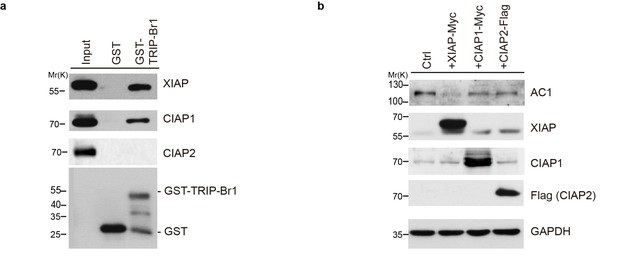
cIAP1 and cIAP2 do not affect AC1 expression.
(a) GST-TRIP-Br1 fusion protein or GST alone was used to pull down endogenous XIAP, cIAP1, and cIAP2 in Hela cells. (b) Overexpression of XIAP but not cIAP1 or cIAP2 degraded endogenous AC1 in Hela cells.
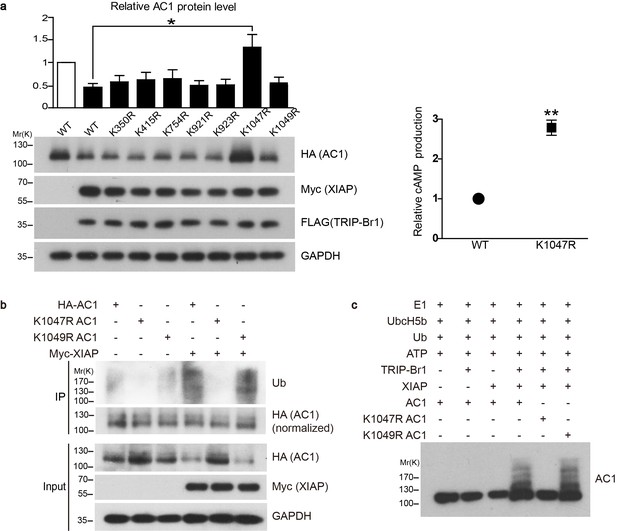
Conserved residue K1047 is the XIAP ubiquitin-conjugation site in AC1.
(a) Left panel: Effect of Lys-to-Arg mutations in HA-tagged AC1 (HA-AC1) on XIAP-induced AC1 degradation in HEK293T cells. Quantification of the western blots is shown at the top: *, different from wild-type (WT) AC1 in the presence of XIAP, p=0.013, n = 3–6 independent experiments for various AC1 mutants. Right panel: relative cAMP production of WT and K1047R AC1 in the presence of XIAP. **p=0.0038. (b) Effect of K1047R and K1049R mutations in HA-AC1 on XIAP-induced ubiquitination and degradation of HA-AC1 in HEK293T cells. (c) In vitro ubiquitination of AC1 and its K1047R and K1049R mutants. E1, ubiquitin-activating enzyme; Ub, ubiquitin. All experiments shown here are representative of 3 independent experiments.
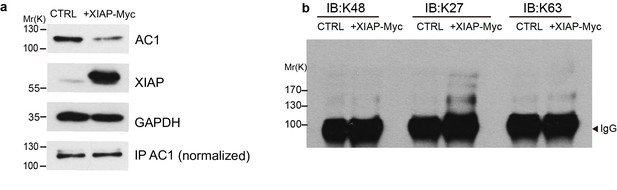
XIAP induces K27-linked polyubiquitination of AC1.
HEK293T cells were transfected with AC1 plus either XIAP-Myc or empty vectors (a, top and second panels). AC1 was immunoprecipitated from the two groups of cells and normalized to equal amounts (a, bottom panel) before subject to western blotting with K48, K27, or K63-linkage specific antibodies in panel b. AC1 polyubiquitinaton chains induced by XIAP appeared predominantly K27-linked. GAPDH, loading control; IgG, the heavy chains of anti-AC1 IgG used for IP are shown as a loading control. Shown here is representative of 3 independent experiments.
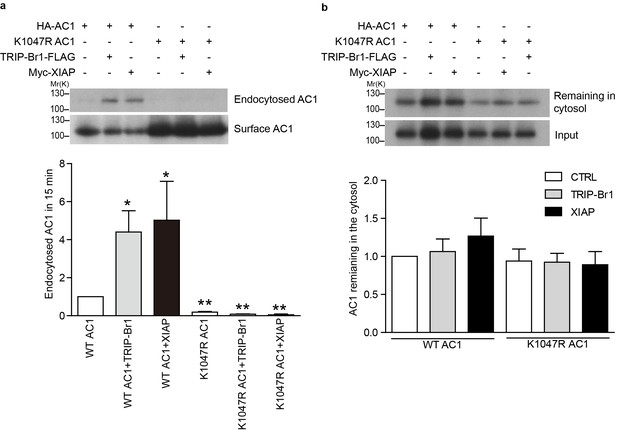
Endocytosis and recycling of HA-tagged wild-type and K1047R AC1 expressed in HeLa cells.
(a) Upper panel: Effect of TRIP-Br1 and XIAP on the endocytosis of cell-surface wild-type and K1047R AC1. Lower panel: Summary data of 4 independent experiments similar to that shown in the upper panel. ‘Endocytosed AC1’ in the lower panel is the ratio of endocytosed AC to cell-surface AC in the upper panel. All data are normalized relative to data obtained for HA-AC1 alone. *, different from wild-type AC1, p≤0.0232; **p≤0.009. (b) Upper panel: Effect of TRIP-Br1 and XIAP on the recycling of endocytosed wild-type and K1047R AC1. Lower panel: Summary data of 3 independent experiments similar to that shown in the upper panel. ‘AC remaining in the cytosol’ is the ratio of remaining AC to ‘input’ (endocytosed AC) in the upper panel. All data are normalized relative to the data obtained for HA-AC1 alone.
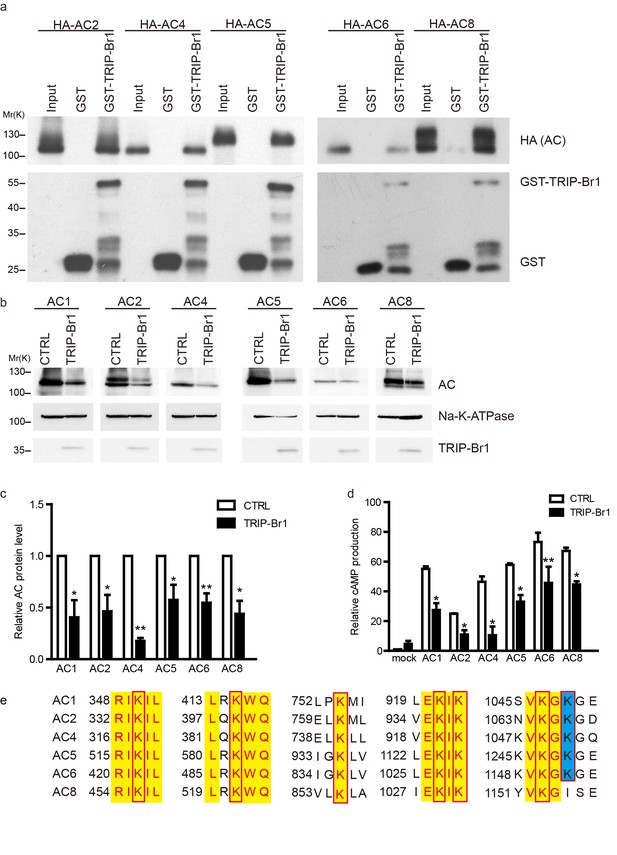
TRIP-Br1 binds to multiple AC isoforms and reduces their protein level and cAMP production.
(a) GST-TRIP-Br1 captured ACs 2, 4, 5, 6, and 8. (b) Stable expression of TRIP-Br1-V5 reduced the protein expression of ACs 1, 2, 4, 5, 6, and 8 that were transiently expressed in HEK293T cells. (c–d) Quantification of protein levels of various AC isoforms in panel b and their forskolin-stimulated cAMP production. cAMP production is normalized relative to that of the control of mock cells. *, different from control, p≤0.048; **p≤0.009; ***p=0.001; n = 3–5. (e) Sequence alignment of the AC isoforms used in panel b to identify conserved Lys residues (boxed). Yellow highlight: identical residues; blue highlight: highly conserved.
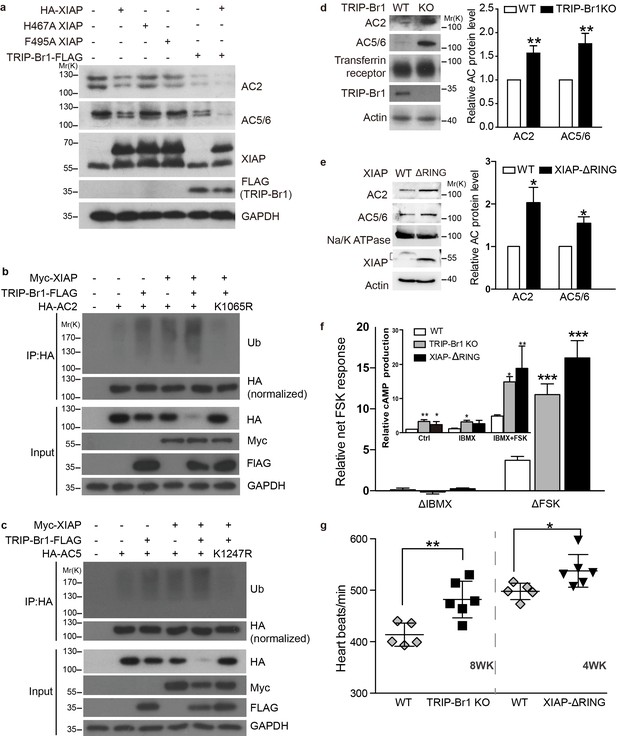
XIAP/TRIP-Br1 attenuates the expression of multiple AC isoforms.
(a) Total endogenous AC2 and AC5/6 in HeLa cells were examined following overexpression of HA-XIAP and its H467A and F495A mutants and TRIP-Br1-FLAG (quantification in Figure 7—figure supplement 1). (b–c,) Mutation to Arg of K1065 in HA-AC2 and K1247 in HA-AC5 abolished XIAP/TRIP-Br1-induced ubiquitination and degradation of HA-AC2 (b) and HA-AC5 (c), respectively, in HEK293T cells. The experiments shown here are representative of 3 independent experiments. (d–e) Knocking out TRIP-Br1 or the RING domain of XIAP in mice increased the expression of AC2 and AC5/6 in heart tissue. Transferrin receptor or Na/K ATPase: membrane-protein negative control; actin: loading control. *, different for wild-type (WT), p≤0.036, n = 3; **p≤0.006, n = 8. (f), Net cAMP production in response to IBMX (ΔIBMX, 100 μM) or forskolin (ΔFSK, 10 μM) in heart tissues of wild-type (WT), TRIP-Br1 knockout, and XIAP ΔRING mice. ΔIBMX and ΔFSK are the differences between IBMX-stimulated and control cAMP production, and between forskolin/IBMX- and IBMX-stimulated cAMP production, respectively (inset). *, different from WT, p≤0.04; **p≤0.006; ***p≤0.001, n = 8 wk-old females for all three groups. (g) Basal heart rates of wild-type (WT), TRIP-Br1 knockout, and XIAP ΔRING mice. **, different from WT, p=0.0049; *, p=0.032; 8-wk-old male and female littermates of WT (n = 5) and TRI-BR1 knockout mice (n = 6) or 4-wk-old male littermates of WT (n = 5) and XIAP ΔRING mice (n = 6) were used for experiments. The mice were anesthetized with Avertin. The difference in the basal heart rates of 4-wk-old and 8-wk-old WT mice has been reported before (Moghtadaei et al., 2016).
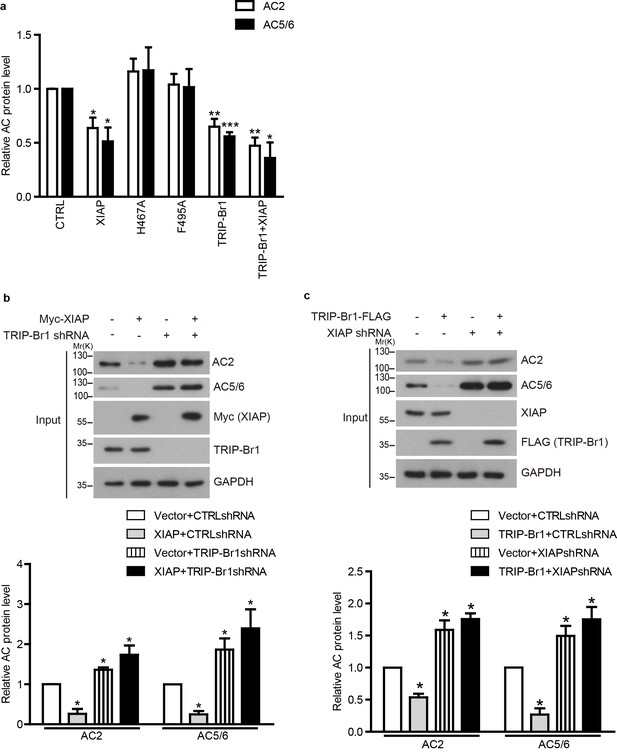
TRIP-Br1 bridges the interaction of AC2 and AC5/6 with XIAP E3 ligase.
(a) Summary data of total endogenous AC2 and AC5/6 in Figure 7a. *, different from control, p≤0.019; **p≤0.008; ***p<0.0001; n = 3. (b–c) Total endogenous AC2 and AC5/6 in HeLa cells were examined following the overexpression of either HA-XIAP without and with TRIP-Br1 knockdown (b), or TRIP-Br1-FLAG without and with XIAP knockdown (c). shRNA, small hairpin RNA, and shTRIP-Br1-1 and shXIAP-1 were used here; GAPDH, loading control. Lower panels in b and c, summary data of their respective upper panels; *p≤0.043; n = 3.
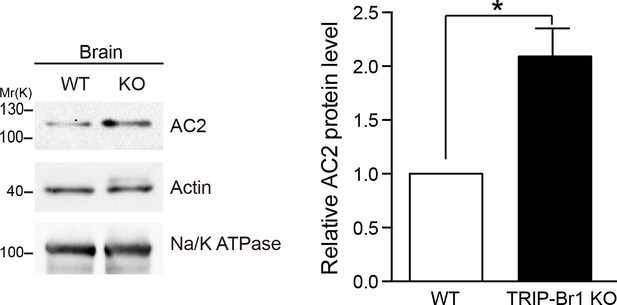
Knocking out TRIP-Br1 in mice increased AC2 expression in the brain.
Na/K ATPase: membrane-protein negative control; actin: loading control. Quantification of the western blots is shown at the right: *, different from wild-type (WT), p=0.0138, n = 3 pairs of female littermates.
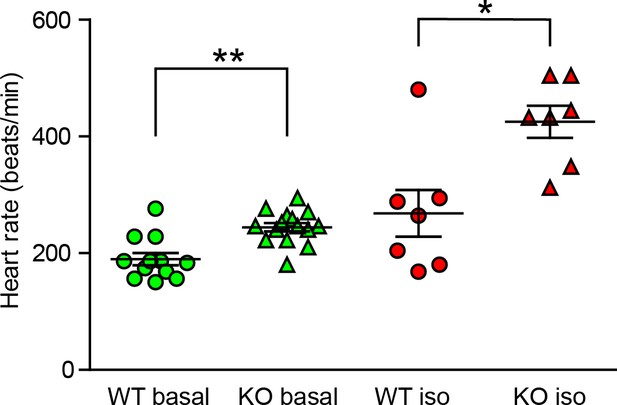
Change in the heart rate of TRIP-Br-1 knockout mice under deep anesthesia.
Heart rates of wild-type (WT) and TRIP-Br1 knockout (KO) mice under basal and isoproterenol-stimulated (iso) conditions; **, different from WT, p=0.002; *p=0.014; n = 12 (WT, basal); 15 (KO, basal); 7 (WT, iso); 7 (KO, iso). Instead of Avertin, the mice in these experiments were anesthetized with ketamine and xylazine, which induce deep anesthesia.
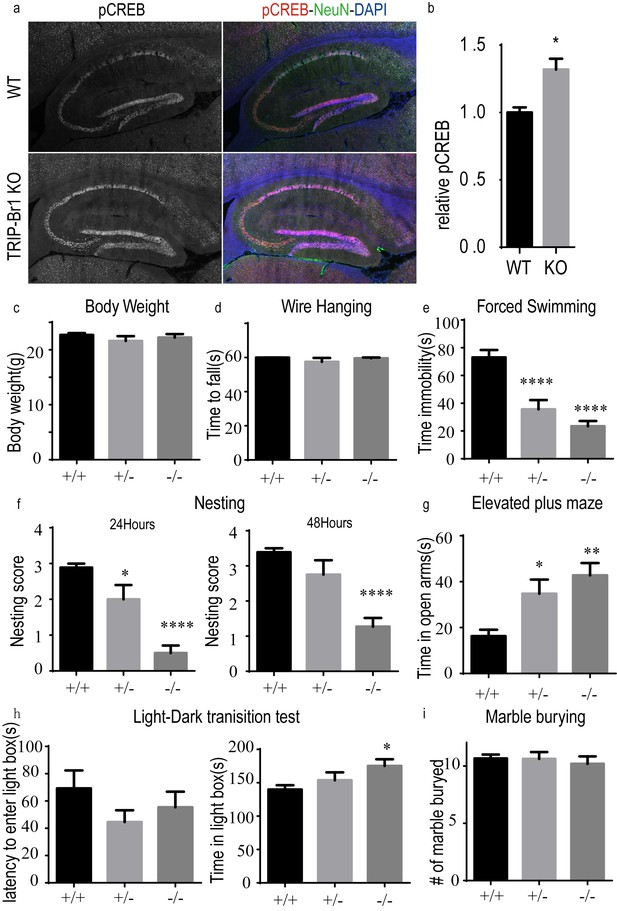
Knocking out TRIP-Br1 induces the phosphorylation of CREB in the hippocampus and mood disorder (especially despair-like behavior) in mice.
(a) Immunostaining of phosphorylated CREB (pCREB, red) in the hippocampus, with NeuN (green, neuronal marker) and DAPI (purple). (b) Quantitation of pCREB immunoreactivity relative to NeuN, *, different from WT, p=0.024, n = 3 10 wk-old male mice for both WT and KO (TRIP-Br1 knockout). (c) Body weight of the mice. (d) Muscle strength in wire hanging test. (e–i) Mood disorders, especially despair-like behavior, in several tests: (e) immobility time in the forced swimming test; (f) Nesting score in 24 (left) and 48 (right) h; (g) time in open arms in an elevated plus maze; (h) latency to enter a light box (left) and time in the light box (right) in the light-dark transition test; (i) number of marble buried in the marble burying test. 9 (±1)-wk-old male and female mice used in the experiments in panels c–I, and ++, +/−, and −/− denote: wild-type (n = 9), heterozygous (n = 8), homozygous (n = 11) TRIP-Br1 knockout mice, respectively. Different from +/+: *p≤0.039; **p=0.007; ****p≤0.0005.
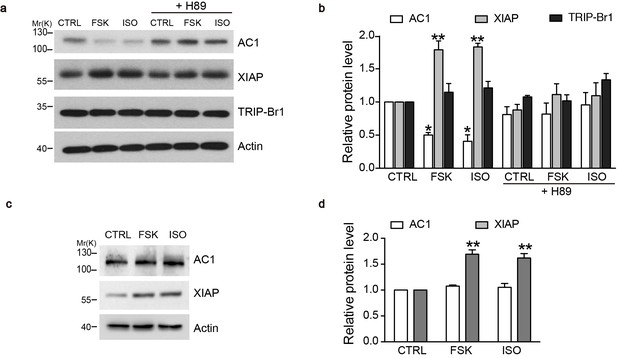
cAMP agonists promote XIAP upregulation and AC1 degradation in a PKA-dependent manner in the macrophages of wild-type but not TRIP-Br1 knockout mice.
Changes in AC1, XIAP, and TRIP-Br1 expression with or without 6 hr forskolin (FSK, 10 μM, in the presence of 100 μM IBMX) or isoproterenol (ISO,10 μM) treatment in the microphages of wild-type (a–b) and TRIP-Br1 knockout (c–d) mice. H89 (10 μM), PKA inhibitor; β-actin, loading control. (b and d), Summary data of panels a and b, respectively, relative to β-actin. Different from control (CTRL), *p≤0.0427; **p≤0.0076, n = 3.
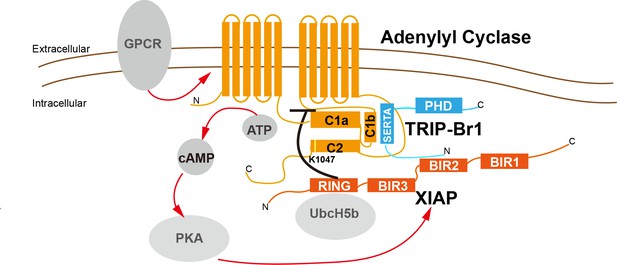
A model depicting a general negative-feedback mechanism of ubiquitination and degradation of multiple AC isoforms.
The SERTA domain of TRIP-Br1 binds to the C1b catalytic domain of AC1 or other AC isoforms and subsequently recruits XIAP, which, together with the E2 enzyme UbcH5b (or UbcH5a or UbcH5c) (Nakatani et al., 2013; Mace et al., 2008), ubiquitinates K1047 in AC1 or an equivalent Lys residue in other AC isoforms. The N-terminus of TRIP-Br1 interacts with XIAP, probably with its BIR2 domain (Hong et al., 2009), and the Ring domain of XIAP interacts with UbcH5b (Nakatani et al., 2013; Mace et al., 2008). The ubiquitination of ACs leads to their endocytosis and degradation. Sustained AC activation and cAMP production under catecholamine stress increase XIAP protein expression through PKA, and elevated XIAP protein expression subsequently dstabilizes ACs and eventually lows cAMP production/signaling. Red arrow, stimulatory effect; black dash: inhibitory effect.
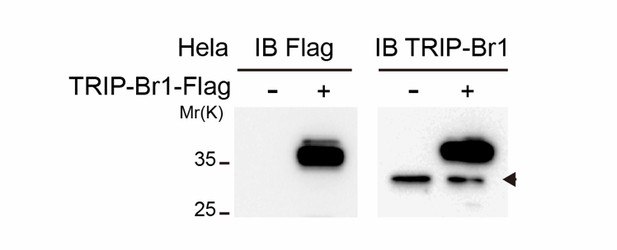
TRIP-Br1-FLAG was detected by TRIP-Br1 antibody.
HeLa cells were seeded in a 60-mm dish the day before the transfection. 5 ug TRIP-Br1-FLAG or control vector were transfected into the HeLa cells. After 48 h culture, cells were lysed by RIPA buffer and the cell lysate was boiled at 95°C for 10 min and added with 200 mM DTT before subject to western blotting by anti-FLAG and anti-TRIP-Br1 antibodies separately. 12% gel was used for better separation of endogenous (arrowhead) and FLAG-tagged TRIP-Br1.





