Mechano-dependent signaling by Latrophilin/CIRL quenches cAMP in proprioceptive neurons
Figures
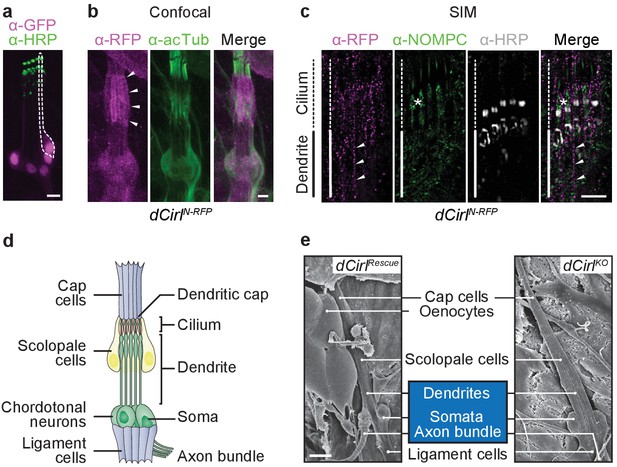
dCIRL is located at the site of ionotropic mechanosensation.
(a) The dCirlpGAL4 driver demonstrates exclusively neuronal expression of dCirl within lch5 ChOs (dCirlpGAL4>UAS-GFP::nls). Rightmost mechanosensory neuron (soma and dendrite) within the organ marked by a dotted line. (b) Maximal projection of a confocal image stack of lch5 (counterstained against acetylated tubulin; green) showing dCIRLN-RFP (magenta) enrichment at the level of the distal dendrites and cilia (arrowheads). (c) SIM imaging shows dCirlN-RFP (magenta) in the distal dendrites (arrowheads) extending to the ciliary compartment, where the receptor is coexpressed in the same subcellular region with the TRP channel NompC (green). lch5 was counterstained with α-HRP, asterisk indicates ciliary dilation. Note that SIM resolves the canal through which the cilium passes. (d) Composition of the larval pentascolopidial organ (lch5). (e) Scanning electron micrographs of lch5 from control and dCirlKO animals. The organ consists of a chain of support cell types that suspend the mechanosensory neurons (blue) between body wall and musculature. No morphological abnormalities are apparent in the mutant. Scale bars, (a–c) 5 µm; (e) 10 µm. See also Figure 1—figure supplements 1 and 2.
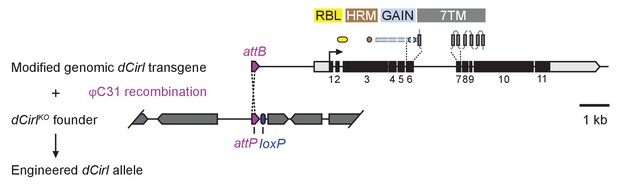
dCirl genomic engineering platform.
Schematic of the genomic engineering platform used to construct dCirl alleles for this study. All dCIRL receptor domains are depicted in approximate relation to the exons of the genomic transgene they are encoded on. The blue half circles represent the GPS (GPCR proteolysis site). RBL, rhamnose-binding lectin domain; HRM, hormone receptor motif domain; GAIN, GPCR autoproteolysis inducing domain; 7TM, heptahelical transmembrane domain. dCirl encoding exons are numbered.
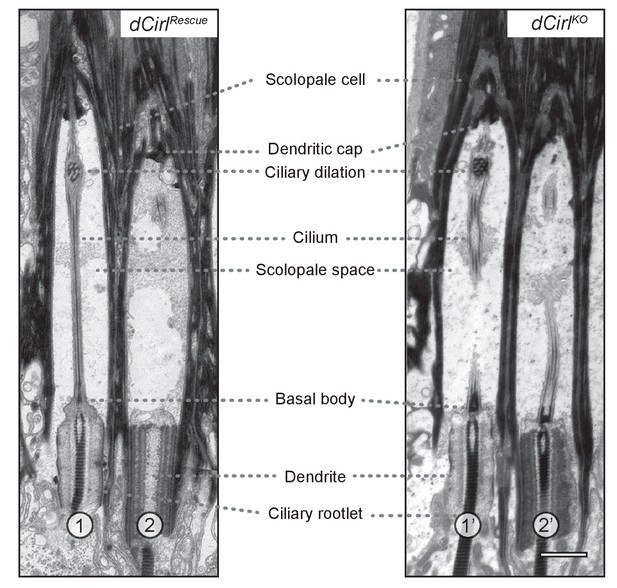
Transmission electron microscopy of ChO in control and dCirlKO.
Electron micrographs of longitudinal ultrathin sections at the distal dendritic region of the two outer (1, 2; 1’, 2’) scolopidia document a complex organization. In scolopidia 1, 1’ and 2’, the section passes through the central dendritic region including ciliary rootlets, ciliary origin with basal bodies and the entire (1) or part of (1’, 2’) the cilium; scolopidium 2 is sectioned peripherally. General cellular architecture and ultrastructural features appear preserved in these distal scolopidia after dCirl removal. Scale bar, 1 µm.
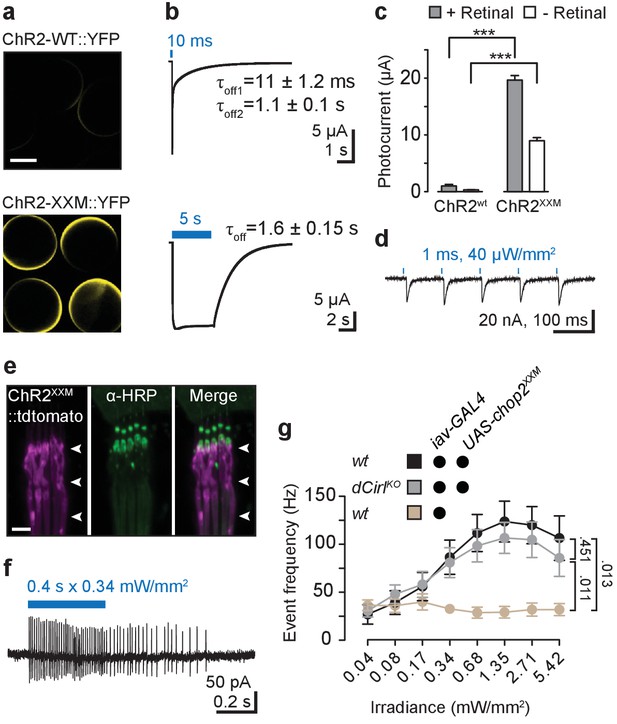
Optogenetic stimulation with ChR2-XXM.
(a) Expression of ChR2-WT::YFP and ChR2-XXM::YFP in Xenopus oocytes (without retinal supplementation) imaged by confocal microscopy. (b) Representative photocurrents of ChR2-XXM::YFP in oocytes (473 nm, ~12.4 mW/mm2). Short light pulses are followed by a rapid biphasic photocurrent decay (𝜏off1: 80%, 𝜏off2: 20%), whereas the longer time constant (𝜏off) dominates upon prolonged photostimulation. Data are presented as mean ± SD, n = 4 recordings from individual oocytes incubated with 1 µM all-trans-retinal. (c) Quantification of photocurrent amplitudes in oocytes with and without retinal supplementation. Data presented as mean ± SEM. ChR2-wt + retinal: 0.999 ± 0.5272 µA, n = 4; ChR2-wt - retinal: 0.317 ± 0.0570 µA, n = 5; ChR2-XXM + retinal: 19.675 ± 1.9458 µA n = 6; ChR2-XXM - retinal: 8.982 ± 1.5718 µA, n = 8; p<0.00001, Student’s t- test. (d) Two-electrode voltage clamp (TEVC) recordings at the NMJ show that photostimulation of motoneurons (440 nm) via ChR2-XXM::tdTomato elicits excitatory postsynaptic currents (EPSCs), which can be stimulus-locked using short, low intensity light pulses. (e) Localization of ChR2-XXM::tdTomato in lch5 dendrites (arrowheads). (f) Example recording from the lch5 axon bundle showing a train of action currents elicited by photostimulation of sensory neurons via ChR2-XXM::tdTomato. The burst gradually decays after the light pulse, reflecting the kinetics of channel closure. (g) Quantification of action current frequencies in lch5 neurons expressing ChR2-XXM::tdTomato upon increasing irradiance. The activity of ChOs scales with light intensity and is independent of dCirl. No light response when the transgene is omitted. Data are presented as mean ± SEM. n = 10 per genotype. Numbers denote p values of comparisons of event frequency at 5.42 mW/mm2 irradiance with a Student’s t- test. Scale bars, (a) 500 µm; (e) 5 µm. See also Figure 2—figure supplements 1 and 2.
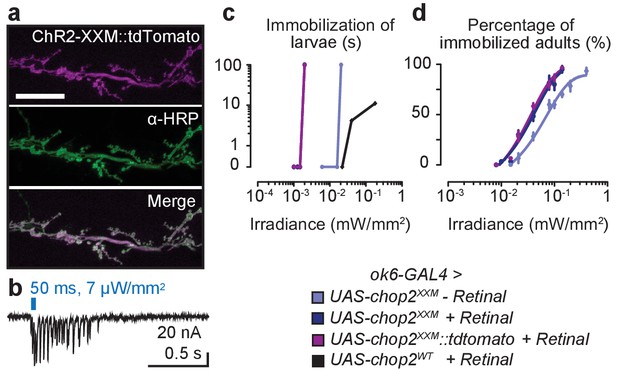
Characterization of ChR2-XXM at the NMJ.
(a) Expression of ChR2-XXM::tdTomato (ok6-GAL4) in motoneuron arborisations at the larval NMJ detected by photoprotein fluorescence (upper panel) and counterstained with α-HRP (middle panel). Scale bar, 25 µm. (b) Photostimulation of motoneurons (440 nm) via ChR2-XXM::tdTomato using longer light pulses elicits a train of EPSCs, which gradually decays. (c) Immobilization of larvae expressing ChR-WT or ChR2-XXM variants in motoneurons during continuous irradiation. The duration of immobilization scales with light intensity. Data are presented as mean ± SEM, n ≥ 6 for each condition and genotype. Larval locomotion was effectively abrogated by motoneuronally-expressed ChR2-XXM, regardless of fusion to a tdTomato moiety and also without dietary retinal supplementation. (d) Efficient immobilization of adult flies via ChR2-XXM at moderate light intensities, irrespective of photoprotein tagging and also without retinal addition (reliable photostimulation by the fusion protein appeared to require retinal supplementation; data not shown). In contrast, low light transmission through the pigmented cuticle prevents a discernible effect of ChR2-wt in this assay (Dawydow et al., 2014). Data are presented as mean ± SEM, n = 6 animals per data point.
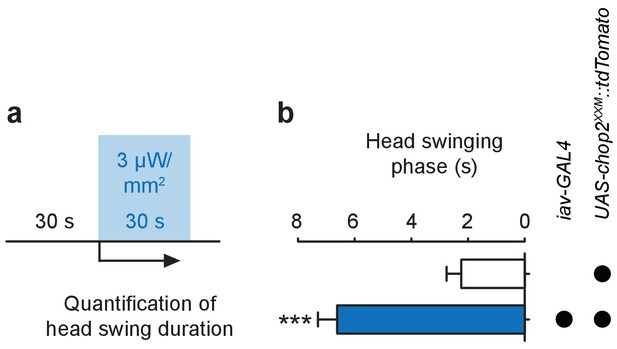
Stimulation of larval ChO neurons via ChR2-XXM in vivo.
(a) Protocol illustrating how head swinging, an efferent motor behavior component that requires afferent ChO activity (Caldwell et al., 2003), was measured. (b) Quantification of head swinging duration elicited through photostimulation of ChR2-XXM::tdtomato expressed in ChO neurons. Illumination reliably induced head swinging phases demonstrating that ChOs are sufficient to trigger this motor response. Note that the effect depends on the presence of the transgene. Data presented as mean ± SEM. iav-GAL4>UAS-chop2XXM::tdTomato: 6.619 ± 0.6778 s; UAS-chop2XXM::tdTomato: 2.238 ± 0.5206 s; p<0.0001, Mann-Whitney U test; n = 21 animals per genotype.
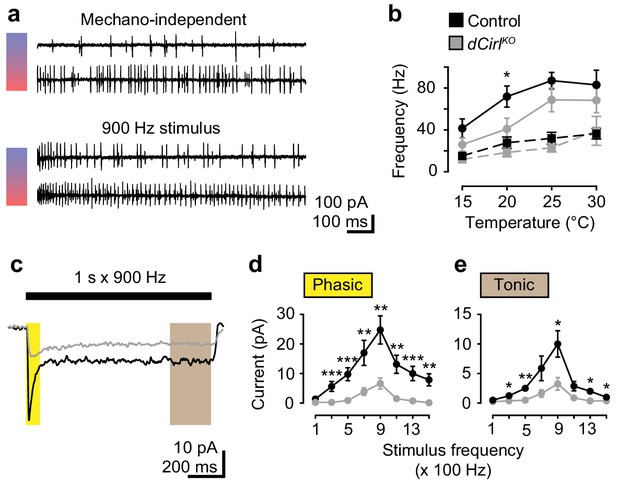
dCIRL shapes mechanosensory signal transduction.
(a) Recordings of wildtype lch5 action currents at 15°C and 30°C without and during mechanical vibration at 900 Hz applied to the cap cell. (b) Quantification of action current frequencies without (dashed line) and with (solid line) mechanical stimulation in control (black) and dCirlKO larvae (gray). Asterisk denotes p≤0.05 comparing event frequency at 20°C with a Student’s t-test. Data are presented as mean ± SEM, n = 8 animals per genotype. (c) Current recordings from lch5 neurons during 900 Hz mechanical stimulation in the presence of TTX (average of 10 sweeps). The wildtype (black) receptor current displays phasic (yellow shaded area) and tonic (gray area) components, both of which are strongly reduced after removal of dCirl (gray). (d) Quantification of phasic and (e) tonic current amplitudes across a stimulation range from 100 to 1500 Hz. Data are presented as mean ± SEM, n = 8 per genotype. Asterisks denote comparisons of current amplitude with a Mann-Whitney U test (*p≤0.05, **p≤0.01).
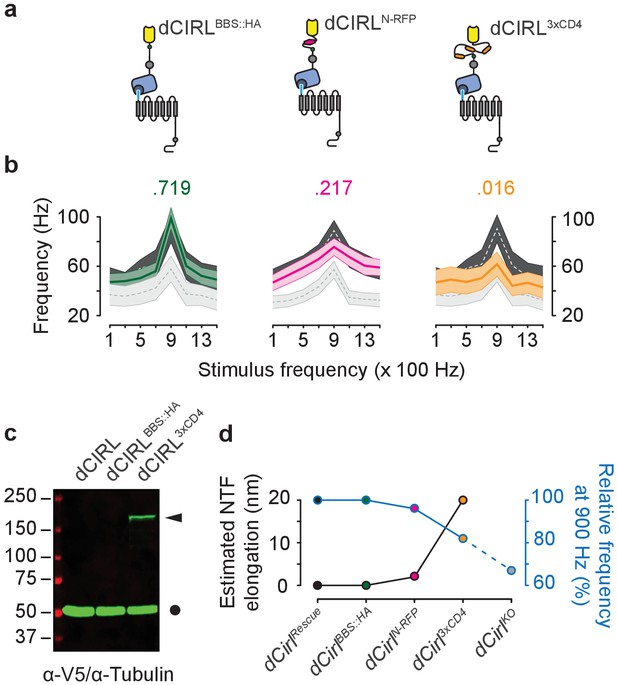
Extending the dCIRL NTF reduces the mechanosensory response.
(a) Upper panel, protein design of dCIRL elongation constructs bestowed with an HA::BBX fusion tag (left, green circle), an mRFP moiety (middle, magenta hexagon), or a triple CD4 immunoglobulin repeat cassette (right, orange ovals). All spacers were integrated into the same site within the dCIRL NTF just C-terminal of the RBL (rhamnose-binding lectin) domain. Schematics not to scale. (b) Action current frequencies plotted against mechanical stimulation. Response curves of wildtype (dCirlRescue; dark gray) and knockout (dCirlKO; light gray) lch5 neurons recorded in the same experiment are displayed for comparison. Data are presented as mean ± SEM. dCirlBBS::HA/dCirlRescue/dCirlKO (n = 10/20/20); dCirlN-RFP/dCirlRescue/dCirlKO (n = 20/20/20); dCirl3xCD4/dCirlRescue/dCirlKO (n = 10/20/20). Numbers above plots denote p values of comparisons with a Student’s t-test between dCirlRescue and respective elongated dCirl variants at 900 Hz stimulation, n denotes number of larvae. (c) Western blot showing stable expression of the dCIRL3xCD4 fusion protein in vivo. Protein extracts from animals (10 per genotype) were blotted and immunostained with an α-V5 antiserum specifically detecting the elongated NTF of dCIRL3xCD4 (ca. 177 kDa) bestowed with poly-V5-tags (arrowhead). Consistent with previous results on the high efficiency of GAIN-mediated dCIRL autoproteolysis (Scholz et al., 2015), no full-length receptor was found. α-Tubulin staining was used as loading control (circle). (d) Relationship between estimated NTF elongation (black curve) and lch5 response frequency (blue curve), normalized to respective dCirlRescue responses.
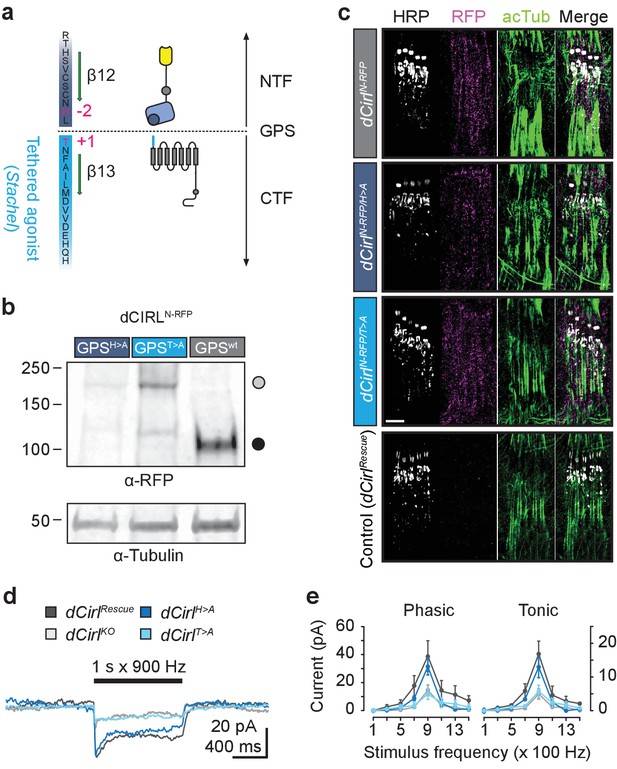
Differential effect of GPS mutations on mechanosensitivity.
(a) Structure of the dCIRL GPS region. The GPS separates NTF from CTF in proteolyzable aGPCRs. The C-terminal cleavage component contains the Stachel sequence, a potent receptor agonist in many aGPCRs (light blue). Magenta: conserved, mutated residues that are necessary for GPS cleavage. (b) Western blot of whole fly protein extracts containing wildtype or proteolysis-defective GPS variants of dCIRL probed against an mRFP tag in the NTF. The dCIRL-GPSwt sample displays only a fragment corresponding to the cleaved NTF (ca. 106 kDa; filled circle), while the two GPS mutants contain a band representing the full-length receptor (ca. 218 kDa; open circle). (c) SIM images of dCIRLN-RFP fusion proteins with wildtype and proteolysis-resistant GPS in lch5. The protein is trafficked into dendrites and cilia, regardless of autoproteolytic cleavage. Scale bar 5 µm. (d) Receptor current recordings (average of 8 sweeps) of lch5 neurons under TTX inhibition highlight the divergent effects of the GPS mutations on mechanosensitivity (dark blue, dCirlH>A; light blue, dCirlT>A). (e) Quantification of tonic and phasic receptor current components. Despite abrogating GPS cleavage, the response profile of the dCirlH>A receptor variant is unaffected (900 Hz, phasic: p=0.464, tonic: p=0.460, Student’s t-test vs. dCirlRescue). In contrast, changing the first residue of the Stachel sequence in dCirlT>A mutants abolishes the receptor’s mechanosensory function, resulting in a dCirlKO response profile (900 Hz, phasic: p=0.030, tonic: p=0.023, Student’s t-test vs. dCirlRescue). Data are presented as mean ± SEM, n = 8 larvae per genotype.
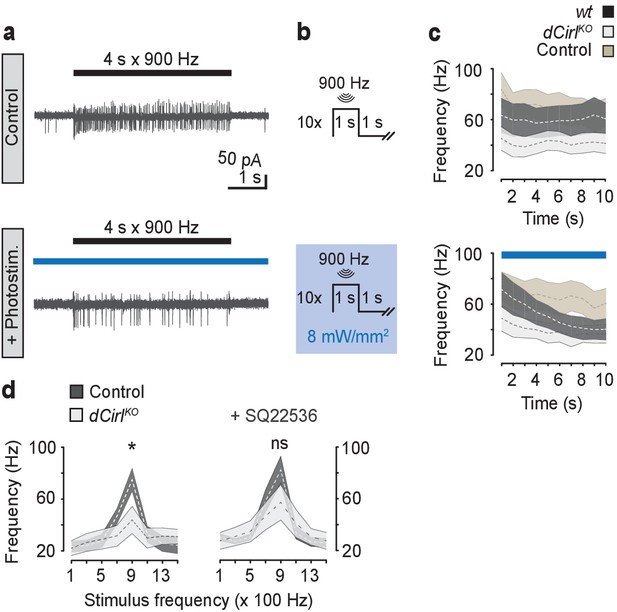
cAMP signaling by dCIRL.
(a) Example current recordings from wildtype lch5 neurons during only mechanical (upper panel) and combined mechanical-light stimulation (lower panel) demonstrate the suppressive effect of cAMP elevation by bPAC on the mechanically-evoked action current frequency. (b) Protocol for combined mechanical stimulation and optogenetic cAMP production via bPAC photoactivation. (c) The mechanosensory response (action current frequency) of wildtype lch5 neurons is decreased to the level of dCirlKO larvae by increasing cAMP concentrations through light-induced bPAC stimulation (blue bar). In contrast, dCirlKO neurons are unaffected by light stimulation. Data are presented as mean ± SEM, n denotes number of animals. iav-GAL4>UAS-bPAC; wt (black, n = 9); iav-GAL4>UAS-bPAC; dCirlKO (gray, n = 10); iav-GAL4; wt (brown, n = 9). (d) Pharmacological inhibition of adenylyl cyclase activity using 100 µM SQ22536 rescues mechanically-evoked action current frequencies in dCirlKO lch5 neurons. Data are presented as mean ± SEM. Event frequency at 900 Hz without inhibitor: Control: 74.9 ± 8.67 Hz; dCirlKO: 43.88 ± 10.48 Hz; p=0.0287, Student’s t-test. Event frequency at 900 Hz with inhibitor: Control: 82.63 ± 10.51 Hz; dCirlKO: 57.25 ± 13.69 Hz; p=0.2103; n = 8 per genotype and condition.
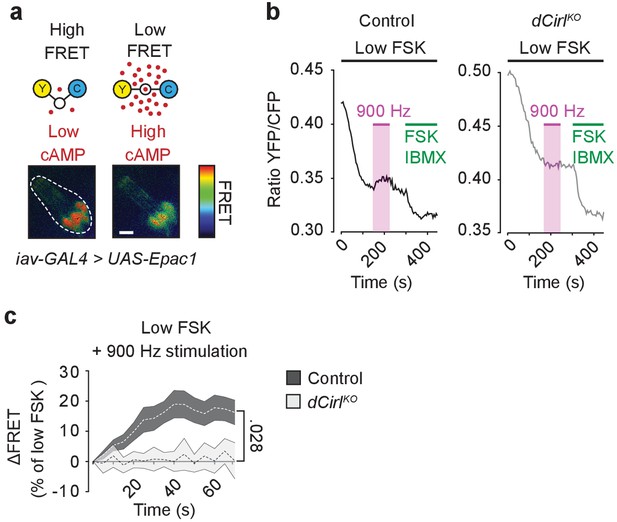
dCIRL reduces cAMP levels in sensory neurons in response to mechanical stimulation.
(a) Schematic structure of the cAMP sensor Epac1-camps, which changes its conformation and fluorescence property upon binding of cAMP. Corresponding pseudocolor FRET images (YFP/CFP ratios) of Ich5 neurons (iav-GAL4>UAS-Epac1-camps) at low and high cAMP concentrations. Scale bar 10 µm. (b) Absolute FRET values (YFP/CFP ratios) recorded in control and dCirlKO Ich5 neurons, corresponding to the region of interest depicted in (a). In order to ensure a dynamic sensor range, 0.5 µM FSK was first added to the preparation (Maiellaro et al., 2016). Mechanical stimulation (900 Hz, pink bar) decreases cAMP levels in control but not in dCirlKO Ich5 neurons. At the end of the experiment, maximal FRET responses are induced by 10 µM FSK and 100 µM IBMX (3-Isobutyl-1-methylxanthin), a non-selective phosphodiesterase inhibitor. (c) Average time course of piezo-induced FRET changes in control and dCirlKO Ich5 neurons. Data are expressed as percentages of the low forskolin response and presented as mean ± SEM. ΔFRET at 70 s: Control: 16.28 ± 4.05%, n = 14; dCirlKO: 0.147 ± 3.78%, n = 6 larvae. Number denotes p value of comparison at 70 s with a Student’s t-test. See also Figure 7—figure supplements 1 and 2.
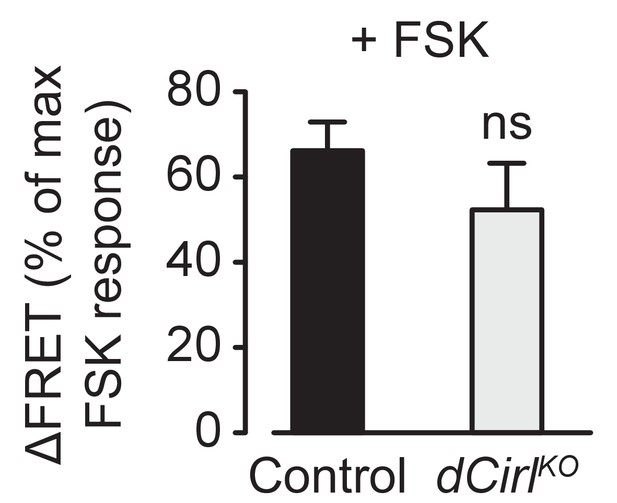
Basal cAMP levels in ChO neurons.
Activation of adenylyl cyclase by low FSK concentrations triggers similar cAMP accumulations in control and dCirlKO neurons. FSK-induced ΔFRET values are expressed as percentages of the maximal FRET response and presented as mean ± SEM. Control: 66.17 ± 6.77%, n = 12; dCirlKO: 52.33 ± 10.89%, n = 6; p=0.276, Student’s t-test.
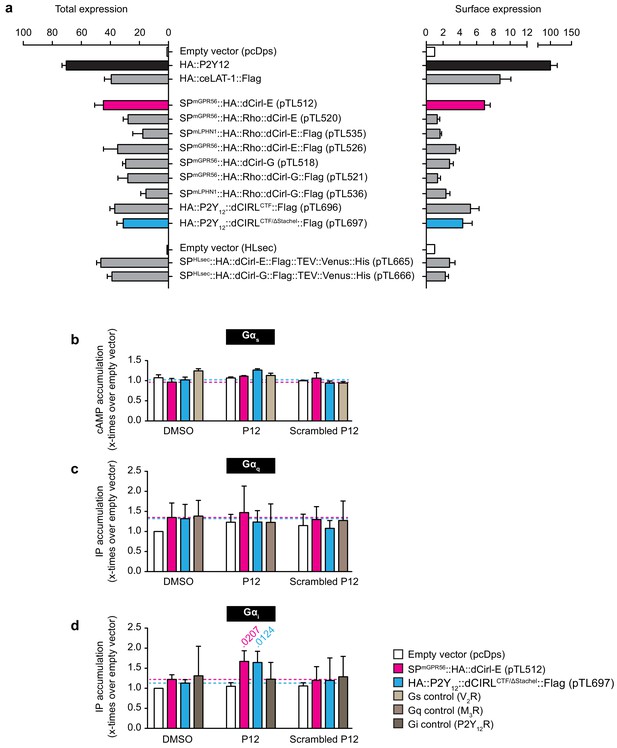
A synthetic peptide mimicking dCIRL’s tethered agonist stimulates Gαi coupling.
(a) Total and cell surface expression of various dCirl constructs. COS-7 cells were transfected with 1 µg (total expression)/0.5 µg (cell surface expression) of empty vector (pcDps or pHLSec) or plasmid encoding either dCirl constructs, the C. elegans Latrophilin homolog lat-1 or the human ADP receptor P2Y12 which served as controls. Expression levels were measured 48 hr post transfection using ELISA on lysed cells (total expression) or intact cells (cell surface expression). Data are displayed as x-fold of empty vector and displayed as mean ± SEM of at least three independent experiments, each performed in triplicate. (b) Peptide-stimulated cAMP response of dCIRL. COS-7 cells transfected with 0.2 µg empty control vector (pcDps) or plasmid encoding dCirl were stimulated with 1 mM putative Stachel sequence-derived peptide of 12 aa length and cAMP levels were measured by cAMP accumulation assay. A scrambled peptide of the same length and amino acid composition (scrambled P12) served as negative control. To control for peptide specificity, the human vasopressin type 2 receptor (V2R) was used, which does not respond to the peptides tested. Basal cAMP levels (empty vector, no peptide) are 3.2 ± 0.7 nM. Data are normalized to respective non-stimulated controls and are given as mean ± SEM. (c) dCIRL does not signal via a Gq protein signaling cascade upon treatment with Stachel sequence-derived peptides. COS-7 cells transfected with 0.2 µg empty control vector (pcDps) or plasmid encoding dCirl were stimulated with 1 mM peptide and IP accumulation assays were performed. Neither the potentially activating peptide nor the scrambled peptide show any effect on dCIRL. The rat muscarinic M3 acetylcholine receptor M3R was used as a control for peptide specificity. Basal IP levels (empty vector, no peptide) are 434 ± 52 nM. Data normalized to respective non-stimulated controls are given as mean ± SEM of four independent experiments, each performed in triplicate. (d) dCIRL is activated by P12 to mediate a Gi protein signal. To measure functional coupling of dCIRL to Gαi upon stimulation with P12, a chimeric Gαqi4 protein was applied to reroute a Gi-mediated signal to a Gαq protein cascade. 0.2 µg of plasmid containing pcDps or dCirl were co-transfected with 0.02 µg plasmid with the chimeric protein DNA into COS-7 cells. IP accumulation assays were performed detecting Gαq-mediated increase in IP levels upon treatment with 1 mM peptide P12 or scrambled P12. A significant increase in IP was detected upon P12 challenge. The human P2Y12 receptor served as a control for peptide specificity. Basal IP levels (empty vector, no peptide): 385 ± 32 nM. Data normalized to respective non-stimulated controls are given as mean ± SEM of four independent experiments, each performed in triplicate. p values < 0.05 are indicated above the respective columns, all others were > 0.05.





