Striatal adenosine A2A receptor neurons control active-period sleep via parvalbumin neurons in external globus pallidus
Figures
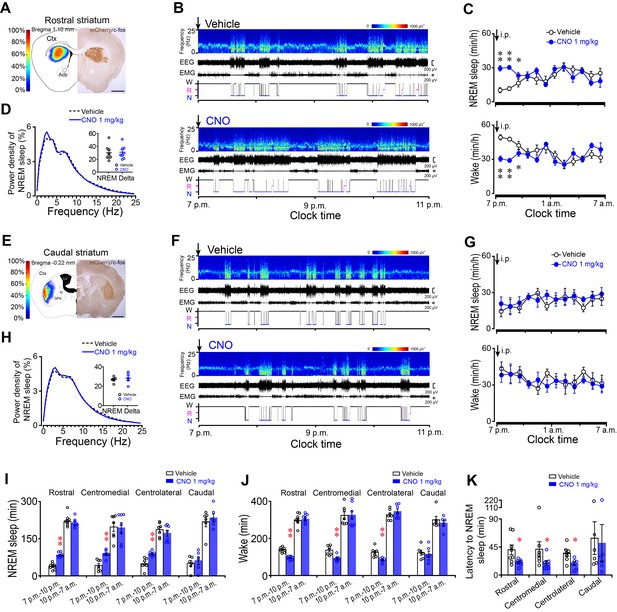
Chemogenetic activation of A2AR neurons in the rostral, centromedial and centrolateral, but not caudal, striatum increased NREM sleep during active period.
(A, E) Heat map (left) shows the virus-injected area, and immunostaining micrograph (right) represents hM3Dq-expressing neurons (mCherry+) in the rostral (A) or caudal (E) striatum of Adora2a-Cre mice. Scale bar, 1 mm. Ctx, cortex; GPe, external globus pallidus; ic, internal capsule; LV, lateral ventricle. (B, F) Typical examples of compressed spectral array (0–25 Hz) EEG, EMG and hypnograms over 4 hr following intraperitoneal (i.p.) administration of vehicle (top panel) or CNO (bottom panel) in a mouse with bilateral hM3Dq receptor expression in A2AR neurons of the rostral (B) or caudal (F) striatum. (C, G) Time course of NREM sleep (top panel) and wakefulness (bottom panel) following vehicle (open black circle) and CNO (closed blue circle) injections (i.p.) in Adora2a-Cre mice with hM3Dq-expressing neurons in the rostral (C, NREM: two-way repeated measures ANOVA, n = 8, F1,14 = 45.113, p=9.836E-6; paired t test, **p=4.999E-5, **p=3.613E-5, *p=0.022. wake: two-way repeated measures ANOVA, n = 8, F1,14 = 36.632, p=2.975E-5; paired t test, **p=5.919E-5, **p=3.613E-5, *p=0.034.) or caudal (G, NREM: two-way repeated measures ANOVA, n = 6, F1,10 = 0.3, p=0.596; wake: two-way repeated measures ANOVA, n = 6, F1,10 = 0.16, p=0.697) striatum. (D, H) Relative average EEG power density of NREM sleep during the 3 hr period after CNO or vehicle injections and quantitative changes in power for the delta (0.5–4.0 Hz) frequency bands (insert) following CNO or vehicle in Adora2a-Cre mice with hM3Dq-expresssing neurons in the rostral (D, paired t test, n = 8, p=0.051) or caudal (H, paired t test, n = 6, p=0.356) striatum. (I, J) Total amount of NREM sleep (I) (paired t test, Rostral: n = 8, **p=1.518 × 10−5, p=0.052; Centromedial: n = 7, **p=1.144E-4, p=0.716; Centrolateral: n = 7, **p=0.002, p=0.194; Caudal: n = 6, p=0.169, p=0.366) and wakefulness (J) (paired t test, Rostral: n = 8, **p=2.096E-5, p=0.345; Centromedial: n = 7, **p=2.449E-4, p=0.84; Centrolateral: n = 7, **p=0.004, p=0.137; Caudal: n = 6, p=0.276, p=0.362) during the 3 hr post-injection period (7 p.m.–10 p.m.) and the subsequent 9 hr of the active period (10 p.m.–7 a.m.) following vehicle or CNO injections in Adora2a-Cre mice expressing hM3Dq receptors in the rostral, centromedial, centrolateral, and caudal striatum. (K) The latency of NREM sleep (paired t test, Rostral: n = 8, *p=0.026; Centromedial: n = 7, *p=0.048; Centrolateral: n = 7, *p=0.032; Caudal: n = 6, p=0.313) following vehicle or CNO injections in Adora2a-Cre mice expressing hM3Dq receptors in the rostral, centromedial, centrolateral, and caudal striatum. See Figure 1—source data 1.
-
Figure 1—source data 1
Sample size (n), mean and SEM are presented for the data in Figure 1.
- https://doi.org/10.7554/eLife.29055.008
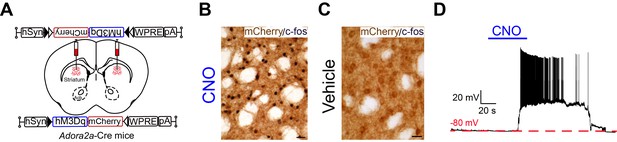
Expression of hM3Dq receptors in the striatum of Adora2a-Cre mice.
(A) Schematic of bilateral virus vector injection sites in the dorsal striatum and expression of hM3Dq receptors in Adora2a-Cre mice. Acb, accumbens nucleus. (B, C) c-fos immunoreactivity (black nuclear) after CNO (B) and vehicle (C) injection, indicating excitation of hM3Dq-expressing neurons (mCherry+) by CNO. Scale bar, 20 μm. (D) Bath applied CNO (5 μM) produced depolarization and firing in hM3Dq-expressing A2AR neurons in brain slice.

Administration of CNO had no effect on the sleep-wake cycle of control mice.
The control mice were injected with hSyn-DIO-mCherry-AAV in the striatum. (A) Time course of changes in NREM sleep, REM sleep and wake following vehicle and CNO injections in mice with bilateral mCherry in A2AR neurons in the striatum. n = 7, two-way repeated measures ANOVA, NREM: F1,12 = 0.509, p=0.489; REM: F1,12 = 0.213, p=0.653; wake: F1,12 = 0.824, p=0.382. (B) Total amounts of NREM sleep, REM sleep and wakefulness during the 3 hr post-injection period (7 p.m.–10 p.m.) and the remainder (9 hr) of the inactive period (11 p.m. –7 a.m.). n = 7, paired t test, NREM: p=0.166, p=0.42; REM: p=0.687, p=0.633; Wake: p=0.163, p=0.519. See Figure 1—figure supplement 2—source data 1.
-
Figure 1—figure supplement 2—source data 1
Sample size (n), mean and SEM are presented for the data in Figure 1—figure supplement 2.
- https://doi.org/10.7554/eLife.29055.009
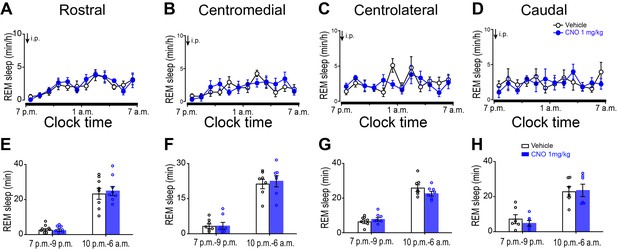
Chemogenetic activation of the A2AR neurons in the rostral, centromedial, centrolateral or caudal striatum did not affect REM sleep.
(A–D) Time course of changes in REM sleep following vehicle and CNO injections in mice with bilateral expression of hM3Dq receptors in A2AR neurons of the rostral (A), centomedial (B), centrolateral (C) or caudal (D) striatum, respectively. n = 6–8, two-way repeated measures ANOVA, rostral: F1,14 = 0.019, p=0.893; centromedial: F1,12 = 0.408, p=0.535; centrolateral: F1,12 = 1.106, p=0.314; caudal: F1,10 = 0.320, p=0.584. (E–H) Total amounts of REM sleep during the 3 hr post-injection period (7 p.m.–10 p.m.) and the remainder (9 hr) during inactive periods (11 p.m.–7 a.m.) following vehicle and CNO injections in mice with bilateral expression of control mCherry in A2AR neurons in the rostral (E), centomedial (F), centrolateral (G) or caudal (H) striatum, respectively. n = 6–8, paired t test, rostral: p=0.879, p=0.401; centromedial: p=0.932, p=0.490; centrolateral: p=0.449, p=0.122; caudal: p=0.143, p=0.870. See Figure 1—figure supplement 3—source data 2.
-
Figure 1—figure supplement 3—source data 2
Sample size (n), mean and SEM are presented for the data in Figure 1—figure supplement 3.
- https://doi.org/10.7554/eLife.29055.010

Chemogenetic activation of A2AR neurons altered the mean duration but not the episode number of NREM sleep.
(A–D) Effect of chemogenetic activation of A2AR neurons in rostral (A, n = 8, paired t test, p=0.758, p=0.381, p=0.769), centromedial (B, n = 7, paired t test, p=0.736, p=0.947, p=0.743), centrolateral (C, n = 7, paired t test, p=0.417, p=0.247, p=0.381), and caudal (D, n = 6, paired t test, p=0.555, p=1, p=0.525) striatum on total episode numbers during the first 3 hr after CNO and vehicle injections. (E–H) Effect of chemogenetic activation of A2AR neurons in rostral (E, n = 8, paired t test, *p=0.011, p=0.389, **p=0.007), centromedial (F, n = 7, paired t test, p=0.143, p=0.691, *p=0.04), centrolateral (G, n = 7, paired t test, p=0.114, p=0.561, p=0.096), and caudal (H, n = 6, paired t test, p=0.735, p=0.265, p=0.056) striatum on mean duration during the first 3 hr after CNO and vehicle injections. See Figure 1—figure supplement 4—source data 3.
-
Figure 1—figure supplement 4—source data 3
Sample size (n), mean and SEM are presented for the data in Figure 1—figure supplement 4.
- https://doi.org/10.7554/eLife.29055.011

Chemogenetic activation of A2AR neurons in the centromedial and centrolateral striatum increased NREM sleep during active period.
(A) Heat map (left) shows the virus-injected area, and immunostaining (right) represents hM3Dq expression in A2AR neurons (mCherry+) in the centromedial striatum. Scale bar, 1 mm. (B) Typical examples of EEG, EMG and hypnograms over 4 hr following administration (i.p.) of vehicle or CNO in a mouse with bilateral hM3Dq receptor expression in A2AR neurons in the centromedial striatum. (C, D) Time course of changes in NREM sleep (C) and wake (D) following vehicle and CNO injections in mice with bilateral hM3Dq receptor expression in A2AR neurons in the centromedial striatum. n = 7, two-way repeated measures ANOVA, paired t test, NREM: F1,12 = 13.705, p=0.003, **p=0.004, **p=0.004, **p=0.002; wake: F1,12 = 12.656, p=0.004, **p=0.003, **p=0.002, **p=0.004 (t-test). (E) Relative average EEG power spectrum of NREM sleep during the 3 hr period after CNO or vehicle injections and the quantitative changes in power for the delta (0.5–4.0 Hz) frequency bands (insert) following CNO or vehicle injections in mice with hM3Dq-expressing in A2AR neurons in the centromedial striatum. n = 7, paired t test, p=0.827. (F) Heat map (left) shows the virus-injected area, and immunostaining (right) represents hM3Dq expression in A2AR neurons (mCherry+) in the centrolateral striatum. Scale bar, 1 mm. (G) Typical examples of EEG, EMG and hypnograms over 4 hr following administration (i.p.) of vehicle or CNO (1 mg/kg) in a mouse with bilateral hM3Dq receptor expression in A2AR neurons in the centrolateral striatum. (H, I) Time course of the amount of NREM sleep (H) and wakefulness (I) following vehicle and CNO injections in mice with bilateral expression of the hM3Dq receptor in A2AR neurons in the centrolateral striatum. n = 7, two-way repeated measures ANOVA, paired t test, NREM: F1,12 = 15.203, p=0.002, **p=2.581E-4, **p=0.002, *p=0.023; wake: F1,12 = 8.509, p=0.015, **p=0.001, **p=0.003. (J) Relative average EEG power spectrum of NREM sleep during the first 3 hr period after CNO or vehicle injections and the quantitative changes in power for the delta (0.5–4.0 Hz) frequency bands (insert) following CNO or vehicle injections in mice with hM3Dq-expressing in A2AR neurons in the centrolateral striatum. n = 7, paired t test, p=0.637. aca, anterior commissure, anterior part; Ctx, cortex. See Figure 1—figure supplement 5—source data 4.
-
Figure 1—figure supplement 5—source data 4
Sample size (n), mean and SEM are presented for the data in Figure 1—figure supplement 5.
- https://doi.org/10.7554/eLife.29055.012
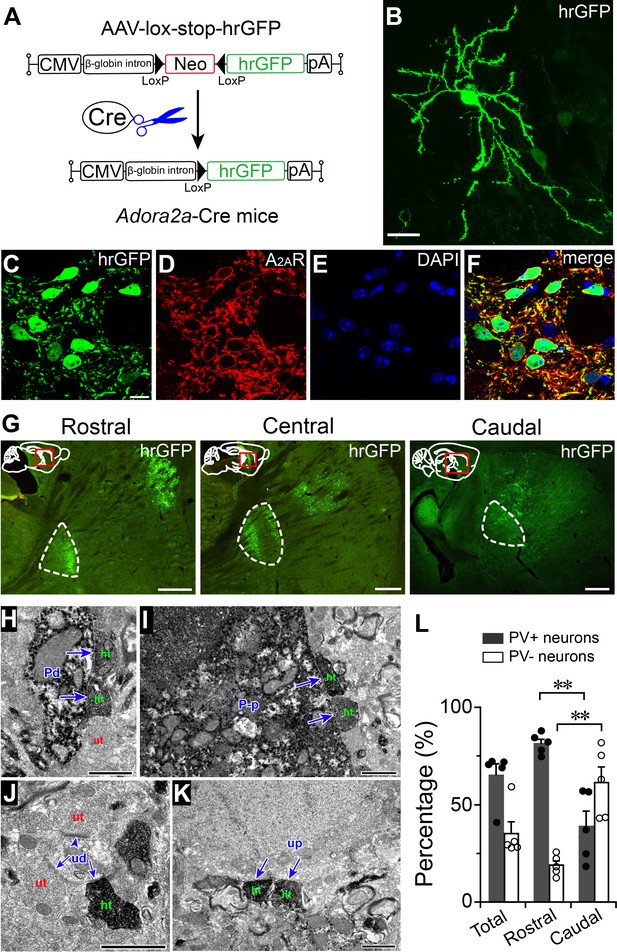
Topographical projections of striatal A2AR neurons in the GPe.
(A) Schematic of Cre-dependent hrGFP expression in Adora2a-Cre mice. (B) Fluorescent micrograph showing a typical A2AR neuron that was labeled by injection of hrGFP, in the striatum. Scale bar, 20 μm. (C–F) Fluorescent micrographs showing colocalization of hrGFP with A2AR immunoreactivity, confirming selective expression of hrGFP in A2AR neurons. Scale bar, 10 μm. (G) Fluorescent micrographs of sagittal brain sections showing that hrGFP-expressing A2AR neurons in the rostral, central, and caudal striatum, send axons to the rostral, rostral plus caudal, and caudal areas of the GPe, respectively. Dotted lines indicate GPe boundaries. Scale bar, 500 μm. (H–K) Electron micrographs showed that hrGFP-IR terminals (ht) formed symmetric synapses (arrow) with a PV-IR dendrite (Pd), (H), a PV-IR perikaryon (P–p), (I), a PV-unlabeled dendrite (ud), (J), and a PV-unlabeled perikaryon (up), (K) in the GPe. In contrast, hrGFP-unlabeled terminals (ut), (H and J) formed both symmetric and asymmetric synapses (arrowhead) with PV-IR (Pd), (H) and PV-unlabeled dendrites (ud), (J). Scale bar, 1 μm (H–K). (L) Percentages of hrGFP-IR terminals that formed synaptic contacts with PV-positive and PV-negative neurons in the whole, rostral, and caudal GPe (n = 5, paired t test, rostral PV+ vs. caudal PV+, **p=0.0025; rostral PV- vs. caudal PV-, **p=0.0034). See Figure 2—source data 1.
-
Figure 2—source data 1
Sample size (n), mean and SEM are presented for the data in Figure 2.
- https://doi.org/10.7554/eLife.29055.014
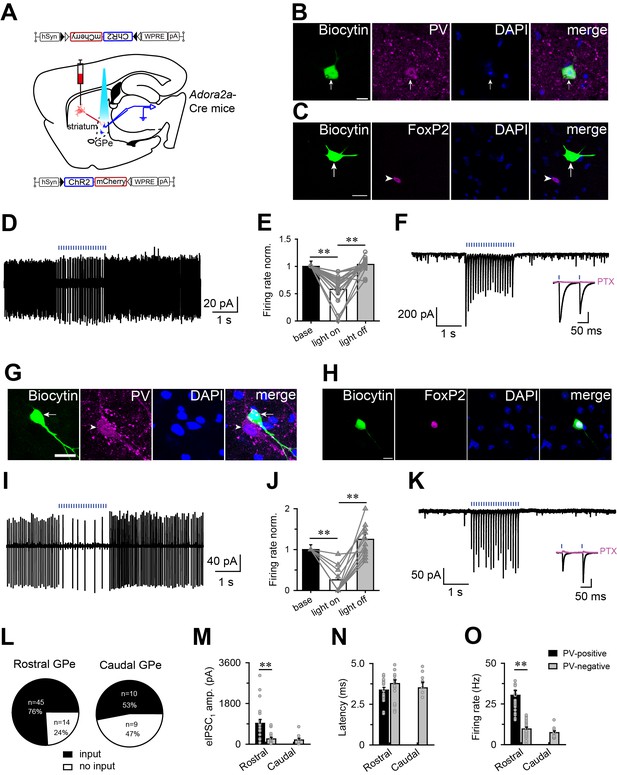
Optogenetic activation of striatopallidial terminals suppressed the firing of PV-positive and PV-negative neurons in the GPe.
(A) Schematic of experiment setup. ChR2-AAV was injected in the striatum of Adora2a-Cre mice, and responses were recorded in the GPe. (B, C) Fluorescence micrographs showing recorded biocytin-filled GPe neurons that expressed PV (B) but not FoxP2 (C). Scale bar, 10 μm. (D, E) Brief light pulses decreased firing rates of PV-positive neurons in the GPe. Typical cell-attached patch recording (D) of a PV-positive neuron. (E) Histograms illustrating the normalized firing rate of PV-positive neurons in the GPe before, during and after blue light stimulation in the cell-attached mode (n = 19, paired t test, **p=0.001, **p=0.002). (F) Photostimulation evoked IPSCs in a GPe PV-positive neuron under voltage-clamp mode. An insert shows that PTX (100 μM; pink line), a GABAA receptor antagonist, completely abolished the IPSCs. (G, H) Fluorescence micrographs showing recorded biocytin-filled GPe neurons that expressed FoxP2 (H) but not PV (G). Scale bar, 10 μm. (I, J) Brief light pulses decreased the firing rate of PV-negative neurons in the GPe. Typical cell-attached patch recording (I) from a PV-negative neuron in the GPe. (J) Histograms illustrating the normalized firing rate of GPe PV-negative neurons before, during, and after blue light stimulation in the cell-attached patch mode. Brief light pulses decreased the firing rate (n = 12, paired t test, **p=2.972E-5, **p=3.465E-4). (K) Photostimulation evoked IPSCs in a GPe PV-negative neuron. An insert shows that PTX (100 μM; pink line) completely abolished the IPSCs. (L) Number and proportion of recorded neurons in the GPe that did respond to the photostimulation of ChR2-expressing terminals from A2AR neurons in the rostral or caudal striatum. (M–O) GPe PV-positive neurons showed larger amplitude IPSCs evoked by blue light stimulation (M) and higher average firing rate (O) with the same latency from light pulse to onset of the evoked IPSC (N) compared with GPe PV-negative neurons that received input from A2AR neurons in the rostral or caudal striatum. IPSCs: n = 37, independent samples t test, **p=0.001; Latency: n = 40, independent samples t test, p=0.154; firing: n = 46, independent samples t test, **p=0.001. Blue bars in D, F, I, K, represent 1 ms blue light pulses at the frequency of 10 Hz. See Figure 3—source data 1.
-
Figure 3—source data 1
Sample size (n), mean and SEM are presented for the data in Figure 3.
- https://doi.org/10.7554/eLife.29055.019
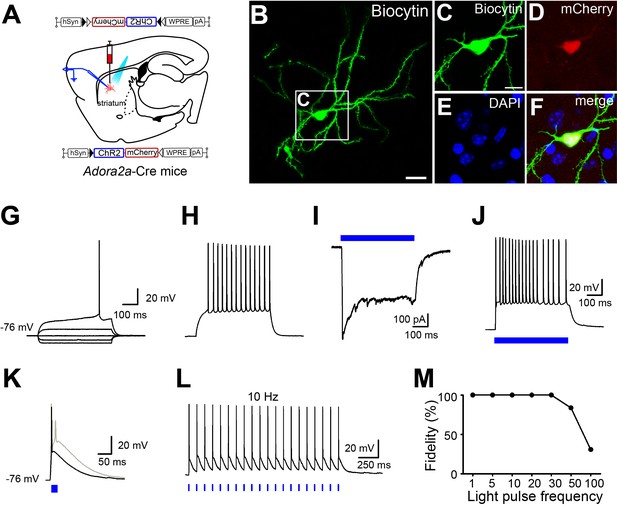
Optogenetic stimulation of A2AR neurons in the striatum in vitro.
(A) Schematic of bilateral virus injection sites in the striatum and the expression of ChR2-mCherry in striatal A2AR neurons of Adora2a-Cre mice. Light stimulation of ChR2-mCherry neurons and striatal whole-cell recordings. (B–F) Fluorescence micrographs showing a recorded biocytin-filled neuron in the striatum (B) and outlined region in (B) showing: biocytin staining (C), mCherry fluorescence (D), nuclei stained with DAPI (E) and the merged image (F). Scale bar, 20 μm (B) and 10 μm (C–F). (G and H) Characteristic membrane properties and spiking pattern of an A2AR neuron expressing ChR2: note the hyperpolarized resting membrane potential (−76 mV), the inward rectification, and the long ramp to the AP threshold leading to a delayed spike discharge (the delay to first spike is 417 ms in this example). Raw traces show individual voltage responses to series of 500 ms current injections from −160 to 160 pA with 80 pA increasing current steps (G). Voltage response to a 500 ms, 240 pA current injection, spike frequency 28 Hz (H). (I and J) Typical traces showing a A2AR neuron expressing ChR2 in response to 500 ms sustained blue light stimulation in a voltage-clamp (I) or current-clamp (J) mode. (K) Brief 1 ms blue light pulse evoked single action potential (black line) in a ChR2 expressing A2AR neuron. A pulse longer than 4 ms resulted in two spikes (gray line). (L) Voltage response of a A2AR neuron expressing ChR2-mCherry to 20 pulses (1 ms) of blue light stimulation at 10 Hz. (M) Fidelity responses of ChR2 expressing neurons to light pulses at frequencies up to 100 Hz (n = 8 cells from five mice).
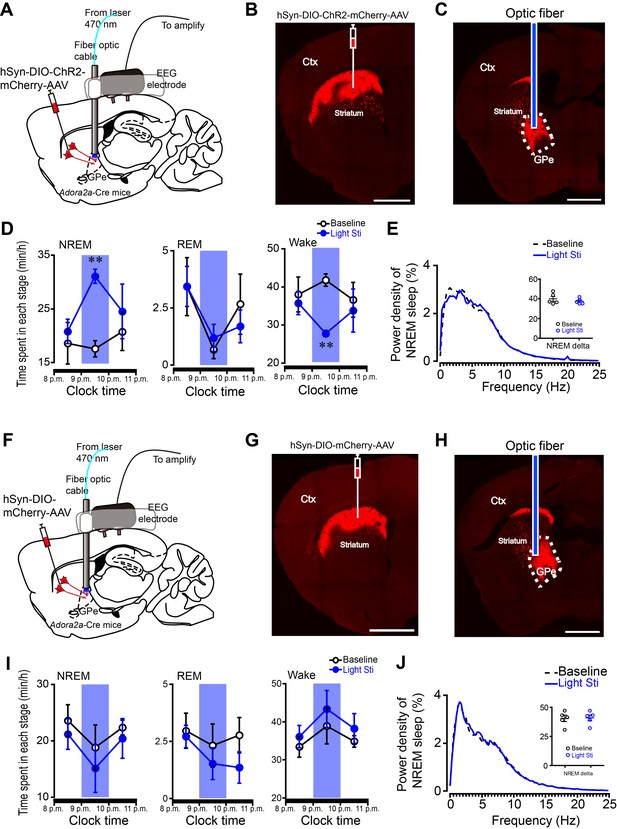
Optogenetic stimulation of the striatopallidal terminals promoted NREM sleep.
(A, F) Schematic of experimental setups. Virus vector carrying either ChR2-mCherry (A) or mCherry (F) was injected into the dorsal striatum of Adora2a-Cre mice, and optical fibers were implanted into the GPe to optically stimulate the striatopallidal terminals. (B, G) Fluorescent micrographs showing ChR2-mCherry expression (B) and only mCherry expression (G) in the dorsal striatum. Scale bar = 1 mm. (C, H) Fluorescent micrographs showing the location of optical fibers implanted in the GPe of the ChR2-mCherry (C) and mCherry transduced mice (H). Ctx, cortex; GPe, external globus pallidus. Scale bar, 1 mm. (D, I) The hourly amount of NREM sleep, REM sleep and wake of baseline and 20 Hz stimulation (5 ms pulses at 20 Hz, 50 s on/40 s off, 40 cycles) in the ChR2-mCherry (D) and mCherry transduced mice (I) at active periods (8 p.m. – 11 p.m.). The blue columns indicate the photostimulation period. ChR2-mCherry group: n = 5, paired t test, NREM, p=0.554, **p=0.0009, p=0.611; REM, p=0.990, p=0.487, p=0.651; wake, p=0.518, **p=0.001, p=0.764. mCherry group: n = 5, paired t test, NREM, p=0.248, p=0.291, p=0.624; REM, p=0.768, p=0.207, p=0.235; wake, p=0.106, p=0.176, p=0.505. (E, J) Relative average EEG power spectrum of NREM sleep and quantitative changes in power for delta (0.5–4 Hz) frequency bands (insert) during the 1 hr period of baseline and photostimulation in the ChR2-mCherry (E) and mCherry transduced mice (J). ChR2-mCherry group: n = 5, paired t test, p=0.426. mCherry group: n = 5, paired t test, p=0.750. See Figure 3—figure supplement 2—source data 1.
-
Figure 3—figure supplement 2—source data 1
Sample size (n), mean and SEM are presented for the data in Figure 3—figure supplement 2.
- https://doi.org/10.7554/eLife.29055.018
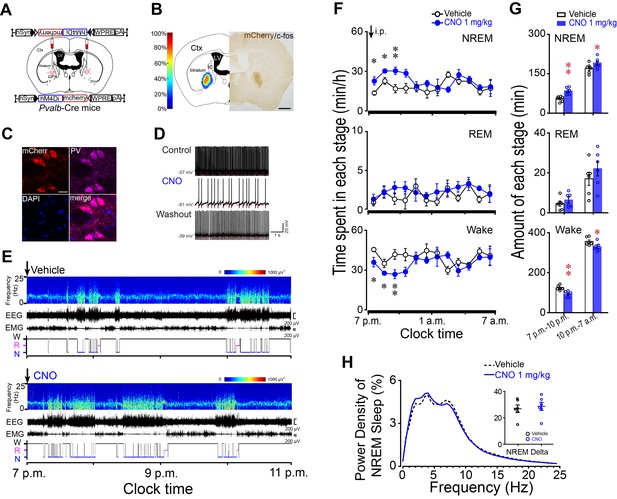
Chemogenetic inhibition of PV neurons in the GPe increased NREM sleep during active period.
(A) Schematic of bilateral virus injection sites in the GPe of Pvalb-Cre mice, with details of hSyn-DIO-hM4Di-mCherry-AAV vector injected. (B) Heat map (left) shows injected areas, and immunostaining (right) represents hM4Di expression (mCherry+) in the GPe. Scale bar, 1 mm. Ctx, cortex; ic, internal capsule; LV, lateral ventricle. (C) Fluorescent micrographs showing colocalization of mCherry with PV immunoreactivity, confirming the selective expression of hM4Di receptors in GPe PV neurons of Pvalb-Cre mice. Scale bar, 10 μm. (D) Bath applied CNO (5 μM) reduced spontaneous firing rate in hM4Di-expressing PV neurons in the GPe of brain slices. (E) Typical examples of compressed spectral array (0–25 Hz) EEG, EMG and hypnograms over 4 hr following administration (i.p.) of vehicle or CNO in a mouse with bilateral hM4Di receptor expression in GPe PV neurons. (F) Time course of changes in NREM sleep, REM sleep, and wakefulness following vehicle (open circle) and CNO (closed blue circle) injections during active period in mice expressing hM4Di receptors in PV neurons of the GPe. n = 6, two-way repeated measures ANOVA, paired t test, NREM: F1,10 = 18.698, p=0.002 (ANOVA), *p=0.026, *p=0.033, **p=0.001 (t-test); REM: F1,10 = 1.209, p=0.297 (ANOVA); Wake: F1,10 = 14.614, p=0.003 (ANOVA), *p=0.036, *p=0.043, **p=0.002 (t-test). (G) Total amounts of NREM sleep, REM sleep, and wakefulness during the 3 hr post-injection period (7 p.m.–10 p.m.) and the following 9 hr of the active period (10 p.m.–7 a.m.) following vehicle or CNO injections in mice expressing hM4Di receptors in GPe PV neurons. n = 6, paired t test, NREM: **p=1.972E-4, *p=0.027; REM: p=0.088, p=0.106; Wake: **p=2.508E-4, *p=0.028. (H) Relative average EEG power spectrum of NREM sleep and quantitative changes in power for delta (0.5–4.0 Hz) frequency bands (insert) during the 3 hr period after CNO and vehicle injections. n = 6, paired t test, p=0.258. See Figure 4—source data 1.
-
Figure 4—source data 1
Sample size (n), mean and SEM are presented for the data in Figure 4.
- https://doi.org/10.7554/eLife.29055.021
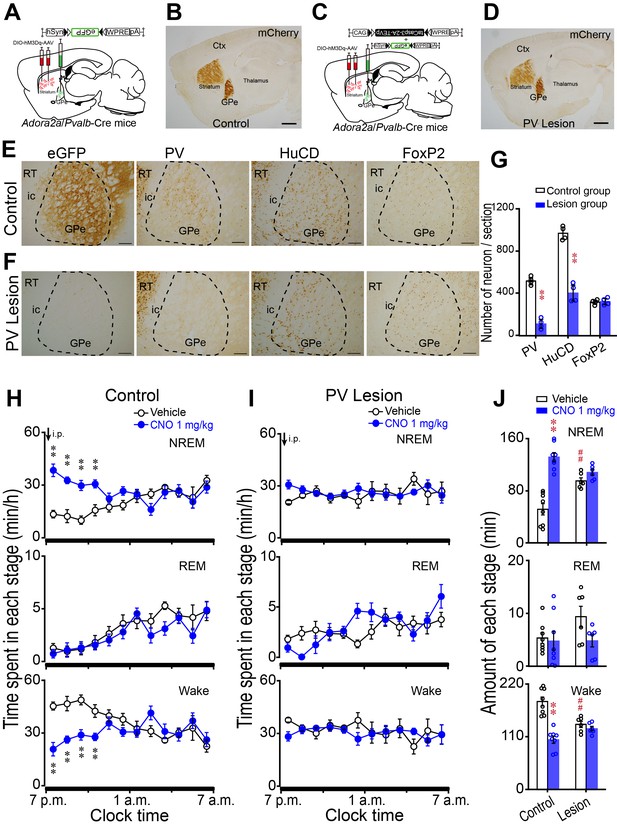
Lesion of GPe PV neurons abolished the increase in NREM sleep caused by activation of striatal A2AR neurons.
(A, C) Schematic of control group (A) or lesion group (C) by injecting AAV-DIO-hM3Dq in the striatum and AAV-DIO-eGFP in the GPe (A) or AAV-DIO-hM3Dq in the striatum and a mixture of AAV-Flex-taCasp3-TEVp and AAV-DIO-eGFP in the GPe (C) of Adora2a/Pvalb-Cre mice. (B, D) Immunostaining micrographs showing hM3Dq expression in A2AR neurons (mCherry+) in the striatum and projections of A2AR neurons in Adora2a/Pvalb-Cre mice of the control group (B) and lesion group (D). Scale bar, 1 mm. Ctx, cortex; GPe, external globus pallidus. (E, F) Immunostaining micrographs represent expression of eGFP, PV, HuCD and FoxP2 in the GPe of a Adora2a/Pvalb-Cre mouse of control group (E) or PV lesion group (F). Scale bar, 200 μm. ic, internal capsule; RT, reticular thalamic nucleus. (G) The number of PV, HuCD, and FoxP2 neurons within the GPe of a representative coronal section (30 μm thick) in control and lesion mice. n = 4, paired t test, PV: **p=0.002; HuCD: **p=0.002; FoxP2: p=0.809. (H, I) Time course of changes in NREM sleep, REM sleep, and wakefulness following vehicle (open circle) and CNO (closed blue circle) injections in the control group (H, n = 8) and lesion group (I, n = 6). Control: n = 8, two-way repeated measures ANOVA, NREM: F1,14 = 11.218, p=0.005, paired t test, **p=0.002, **p=0.003, **p=1.783E-5, **p=7.75E-6; REM: F1,14 = 2.287, p=0.153; Wake: F1,14 = 7.511, p=0.016, **p=0.002, **p=0.003, **p=5.551E-5, **p=9.232E-5. Lesion: n = 6, NREM: F1,10 = 0.461, p=0.512; REM: F1,10 = 0.387, p=0.548; Wake: F1,10 = 0.532, p=0.483. (J) Total amounts of NREM sleep, REM sleep and wakefulness during the 4 hr post-injection period (7 p.m.–11 p.m.) following vehicle or CNO injections in the control and lesion groups. Vehicle v.s. CNO, control: n = 8, paired t test, NREM: **p=5.778E-5; REM: p=0.77; Wake: p=1.21E-5. Lesion: n = 6, paired t test, NREM: p=0.171; REM: p=0.078; Wake: p=0.413. Control vehicle v.s. lesion vehicle, independent-samples Student’s t test, NREM: ## p=0.001; REM: p=0.110; ##Wake: p=3.187E-4. See Figure 5—source data 1.
-
Figure 5—source data 1
Sample size (n), mean and SEM are presented for the data in Figure 5.
- https://doi.org/10.7554/eLife.29055.023
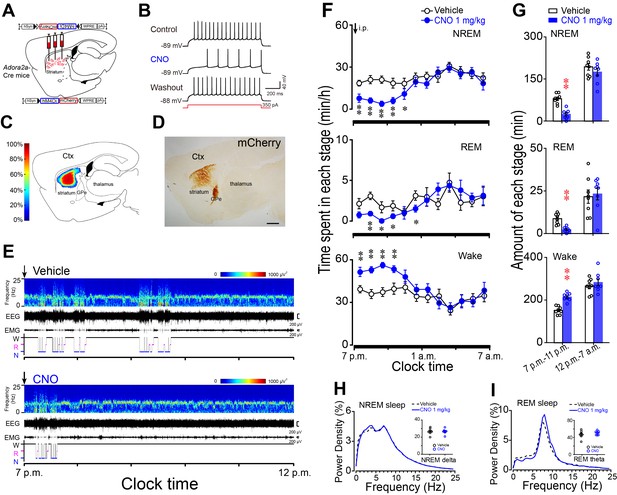
Chemogenetic inhibition of striatal A2AR neurons reduced NREM sleep during active period.
(A) Schematic of virus injection into the striatum of Adora2a-Cre mice and the expression of hM4Di receptors in A2AR neurons. (B) Bath applied CNO (5 μM) reduced firing rate in response to 350 pA current injection in hM4Di-expressing A2AR neurons of brain slices. (C, D) Heat map (C) shows the virus-injected areas in the dorsal striatum, and immunostaining (D) represents hM4Di-expressing neurons (mCherry+) in striatum. Scale bar, 1 mm. Ctx, cortex; GPe, external globus pallidus. (E) Typical examples of compressed spectral array (0–25 Hz) EEG, EMG, and hypnograms over 5 hr following administration (i.p.) of vehicle (top panel) or CNO (bottom panel) in a mouse with bilateral hM4Di expression in striatal A2AR neurons. (F) Time course of changes in NREM sleep, REM sleep, and wakefulness following vehicle (open circle) and CNO (closed blue circle) injections in Adora2a-Cre mice. n = 8, two-way repeated measures ANOVA, and paired t test, NREM: F1,14 = 19.350, p=6.062E-4, **p=0.003, **p=0.001, **p=2.208E-4, **p=0.003, *p=0.042; REM: F1,14 = 15.221, p=0.002, **p=0.006, *p=0.045, *p=0.015; *p=0.036; Wake: F1,14 = 20.302, p=4.937E-4, **p=0.004, **p=0.001, **p=4.055E-4, **p=0.003. (G) Total amounts of NREM sleep, REM sleep and wakefulness during the 4 hr post-injection period (7 p.m.–11 p.m.) and the following 8 hr of the active period (11 p.m.–7 a.m.) following vehicle or CNO injections in Adora2a-Cre mice expressing hM4Di receptors in the striatum. n = 8, paired t test, NREM: **p=2.607E-6; REM: **p=1.653E-4; Wake: **p=1.597E-6. (H and I) Relative average EEG power density of NREM sleep (H) and REM sleep (I) during the 4 hr period and quantitative changes in power for delta (0.5–4.0 Hz) frequency bands during NREM sleep (H) and theta (6–10 Hz) frequency bands (insert) during REM sleep (I) after CNO or vehicle injections in Adora2a-Cre mice with hM4Di-expresssing neurons in the striatum. See Figure 6—source data 1.
-
Figure 6—source data 1
Sample size (n), mean and SEM are presented for the data in Figure 6.
- https://doi.org/10.7554/eLife.29055.027

Chemogenetic inhibition of A2AR neurons of the striatum did not alter sleep-wake profiles during inactive period.
(A) Time course of changes in NREM sleep, REM sleep, and wakefulness following vehicle (open black circle) and CNO (closed blue circle, 1 mg/kg) injections in Adora2a-Cre mice. n = 8, two-way repeated measures ANOVA, NREM: F1,14 = 0.066, p=0.8; REM: F1,14 = 0.114, p=0.741; Wake: F1,14 = 0.029, p=0.867. (B) Total amounts of NREM sleep, REM sleep and wakefulness during the 4 hr post-injection period (9 a.m.–1 p.m.) and the remainder (6 hr) of the inactive period (1 p.m. –7 p.m.). n = 8, paired t test, NREM: p=0.481, p=0.471; REM: p=0.606, p=0.838; Wake: p=0.612, p=0.472. (C) Relative average EEG power density of NREM sleep, REM sleep and wakefulness and quantitative changes in power for the delta (0.5–4.0 Hz) frequency bands during NREM sleep, theta (6–10 Hz) frequency bands during REM sleep, and alpha (12–14 Hz) and beta (15–25 Hz) frequency bands (insert) during wakefulness during the first 4 hr period following CNO or vehicle injections. n = 8, paired t test, NREM delta: p=0.214; REM theta: p=0.104; wake alpha: p=0.481; wake beta: p=0.159. See Figure 6—figure supplement 1—source data 1.
-
Figure 6—figure supplement 1—source data 1
Sample size (n), mean and SEM are presented for the data in Figure 6—figure supplement 1.
- https://doi.org/10.7554/eLife.29055.026
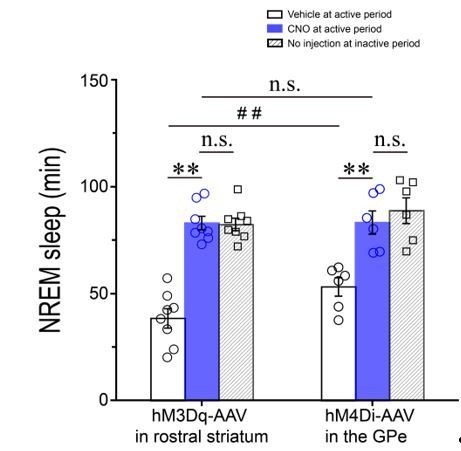
Total amounts of NREM sleep during the 3–h post–injection period (7 p.m.–10 p.m.) following vehicle (open columns) or CNO (blue columns) injections and during the first 3–h (7 a.m.–10 a.m.) after light–on (diagonal columns) in Adora2a–Cre mice expressing hM3Dq receptors in the rostral striatum and Pvalb–Cre mice expressing hM4Di receptors in the GPe. **P < 0.01; #P < 0.05; n.s.: no significance, P > 0.05.
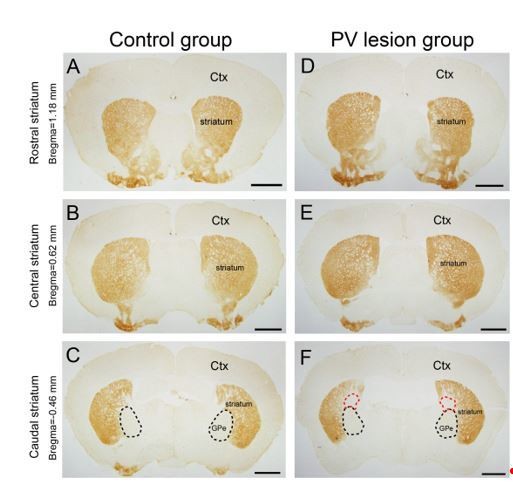
Micrographs of A2AR immunostaining in the rostral.
(A, D), central (B, E), and caudal (C, F) striatum in the control group (A–C) injected eGFP–AAV and lesion group (D–F) injected taCasp3–AAV into the GPe of Adora2a/Pvalb–Cre mice. The black dotted line showed the boundary of the GPe, and the red dotted line showing the region of striatum infected by the taCasp3–AAV. Scale bar, 1 mm. Ctx, cortex; GPe, external globus pallidus.
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.29055.028





