Fundamental constraints in synchronous muscle limit superfast motor control in vertebrates
Figures
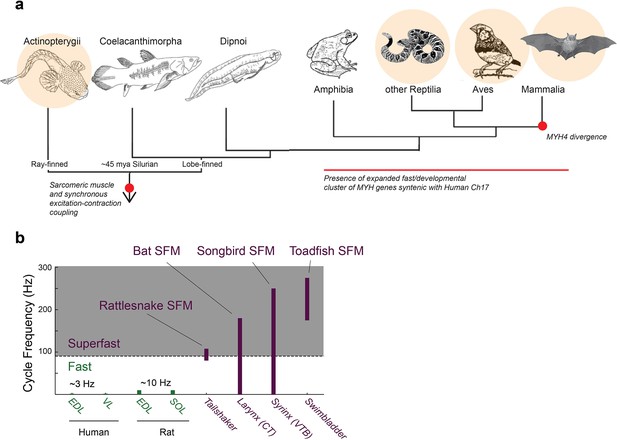
Superfast muscles are present in multiple vertebrate lineages.
(a) Distribution of identified superfast muscles (SFMs, beige disks) in vertebrates relative to key evolutionary events: excitation-contraction coupling, and myosin heavy chain (MYH) gene evolution. Modified after (Bass, 2014). (b) Ranges of in vivo cycling frequency. SFM (labeled purple) has been defined as synchronous muscle capable of producing work at cycling frequencies in excess of 90 Hz (grey box) (Rome, 2006). Bat, songbird, and toadfish SFM approach or exceed 200 Hz. CT, cricothyroid; VTB, m. tracheobronchialis ventralis; EDL, m. extensor digitorum longus; SOL, soleus; VL, vastus lateralis.
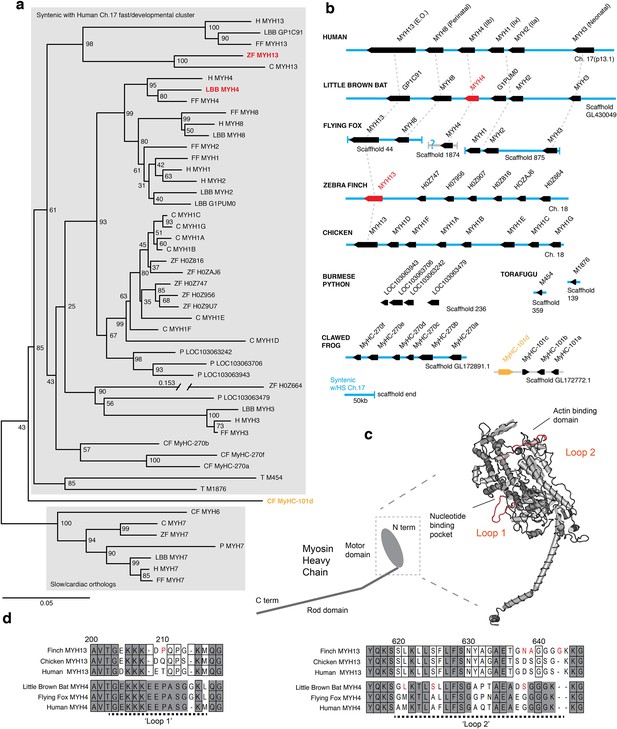
Mammals and avians possess orthologous clusters of myosin heavy chain genes.
(a) Neighbor-joining tree of chicken (C), clawed frog (CF), flying fox (FF), human (H), little brown bat (LBB), Burmese python (P), torafugu (T), and zebra finch (ZF) predicted myosin heavy chain rod domain amino acid sequence from genomic regions syntenic with the human fast/developmental cluster on chromosome 17. Also included are representative cardiac genes, as well as a fast laryngeal myosin gene from the clawed frog (yellow). MYH expressed in bat and finch SFM indicated in red. Human non-muscle MYH9 was used to root the tree (not shown). Branch lengths shown are derived from a maximum likelihood analysis of aligned amino acid sequence. Bootstrap values from a 1000-replicate analysis are given at nodes in percentages. (b) Relative local positions of predicted MYH genes in genomes of the above species. Synteny with human Ch17 is shown in blue. Red and yellow color-coding as in (a). Orthologs indicated by vertical dotted lines. (c) Homology model of human MYH3 myosin motor domain indicating position of loop subdomains and the nucleotide binding pocket. (d) Hypervariable surface loops of MYH13 and MYH4 motor-domains that likely influence actomyosin crossbridge kinetics. Grey shading indicates conservation with/among MYH4 orthologs. Outlines indicate conservation with/among MYH13 orthologs. Substitutions and insertions unique to SFM species in red. Horizontal black dashed lines indicate ‘Loop 1’ and ‘Loop 2’ subdomains.
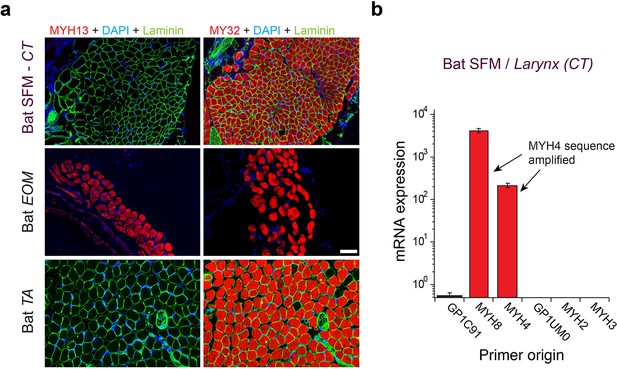
Mammalian superfast muscles are enriched with MYH4 ortholog.
(a) Immunohistochemistry labeling shows absence of MYH13 (left column) in Daubenton’s bat larynx SFM (top) with positive control in extraocular muscle (EOM) (middle) and negative control in body skeletal muscle (m. tibialis anterior; TA) (bottom). Fast twitch MYHs (right column) are present in SFM, EOM and TA muscles. (b) qPCR analysis identifies MYH4 (MyHC-2b) to be dominating in bat SFM. Both MYH4 and MYH8 primer pairs amplified MYH4 mRNA as confirmed by cDNA sequencing.
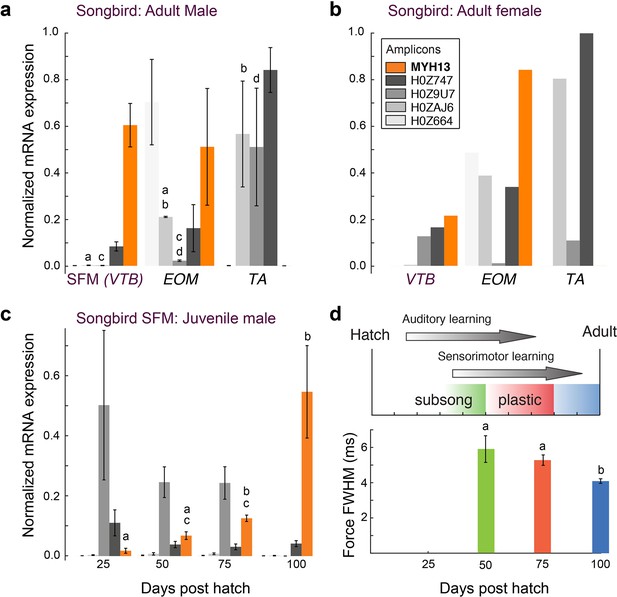
Avian superfast muscles are enriched for the myosin MYH13 ortholog.
(a) qPCR analysis of syringeal SFM of adult male (N = 3) and (b) female (N = 3, pooled) zebra finch show high enrichment for the MYH13 ortholog in singing males. Legend for color code of bars in b. Unshared lowercase letters a-d indicate post-hoc differences with significance level p<0.05. Fast myosin (MY-32) immunohistochemistry results are shown in Figure 4—figure supplement 1. (c) During the sensorimotor phase of vocal imitation learning in male zebra finches MYH13 is significantly upregulated (N = 3 for each timepoint). Legend for color code of bars in b. Unshared lowercase letters a-c indicate differences with significance level p<0.05. (d) Syrinx SFM significantly increases their speed during song development (N = 4 for each timepoint). FWHM; full width at half maximum.
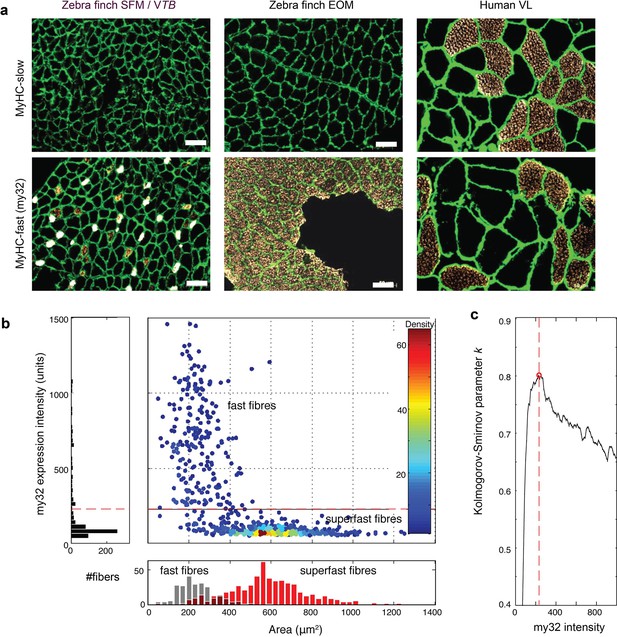
Zebra finch SFM contains two fiber type populations.
(a) Labeling of slow and fast (MY-32) myosin antibodies in adult male zebra finch SFM shows that it contains some myofibrils that express or co-express fast MYH genes, but predominantly fibers that do not react. The latter fibers express the avian MYH13 ortholog. (b) FACS analysis of 801 cells of 4 individuals showing the two fiber populations separate c) best at intensity of 220 as shown by a Kolmogorov-Smirnov test.
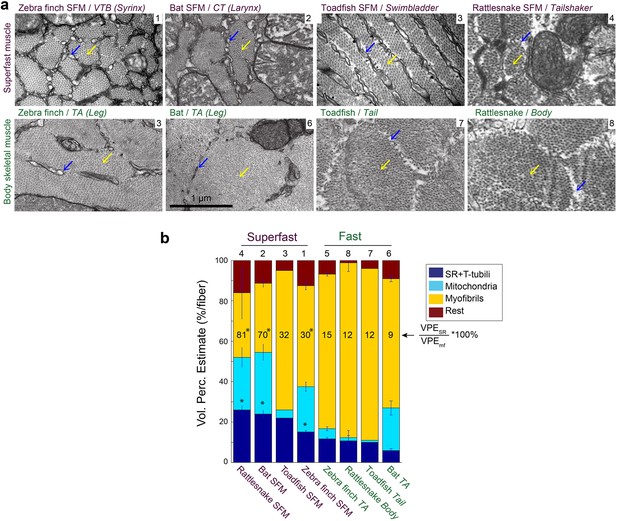
Vertebrate superfast muscles share morphometric cellular adaptations.
(a) Representative transmission electron microscopy images of zebra finch, Daubenton’s bat, Oyster toadfish and Diamondback rattlesnake SFMs (upper row) and intraspecific skeletal muscles (lower row). Blue arrows indicate SR, yellow arrows myofibrils. Rattlesnake muscle images adapted from Schaeffer et al., 1996. (b) Volumetric percentage estimates (VPE) of myofiber composition (see Materials and methods) show that all SFM have VPESR ≥15%, and SR per myofibril ratio ≥30%. Both VPESR and SR per myofibril ratio are significantly higher compared to fast intraspecific skeletal fibers for zebra finch, bat and rattlesnake (*, p<0.05). Data presented in descending order of SR per myofibril ratio. The numbers 1–8 above the bars refer to images in panel (a).
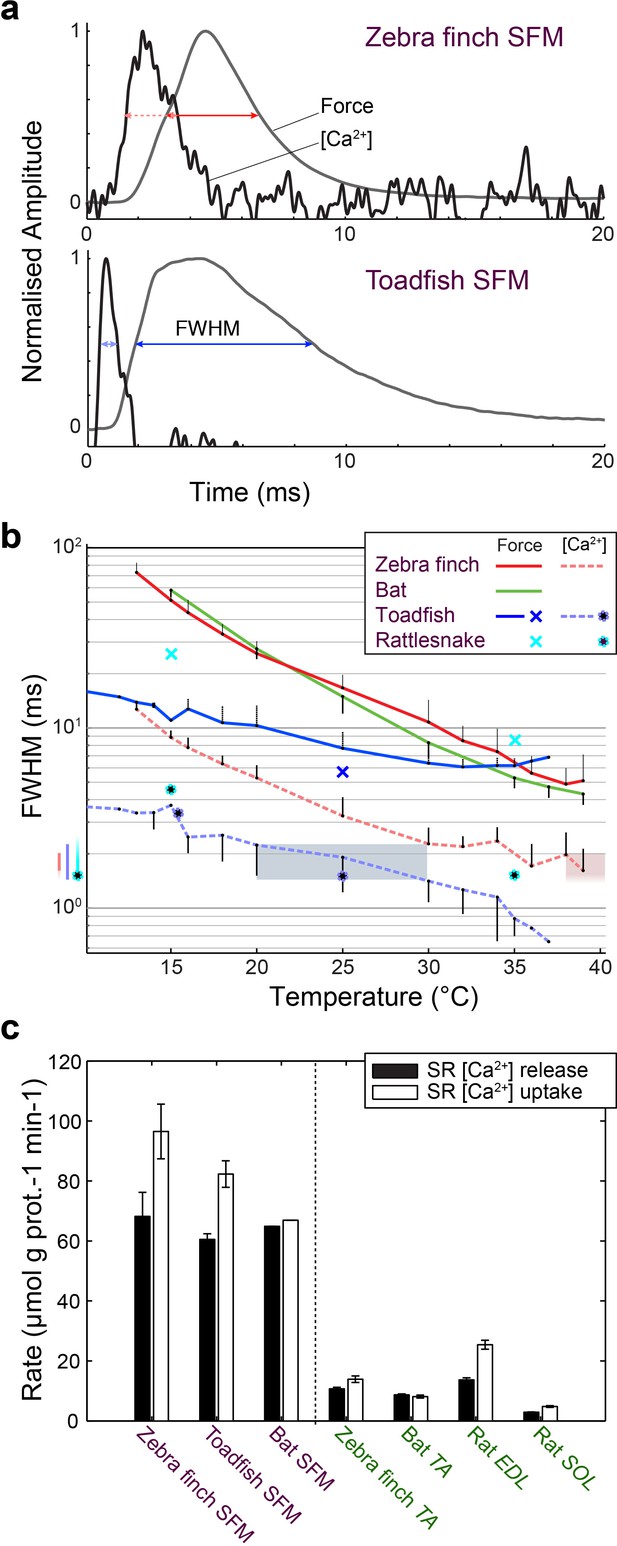
Superfast muscles have convergent elevated myoplasmic calcium transient dynamics.
(a) Representative force development and myoplasmic calcium concentration ([Ca2+]i) transients in songbird and toadfish SFMs. (b) Force and [Ca2+]i transient full-width-at-half-maximum (FWHM) values as a function of temperature. [Ca2+]i FWHM values converge to 1.5–2.0 ms for all SFMs (color left of ordinate) at their operating temperatures (shaded areas). Filled circle and crosses refer to toadfish and rattlesnake data from ref 6. (c) Calcium loading and unloading kinetics in isolated SR vesicles (see Materials and methods) yield similar values for bat SFMs compared to songbird and toadfish SFMs, and were significantly elevated (p<0.01, ANOVA) 5–10 times compared to intraspecific skeletal muscle in zebra finch and bat.
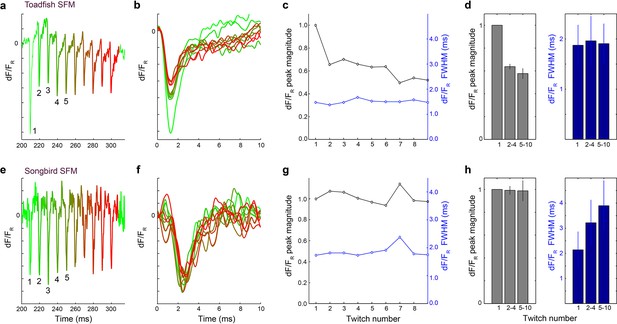
Calcium transient speed is stable over multiple stimulations.
(a) Representative traces of changes in fluorescence (dF) scaled to resting fluorescence (FR) during multiple stimulations in toadfish SFMs. The dF/FR signal inversely relates to the myoplasmic calcium concentration ([Ca2+]i). The first transient clearly shows an increased peak magnitude compared to the consecutive transients. Color coding from green (twitch 1) to red (twitch 10). (b) dF/FR transients overlaid for the 10 twitches and (c) corresponding dF/FR peak magnitude and FWHM as a function of twitch number. (d) In toadfish SFMs, dF/FR peak magnitude is higher for the first twitch compared to the consecutive ones confirming earlier observations (Harwood et al., 2011), but dF/FR FWHM does not change as a function of twitch number. (e–g) Results for zebra finch SFM. (h) Both dF/FR peak magnitude and FWHM do not significantly change as a function of twitch number (KW, p>0.05). The trend to longer duration FWHM with twitch number may be explained by measurement inaccuracy due to low signal to noise ratio.
Additional files
-
Supplementary file 1
- https://doi.org/10.7554/eLife.29425.011
-
Transparent reporting form
- https://doi.org/10.7554/eLife.29425.012





