An FGF-driven feed-forward circuit patterns the cardiopharyngeal mesoderm in space and time
Figures
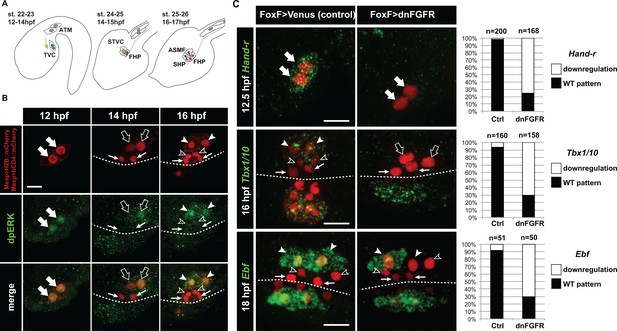
Spatio-temporal restriction of ERK activity reflects FGF requirement for the specification of cardiopharyngeal progenitors.
(A) Schematic of Ciona development showing asymmetric cell divisions and resulting cell fates of the cardiopharyngeal mesoderm (CPM). Embryonic and larval stages (St) according to (Hotta et al., 2007) with hours post fertilization (hpf) at 18°C. Anterior tail muscle (ATM, gray), trunk ventral cell (TVC, green), secondary TVC (STVC, green), first heart precursor (FHP, red), second heart precursor (SHP, orange), atrial siphon founder cell (ASMF, blue). Black bars link sister cells. Dashed lines: ventral midline. The first stage presents a quasi-lateral view while the second and third stages present quasi-ventral views. Anterior is to the left. Scale bar, 50 µm. (B) ERK activity visualized by anti-dpERK antibody (green). TVCs and their progeny are marked by mCherry driven by Mesp and revealed by anti-mCherry antibody (red). H2B::mCherry and hCD4::mCherry accumulate in the nuclei and at the cell membrane, respectively. Arrowheads indicate STVCs and ASMFs at 14 and 16 hpf, respectively. Arrows indicate FHPs and open arrowheads mark SHPs. Anterior to the left. Scale bar, 10 µm. See also Figure 1—figure supplement 1 for broader time series of dpERK immunostaining in the B7.5 lineage. (C, D) TVC-specific overexpression of dnFGFR induces loss of expression of key lateral CPM markers visualized by in situ hybridization. (C) Representative expression patterns of key CPM genes (Hand-related, Tbx1/10, Ebf) in control embryos (Ctrl, electroporated with Foxf(TVC):bpFOG-1>Venus) and TVC-specific dnFGFR expression (electroporated with Foxf(TVC):bpFOG-1>dnFGFR::mCherry) individuals. TVCs and progeny are marked with Mesp > NLS::lacZ (red). Loss of expression in half of the TVC progeny, as presented for Ebf, is assumed to be due to left-right mosaicism. Arrowheads mark the ASMFs. Anterior is to the left. Scale bar, 10 µm. (D) Corresponding histograms with the phenotype proportions. For simplicity, loss of gene expression in half or all of the TVCs and their progeny were combined in the same category. ‘n’ corresponds to the number of individual halves documented per condition.
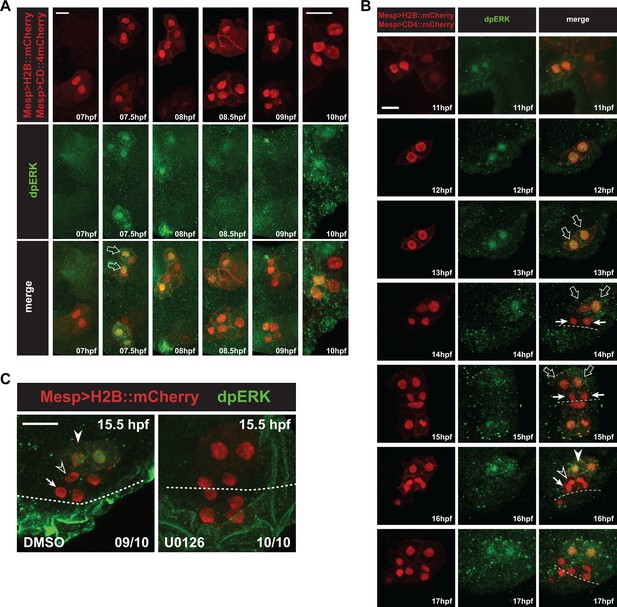
Detailed patterns of MAPK activity during early cardiopharyngeal development.
(A) MAPK activation during TVC induction. Close-up views of B7.5 lineage cells marked with Mesp > H2B::mCherry (nuclei) and Mesp > hCD4::mCherry (membranes) and immunostained for dpERK at indicated successive time points between 7 and 10hpf. DpERK staining was not detected in the founder cells at 7hpf, but increased sharply and specifically in the smaller trunk ventral cells (TVCs, open arrows) at 7.5hpf, but not in the larger anterior tail muscles (ATMs). DpERK staining persisted throughout TVC migration (see also B). (B) MAPK activation patterns during cardiopharyngeal fate diversification. DpERK staining was clearly detected in migrating TVCs (open arrows, 11 to 13hpf); in lateral large STVCs (open arrows, 14 to 15hpf), but not in the small median first heart precursors (FHPs, arrows, 14 to 15hpf); in the large lateral atrial siphon muscle founder cells (ASMFs, solid arrowheads, 16 to 17hpf), but neither in the FHPs (arrows), nor in the second heart precursors (SHPs, open arrowheads). (C) Treatment with the MEK inhibitor U0126 from 12 to 15.5hpf abolished dpERK staining in the lateral STVCs, compared to a control treatment with DMSO. Numbers of embryos showing the presented pattern out of the total numbers of embryos are shown.
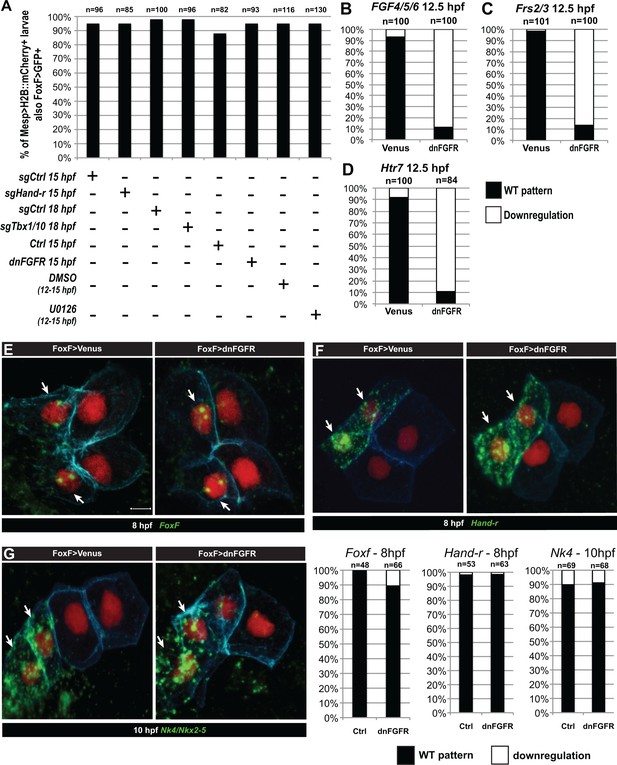
Late TVC-specific inhibition of FGF-MAPK signaling does not alter early TVC induction .
(A) Effects of perturbations on Foxf TVC-specific enhancer activity. Proportions of Mesp >H2B:mCherry-positive embryos showing Foxf::bpFog-1>NLS:GFP activity (i.e. GFP+) in the indicated conditions: TVC-specific CRISPR/Cas9 mediated loss-of-function of Hand-r (sgHand-r), and corresponding control (Neurogenin/sgCtrl) at 15 hpf; TVC-specific CRISPR/Cas9 mediated loss-of-function of Tbx1/10 (sgTbx1/10) and corresponding control (Neurogenin/sgCtrl) at 18 hpf; TVC-targeted dnFGFR embryos (FoxF::bpFog-1>dnFGFR) and corresponding control (FoxF::bpFog-1>NLS::LacZ) at 15 hpf; Inhibition of MAPK activity with 4 hr incubations in U0126 (DMSO as vehicle control) at indicated times. TVCS and their progeny marked with Mesp >H2B::mCherry and possible effects on Foxf enhancer activity of these perturbations have been verified with TVC-specific green staining. There were no significant difference in the proportions of GFP +embryos between each perturbations and controls. (B–D) Other markers expressed in the TVC need continuous FGF-MAPK inputs for maintenance. All panels show the proportions of 12.5hpf embryos halves showing expression of the indicated genes in late TVCs, following electroporation of either a Foxf(TVC)>Venus control of a Foxf(TVC)>dnFGFR construct that inhibits signaling through FGFR. Wild-type pattern were first reported in (Razy-Krajka et al., 2014). (E–G) Foxf>dnFGFR does not inhibit TVC induction. TVC-specific expression of dnFGFR (FoxF::bpFog-1>dnFGFR) do not show difference in TVC markers expression in comparison to control (FoxF::bpFog-1>Venus) in early TVC (Foxf and Hand-r at 8 hpf, Nkx2-5 at 10 hpf). Arrows indicate anterior TVCs, adjacent cells on the right are ATMs, which do not express TVC markers.
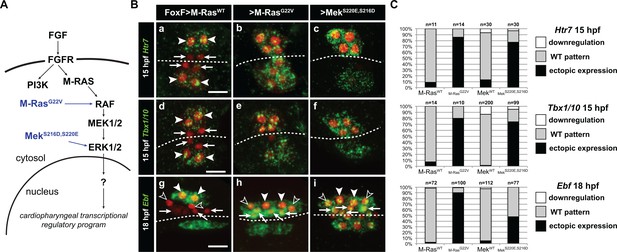
Constitutively active M-Ras and MEK are sufficient to impose a pharyngeal muscle fate in the cardiopharyngeal lineage.
(A) Diagram of the FGF-MAPK transduction pathway with constitutive activation by M-RasG22V and MEKS216D,S220E mutants. (B) Expression patterns of markers of the lateral TVC progeny, Htr7 (a, b, c,), Tbx1/10 (d, e, f) and Ebf (g, h, i), visualized by in situ hybridization following TVC-specific over-expression of M-RasWT (as control), M-RasG22V and MEKS216D,S220E. M-RasWT overexpression (a, d, g) does not alter the wild-type spatial expression patterns of Htr7, Tbx1/10 and Ebf in lateral TVC derivatives (STVC and ASMF) and excluded from the median heart precursors. TVC-specific over-expression of M-RasG22V (b, e, h) or MEKS216D,S220E (c, f, i) induces ectopic expression of STVC and/or ASMF markers (Htr7, Tbx1/10 and Ebf) in the more median cells, that normally form cardiac precursors. Arrowheads indicate STVCs and ASMFs at 15 and 18 hpf, respectively. Arrows indicate FHPs and open arrowheads mark SHPs. At 18 hpf, the FHPs start dividing or have divided into 4 cells. Anterior to the left. Scale bar, 10 µm. (C) Corresponding histograms: Larvae with TVC-specific over-expression of MEKWT retain the wild-type expression patterns. For simplicity, ectopic expressions in half to all of the cardiac precursors were combined in the same phenotype category. ‘n’ corresponds to the number of embryo halves documented per condition. See also Figure 1—figure supplement 2.
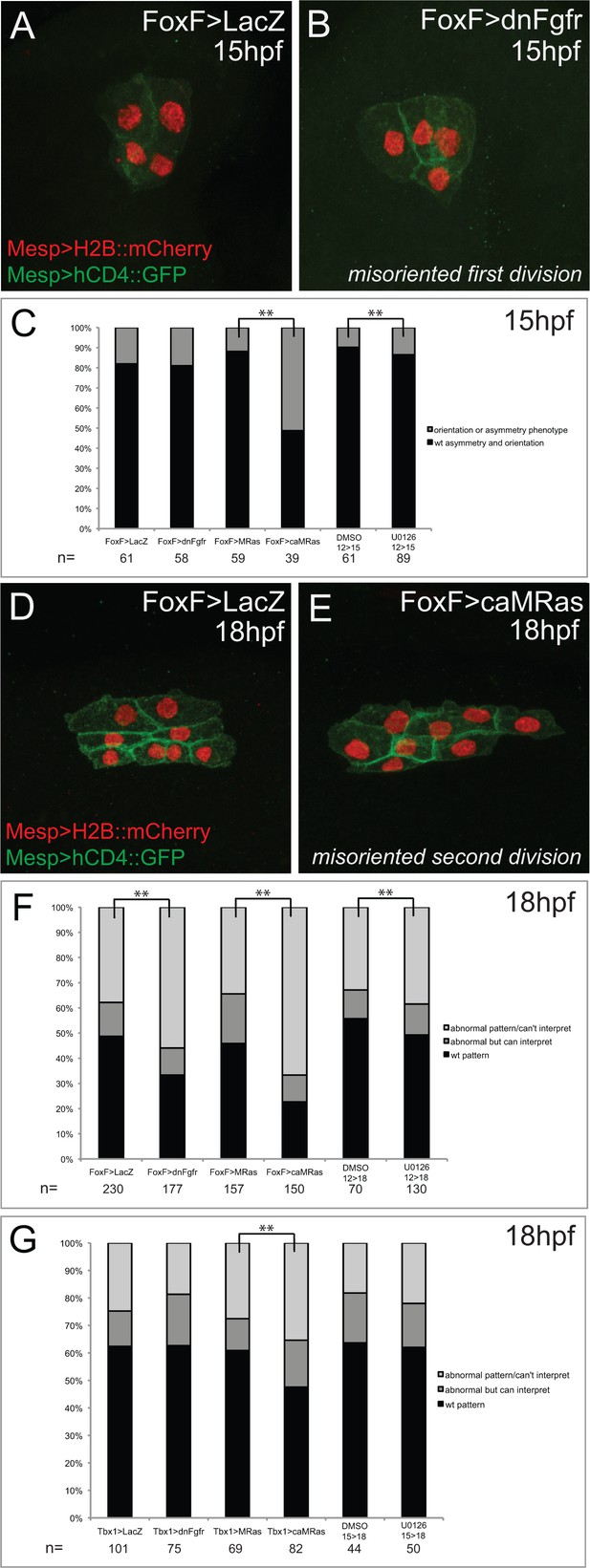
Effects of defined FGF-MAPK signaling perturbations on cell division patterns in the cardiopharyngeal lineage.
(A) Representative phenotype in unperturbed conditions (control plasmid: Foxf>LacZ) at 15hpf. TVCs divide asymmetrically and in a medio-lateral orientation. (B) Representative misoriented TVC division phenotype (Foxf>dnFGFR). One TVC divided improperly in the antero-posterior axis. (C) Proportions of embryo halves showing wild-type vs. orientation or symmetry phenotype, scored at 15hpf. For pharmacological treatments, embryos were incubated in DMSO or U0126 from 12hpf-15hpf. Proportions differ significantly between Foxf>MRas and Foxf>caMRas, and between DMSO and U0126 treatment conditions (** indicates p-value<0.01, Chi2 test). (D) Representative phenotype in unperturbed conditions (FoxF>LacZ) at 18hpf. STVCs divide in a medio-lateral orientation. (E) Representative misoriented STVC division phenotype (FoxF>caMRas), scored as ‘abnormal but can interpret.’ STVCs likely divided improperly in the antero-posterior axis. (F) Proportions of larva halves in Foxf(TVC)-driven perturbations showing wild-type pattern vs. orientation and/or symmetry phenotypes, patterns scored at 18hpf as abnormal but interpretable or abnormal/can’t interpret. For pharmacological treatments, larvae were incubated in DMSO or U0126 from 12hpf-18hpf. Proportions differ significantly in all conditions in comparison to the corresponding control (** indicates p-value<0.01, Chi2 test). (G) Proportions of larva halves in Tbx1-driven perturbations showing wild-type pattern vs. orientation and/or symmetry phenotype, patterns scored at 18hpf as abnormal but interpretable or abnormal/can’t interpret. For pharmacological treatments, larvae were incubated in DMSO or U0126 from 15hpf-18hpf. Proportions differ significantly only between Tbx1 >MRas and Tbx1 >caMRas conditions (** indicates p-value<0.01, Chi2 test).
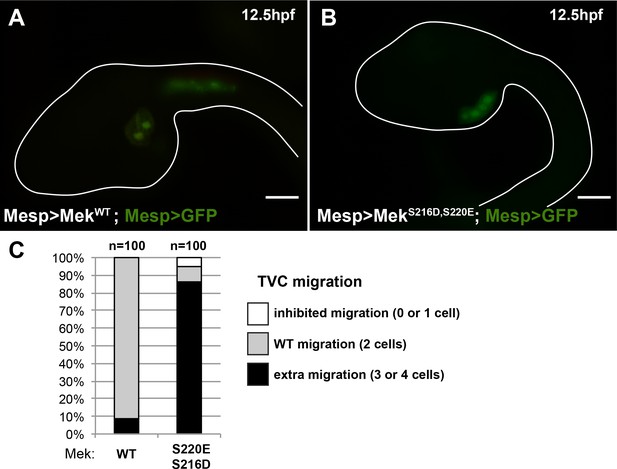
The constitutively active MEKS216D,S220E mutant is sufficient to impose a TVC identity to the whole B7.5 lineage.
(A) Control late tailbud embryo showing the left side B7.5 lineage expressing GFP and a MEKWT control under the control of the Mesp enhancer. Two TVCs and two ATMs are normally induced, and TVCs migrated into the trunk. (B) Late tailbud embryo showing the left side B7.5 lineage expressing GFP and a MEKS216D,S220E mutant under the control of the Mesp enhancer. Four cells are observed as having migrated into the trunk, indicating that they have been induced to acquire a TVC fate and migrate, replicating FGF-MAPK gain-of-function phenotypes as described in (Davidson et al., 2006). (C) Proportions of embryo halves showing the indicated phenotypes. Extra migration is interpreted as ectopic induction of the TVC fate in all B7.5 lineage cells. Scale bar ~ 20 µm.
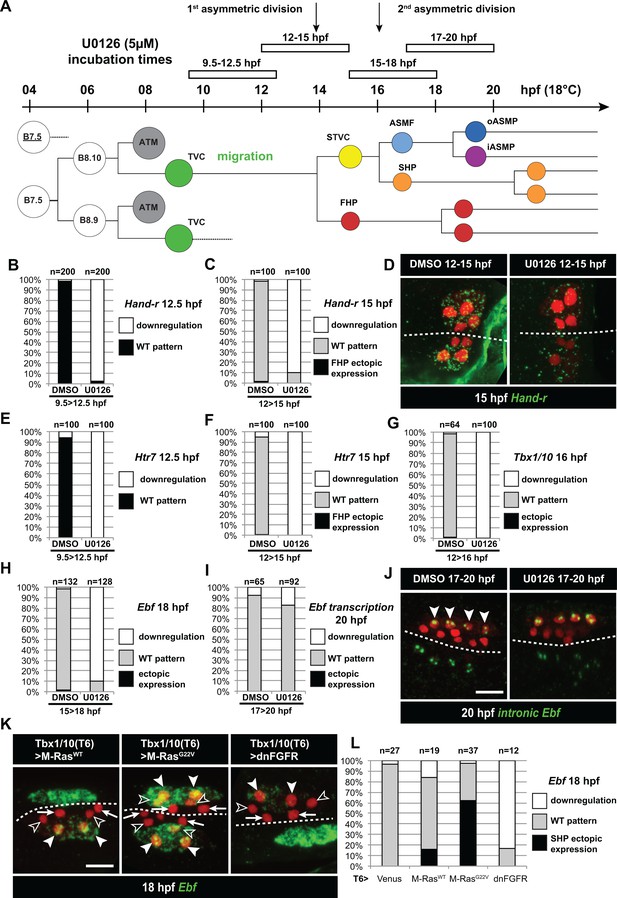
Temporal requirement for MAPK activity permits the progressive deployment of the cardiopharyngeal regulatory program.
(A) Summary of the CPM cell lineage showing the different U0126 treatments with regard to the timing of cell divisions. Abbreviations and color codes as in Figure 1. (B, C) Proportions of embryo halves with wild-type or downregulated expression of Hand-r at 12.5 hpf (B) and 15 hpf (C) following 3 hr incubations in U0126 (with DMSO as control treatment). (D) Hand-r expression visualized by in situ hybridization at 15 hpf in control (DMSO treated) and U0126 treated embryos. In control embryos, Hand-r remains expressed in the STVCs and downregulated in the FHPs. In U0126 (12–15 hpf) treated embryos, downregulation of Hand-r expression is observed throughout the TVC progeny (STVCs and FHPs), suggesting inhibition of transcription and inheritance of remnant transcripts following TVC divisions. (E, F) Proportions of embryo halves with wild-type or downregulated expression of Htr7 at 12.5 hpf (E) and 15 hpf (F) following 3 hr incubations in U0126 (with DMSO as control treatment). (G) Proportions of larvae with wild-type expression or downregulated expression of Tbx1/10 at 16 hpf following 4 hr incubation in U0126 (with DMSO as control). (H) Proportions of larvae with wild-type or downregulated expression of Ebf at 18 hpf following a three hour incubation in U0126 (with DMSO as control). (I) Proportions of larvae with wild-type or downregulated transcription of Ebf at 18 hpf following a three hour incubation in U0126 (DMSO as vehicle control). (J) Pattern of nascent Ebf transcripts visualized by in situ hybridization with intronic probes (green) at 20 hpf. The nuclear dots reveal the active transcription sites in the four ASMPs per side in larvae, both control/DMSO- and U0126-treated from 17 to 20 hpf. (K) Ebf expression (green) in 18hpf larvae expressing control M-RasWT, constitutively active M-RasG22V or dominant negative dnFGFR under the control of the T12 element, an STVC-specific Tbx1/10 enhancer. Arrows: first heart precursors (FHP); open arrowhead: second heart precursors (SHPs); closed arrowheads: ASM founder cells (ASMFs); dotted line: midline. (L) Proportions of larvae with wild-type or downregulated expression of Ebf at 18 hpf in larvae with Venus (control), M-RasWT, M-RasG22, or dnFGFR driven by Tbx1/10 cis-regulatory sequence and overexpressed in the STVCs. ‘n’: number of individual halves documented per condition.
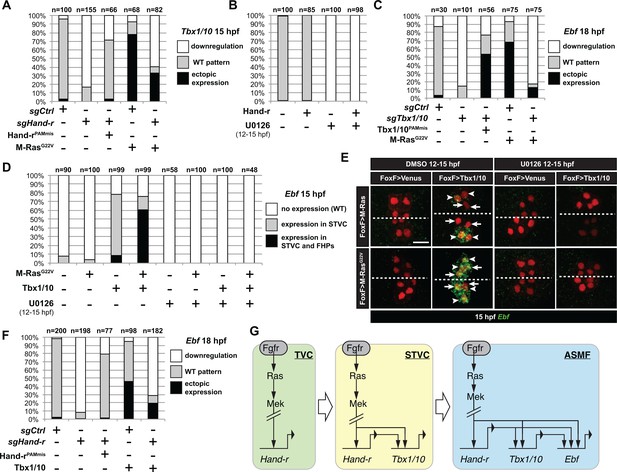
M-Ras/MAPK-driven feed-forward subcircuits control the successive activations of Hand-r, Tbx1/10 and Ebf.
(A) Proportions of embryo halves with indicated Tbx1/10 expression patterns following TVC-specific CRISPR/Cas9-mediated mutagenesis of Neurogenin/Neurog as a control (sgCtrl), and Hand-r (sgHand-r). TVC-specific overexpression of a CRISPR/Cas9-resistant form of Hand-r with mutation in the PAM sequence (Hand-rPAMmis) rescued Tbx1/10 expression in the sgHand-r ‘background’. TVC-specific overexpression of a constitutively active M-Ras mutant (M-RasG22) (control: M-RasWT) was sufficient to induce ectopic expression of Tbx1/10 in the FHPs in sgCtrl embryos but not in sgHand-r embryos indicating that Hand-r is necessary for M-Ras-dependent activation of Tbx1/10 transcription. (B) Proportions of embryo halves with indicated Tbx1/10 expression patterns following TVC-specific overexpression of Hand-r or a neutral reporter (Venus) and treated from 12 to 15hpf with the MEK inhibitor U0126 (+) or with DMSO (-) as control. Hand-r overexpression is not sufficient to rescue loss of Tbx1/10 expression due to MAPK inhibition indicating that M-Ras/MAPK activity is required in parallel of Hand-r expression to activate Tbx1/10 transcription in the TVC progeny. (C) Tbx1/10 is necessary downstream of M-Ras/MAPK activity to activate Ebf transcription in the TVC progeny. Shown are proportions of Ebf expression phenotypes following TVC-specific CRISPR/Cas9-mediated loss of Tbx1/10 function (sgTbx1/10), with Neurog-targeting sgRNA as control (sgCtrl). Specificity of Tbx1/10 loss of function was validated through rescue of Ebf expression with TVC-specific overexpression of a CRISPR/Cas9 resistant form of Tbx1/10 (Tbx1/10PAMmis). Ectopic Ebf expression in SHPs in Tbx1/10PAMmis larvae is explained by precocious misexpression of Tbx1/10 in the TVC as described in Wang et al. (2013). TVC-specific overexpression of M-RasG22 (M-RasG22), with wild type M-Ras (M-RasWT) as control, was sufficient to induce ectopic expression of Ebf in the cardiac precursors in sgCtrl embryos but not in sgTbx1/10 embryos indicating that Tbx1/10 is necessary for M-Ras-dependent activation of Ebf transcription. (D, E) Proportions (D) and examples (E) of 15hpf larvae halves showing indicated Ebf expression phenotypes in sgCtrl and sgHand-r CRISPR/Cas9 conditions combined with TVC-specific overexpression of a neutral reporter (Venus), Hand-rPAMmis, or Tbx1/10, and with MEK inhibition by U0126 (+) or not (DMSO control (-)). Arrowhead: STVCs, Arrows: FHPs, dotted line: ventral midline (F) Loss of Hand-r function impaired the ability of Tbx1/10 to induce ectopic Ebf expression. For simplicity, ectopic expressions in half to all of the cardiac precursors were combined in the same phenotype category. ‘n=": number of individual halves documented per condition. (G) Summary model of the temporal deployment of FGF/MAPK-driven feed-forward sub-circuits leading to the sequential activations of Tbx1/10 and Ebf in the STVCs and ASMFs, respectively.
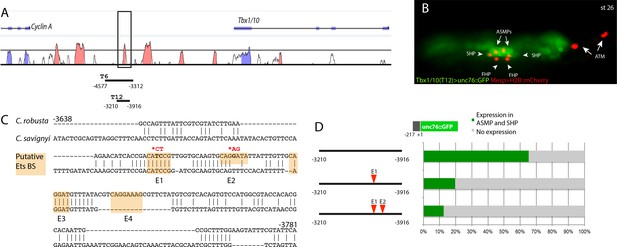
The Tbx1/10 enhancer has conserved putative Ets binding sites required for reporter gene expression.
(A) Alignment of Tbx1/10 locus between Ciona robusta/Ciona savignyi using VISTA. (B) Ciona larva (st 26) expressing GFP driven by the T12 element, a STVC-specific Tbx1/10 enhancer (green) and Mesp > H2B::mCherry to track the B7.5 lineage cell nuclei (red). The Tbx1/10 enhancer drives the unc76::GFP reporter in the STVC progeny, including ASM precursors (ASMPs) (white arrows) and the second heart precursors (SHPs) (white arrowheads). No expression is detected in the first heart precursors (FHPs) (white arrowheads) and anterior tail muscle (ATMs) (orange arrows). (C) Sequence alignment of Tbx1/10 enhancer between Ciona robusta/Ciona savignyi Conserved blocks in the orange boxes with putative Ets binding sites. (D) Proportion of larvae expressing both GFP and mCherry in the STVC progeny when co-electroporated wild-type and mutant Tbx1/10 reporters lacking the indicated putative Ets binding sites and Mesp > H2B::mCherry in comparison to the control (no enhancer, only Tbx1/10 basal promoter driving unc::76GFP). n: number of electroporated larval halves.
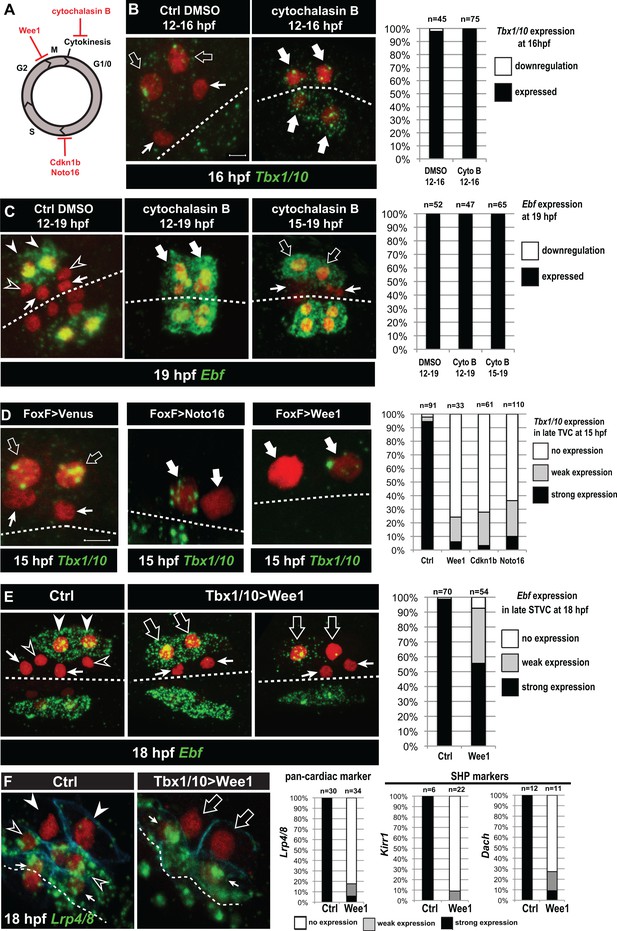
Temporal deployment of the cardiopharyngeal network is partially coupled with cell cycle progression.
(A) Schematic representation of the canonical eukaryotic cell cycle, and actions of the perturbations used in this study. (B,C) Tbx1/10 and Ebf expression at indicated time points, and following inhibition of cytokinesis by cytochalasin B treatment at indicated time points. Note that 15 to 19hpf treatment is applied AFTER the first division and birth the FHPs, which do not activate Ebf at 19hpf (right panel, arrows). (D) Inhibition of G1/S or G2/M blocks TVC division, and reduces Tbx1/10 expression. Pictures shows TVCs that have divided in controls but not in experimental cells, with one cell occasionnally turning on Tbx1/10, but not the other. Left: the proportions of embryos showing strong Tbx1/10 expression is substantially reduced compared to control embryos (e.g. Figure 1, and [Wang et al., 2013]). (E) Inhibition of G2/M in the STVCs by misexpression of Wee1 using the Tbx1/10 T6 enhancer inhibits STVC division, and has a mild impact on Ebf expression at 18hpf. Open arrows indicate STVCs that have not divided, but express high (middle) or low (right) levels of Ebf. Left: control larva showing high Ebf expression in the ASMF (closed arrowheads), but neither in the SHPs (open arrowheads) nor in the FHPs (Arrows). (F) Misexpression of Wee1 using the Tbx1/10 T6 enhancer (Tbx1 >Wee1) inhibits STVC division. Right: the proportions of embryos showing strong Lrp4/8, Kirr1 or Dach expression into late STVCs is reduced compared to SHPs in control embryos (T6 >NLS::LacZ) at 18 hpf. Notably, the pan-cardiac marker Lrp4/8 is still expressed in FHPs (arrows). Nuclei are marked in red with Mesp >NLS::lacZ, membranes in blue with Mesp >hCD4::mCherry, FHP labeled with arrows and SHP with arrowheads. 'n=', number of individual halves scored per condition. Scale bar, 5 µm. In all image panels, dotted line: ventral midline.
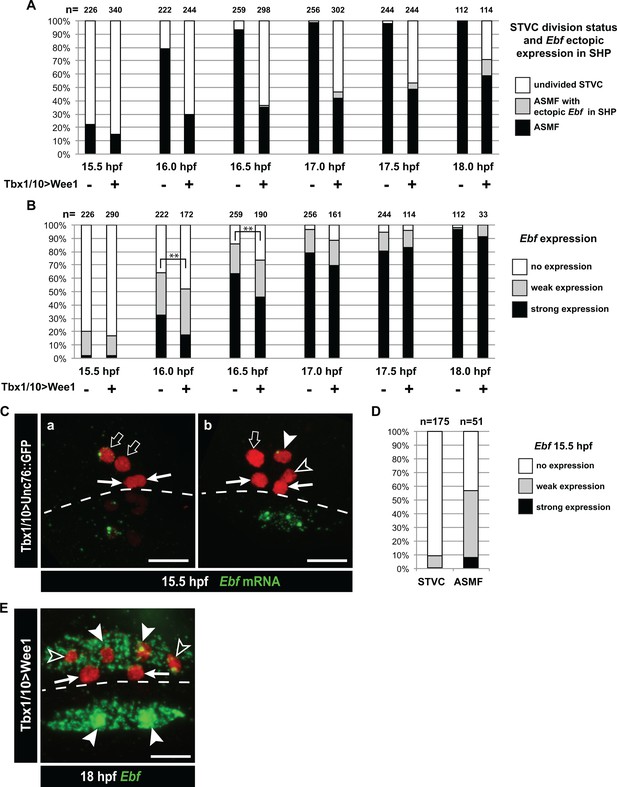
Dynamics of Ebf upregulation and entrainment by the cell cycle.
(A) Proportions of larva halves fixed at successive time points and showing undivided STVCs, or ASMFs with or without ectopic Ebf expression in the SHPs following STVC-specific expression of the G2/M inhibitor Wee1 (+), or a control construct (-). See Figure 5—figure supplement 1E for an example of ectopic Ebf expression in the SHPs (grey labels). Note the sharp increase in % of larva with ASMF nbetween 15.5 and 16hpf, indicating that mitosis occurs primarily during this time window, but is delayed in a majority of larvae upon Wee1 misexpression. (B) Proportions of larva halves with cells showing indicated Ebf expression. The numbers (n) for cells expressing Wee1 focus on cells that have not divided (% shown in E), to estimate the dynamics of Ebf activation in G2/M-inhibited cells. Control cells consist mostly ASMFs after 15.5hpf as shown in (E). Wee1 and controls distributions differ significantly only at 16 and 16.5hpf (**, p<0.01, Chi2 test), suggesting that Wee1 merely delays the accumulation of Ebf transcripts. (A) 15.5hpf Cardiopharyngeal lineage cells expressing Mesp > H2B::mCherry (red) and control Tbx1/10 > unc76::GFP construct (not visible). Rare precocious activation of Ebf transcription in STVCs. (C.a) Green nuclear dot indicates nascent Ebf transcription in an STVC (open arrow), but not the other, and not in the first heart precursors (FHP; arrow). (C.b) left pair of nuclei shows an STVC (open arrow) and an FHP (arrow), neither of which express Ebf, whereas the cousin ASMF (solid arrowhead) shows nascent Ebf transcription (green dot). Dotted line: midline. (D) Proportions of STVCs and ASMFs showing indicated Ebf expression patterns. Note that > 90% of STVCs do not express Ebf, which turns on almost exclusively in ASMFs. (E) Cardiopharyngeal lineage cells with Ebf expression in the ASMFs (solid arrowheads), and ectopically in the SHP (open arrowheads), but not in the FHPs (arrows), following misexpression of Wee1 using the STVC-specific Tbx1/10 T12 enhancer. Dotted line: midline.
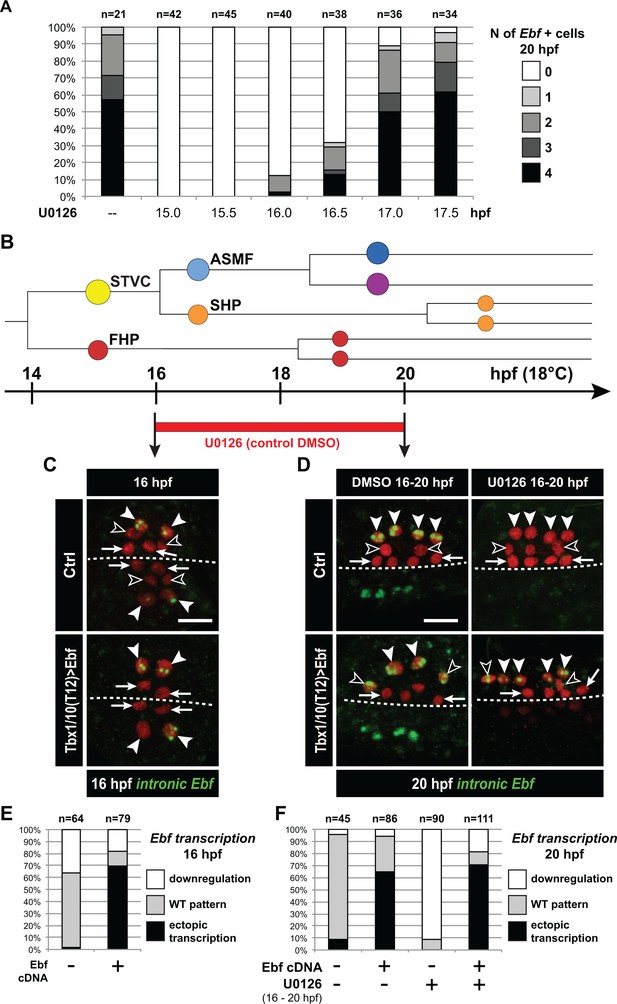
Ebf regulation transitions from MAPK-dependent to autoregulative during the early phase of ASMF cycle.
(A) Proportions of 20hpf larva halves showing the indicated number of Ebf-expressing cells following U0126 treatments started at the indicated time points. This indicates that, by 17hpf, Ebf expression, which started at ~16 hpf, has become largely insensitive to loss of MAPK activity. (B) Summary lineage diagram and time scale indicating the approximate stages for U0126 and DMSO (control) treatments for the results shown in (C, D). (C) Control (Ctrl) and Ebf-misexpressing embryos fixed at 16hpf, prior to chemical treatments, and stained for nascent transcripts with an intronic Ebf probe. In controls, the ASMFs (solid arrowhead), but neither the SHPs (open arrowheads) nor the FHPs (arrows), actively transcribe Ebf (green nuclear dots). In Larvae misexpressing the Ebf cDNA under the control of the STVC-specific Tbx1/10 enhancer, divisions are delayed and STVCs (solid arrowheads) activated transcription of endogenous Ebf loci (green nuclear dots). (D) After 4 hr, U0126 treated ASMFs no longer transcribe Ebf (top right image, solid arrowheads), whereas control DMSO-treated ASMFs do (top left, green nuclear dots). Upon misexpression of the Ebf cDNA in the STVCs and derivatives, ongoing Ebf transcription is detected at 20hpf in both DMSO and U0126-treated cells, and it persists in both ASMFs (solid arrowheads), and SHPs (open arrowheads). (E, F) Proportions of larvae halves showing the indicated Ebf transcription patterns, in indicated experimental conditions, as illustrated in C and D, respectively.
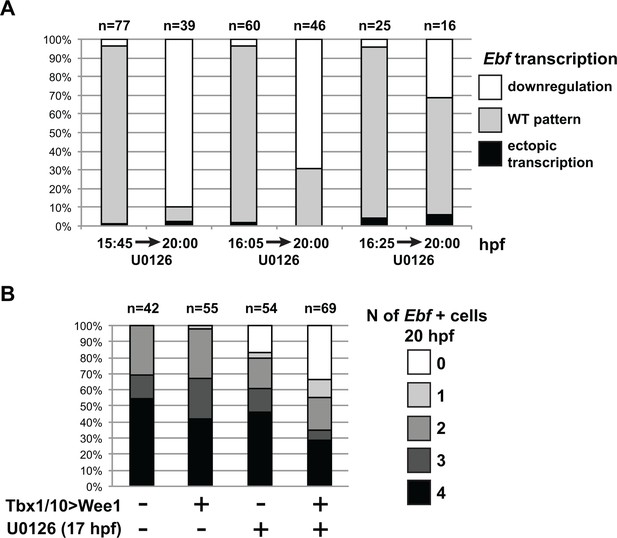
MAPK signaling is necessary for Ebf expression only in early ASMF, and cell cycle inputs shorten the MAPK-dependent period.
(A) Proportions of larva halves showing the indicated Ebf transcriptional activity (assayed using intronic probes). Batches of larvae expressing Mesp > H2B::mCherry were split to be fixed for WMFISH or treated with U0126 at three successive time points (15.75hpf, 16hpf or 16.25hpf), and the treated larvae were fixed at 20hpf. This data shows that, although all batches expressed Ebf at the beginning of the experiment, only when MEK was inhibited later (16.25hpf) did Ebf transcription persist in 20hpf larvae. (B) Proportions of larva halves showing the indicated numbers of Ebf+ cells at 20hpf, following expression of the G2/M inhibitor Wee1 in the STVCs, under the control of the Tbx1/10 T12 enhancer (+). Negative controls (-) were electroporated with a Tbx1/10(T12) > Venus construct. Larvae were also treated with U0126 (+) or DMSO (as negative control, (-)), starting at 17hpf, which corresponds to the transition from a MAPK-dependent to a MAPK-independent autoregulative mode of Ebf expression (see Figure 6A). Wee1-induced delays in cell cycle progression increased the sensitivity of late Ebf expression to MAPK inhibition, further supporting the notion that cell divisions accelerate the transition from MAPK-dependent to MAPK-independent self-activating regulation of Ebf transcription.
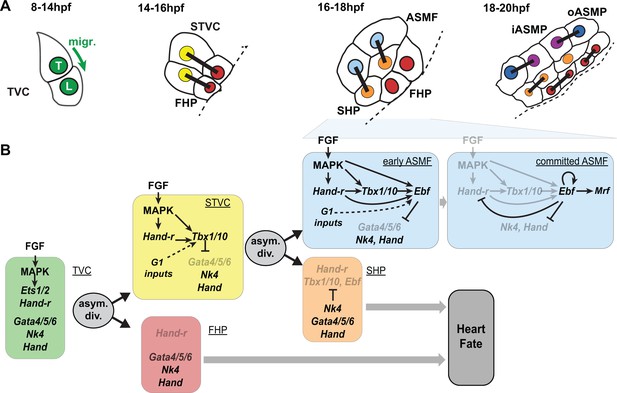
Summary model.
(A) Schematic representation of cardiopharyngeal lineage cells at successive time points representing the main fate transitions. hpf: hours post-fertilization; TVC: trunk ventral cells; L: Leader T: trailer; migr.: migration; STVC: second trunk ventral cells; FHP: first heart precursors; dotted line: midline; black bars link sister cells; ASMF: atrial siphon muscle founder cells; SHP: second heart precursors; iASMP: inner atrial siphon muscle precursors; oASMP: outer atrial siphon muscle precursor (these cells correspond to stem-cell-like Mrf-; Notch+ precursors and Mrf+; Notch- differentiating myoblasts, respectively; see (Razy-Krajka et al., 2014) for details). (B) Lineage diagram and documented regulatory relationships between indicated genes and pathways, as showing here and in (Razy-Krajka et al., 2014; Wang et al., 2013). In TVCs, primed heart and ASM markers are coexpressed, and maintenance of the STVC and ASM markers requires ongoing FGF/MAPK signaling. Following the first oriented and asymmetric cell division, FGF-MAPK is maintained only in the STVCs, which permits the continued expression of Hand-r and the activation of Tbx1/10. Cell division, presumably through G1-specific inputs, contributes to Tbx1/10 activation, and Tbx1/10 function antagonizes Gata4/5/6 expression (Wang et al., 2013). In the FHPs, termination of FGF-MAPK signaling inhibits Hand-r expression and prevents Tbx1/10 activation. Following oriented and asymmetric division of the STVCs, FGF/MAPK signaling persists only in the ASMFs, where it permits the transient maintenance of Hand-r and Tbx1/10, both of which act in parallel to FGF/MAPK to activate Ebf expression, together with contributions from presumed G1 inputs. Ebf activities further antagonize the cardiac program (marked by Gata4/5/6, Nk4/Nkx2.5 and Hand expression; [Razy-Krajka et al., 2014; Stolfi et al., 2010; Wang et al., 2013]). Once Ebf expression reaches ‘high levels’, its regulation becomes MAPK-independent and self-activating (this study). It also feeds back negatively on early activators such as Hand-r, and promotes the expression of the muscle determinant Mrf (Razy-Krajka et al., 2014; Tolkin and Christiaen, 2016). We propose that this transition represents commitment to an ASM fate. In the SHPs, termination of FGF/MAPK signaling prevents maintenance of Hand-r and Tbx1/10 expression, which, together with repressive inputs from Nk4/Nkx2.5, inhibits Ebf activation (Wang et al., 2013), and permits heart fate specification (Wang et al., 2017).
Additional files
-
Supplementary file 1
Primer sequences.
- https://doi.org/10.7554/eLife.29656.016
-
Transparent reporting form
- https://doi.org/10.7554/eLife.29656.017





