Brainstem network dynamics underlying the encoding of bladder information
Figures
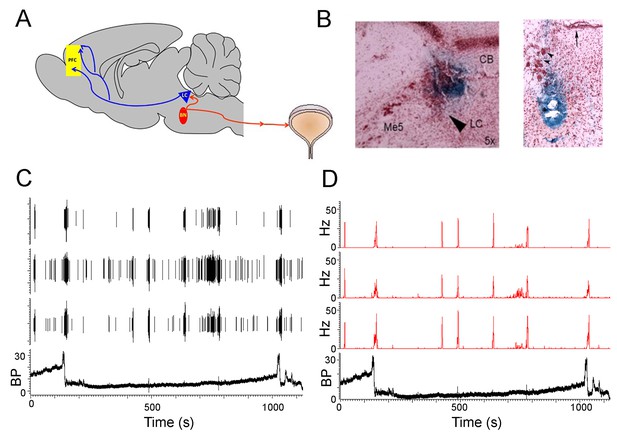
PMC neuronal activity during micturition cycles in unanesthetized rats.
(A) Schematic showing proposed circuitry engaged during micturition and recording sites. The PMC (red oval) projects to lumbosacral preganglionic parasympathetic neurons that give rise to cholinergic input to the detrusor and produce contraction. A population of PMC spinal-projecting neurons are retrogradely labeled from the LC (blue triangle) which is just caudal, dorsal and lateral to the PMC. LC neurons project widely throughout the cortex. In the present study, single unit activity was recorded from PMC neurons and/or LC neurons and local field potential activity was recorded from the medial prefrontal cortex (yellow rectangle). Neuronal recordings were obtained simultaneously with in vivo cystometry recordings from an implanted bladder catheter. (B) Representative Prussian blue histological verification of a recording in the LC (left) and the PMC (right). For the LC photomicrograph CB (Cerebellum), Me5 (mesencephalic trigeminal nucleus) and the arrow points to the LC. For the PMC photomicrograph, the arrow points to the ventricle and the arrowheads point to the mesencephalic trigeminal nucleus. Figure 1—figure supplement 1 shows a section slightly rostral to this with a lesion created by the wires (C) Raw waveform traces showing spikes from three PMC neurons and simultaneously recorded bladder pressure (BP mm Hg) during two micturition cycles. (D) Ratemeter records of the same cells shown in C.
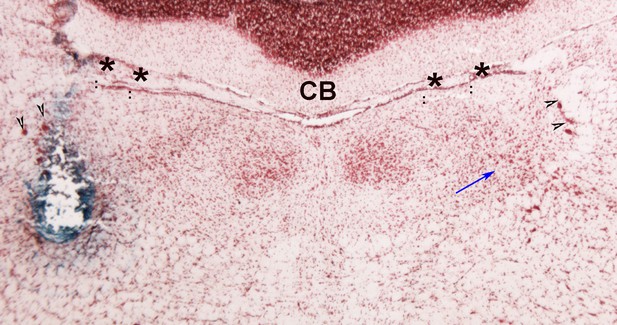
Section through the pons that is just rostral to that shown in Figure 1B (PMC).
The lesion produced by the wire bundle can be seen here on the left and cells of Barrington’s nucleus can be seen on the contralateral side (blue arrow). Arrowheads point the cells of the mesencephalic trigeminal nucleus. Asterisks indicate the ventricle. Cerebellum (CB).
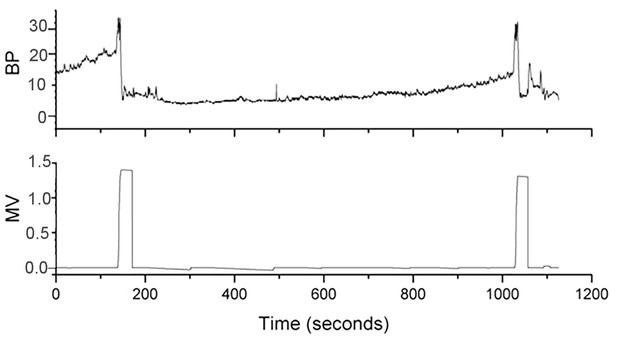
The same bladder pressure trace as shown in Figure 1C is aligned with a trace noting the timing of micturition (bottom panel).
Bladder pressure (BP, mm Hg), micturition volume (MV, ml).
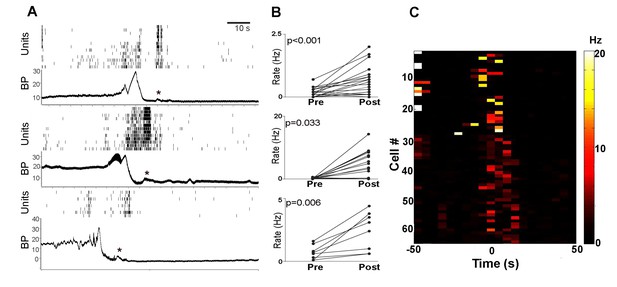
PMC neuronal activity of each rat.
(A) Each panel shows PMC cell rasters and bladder pressure (BP in mm Hg) during a representative micturition cycle from each of the three rats. Note that some PMC units discharge even when BP is relatively low. Activity increases prior to peak micturition pressure and a burst of activity occurs after bladder emptying when BP returns to baseline. A slight rise in BP is temporally associated with this burst (asterisks). (B) Pairwise pre-and post-micturition firing rate changes (Hz) for individual neurons shown in the rasters in A. Pre-micturition rates were determined from the period between 40–50 s prior to peak BP and Post-micturition rates were determined from 0 to 10 s sec after peak micturition pressure. For all three panels the mean post rates were greater than mean pre-rates as indicated by the Student’s t-test value (matched pairs) in the figure. (C) Heatmap of all PMC neurons recorded from the three rats showing discharge rate in Hz (coded by color scale on right) for the period of 50 s before and 50 s after micturition indicated by time = 0. For most cells, data are shown for two micturition cycles indicative of technical reproducibility. Note firing occurring 0–20 s after micturition. Bin size = 10 s.
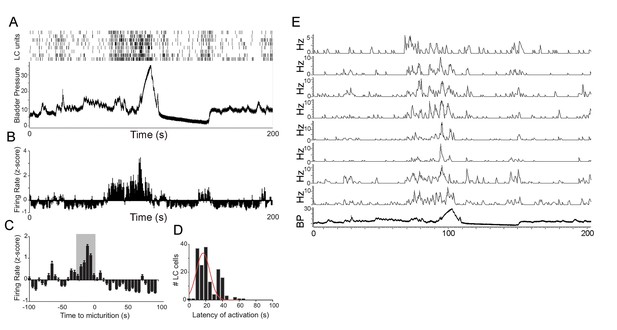
LC neuronal activity during micturition cycles in unanesthetized rats.
(A) Spike rasters of 8 discriminated LC neurons from an individual rat and simultaneously recorded bladder pressure (mm Hg) during a micturition cycle. Abscissa indicates time in seconds. (B) Bars indicate the average z-score firing rate of the LC units shown in A. The abscissa in B is time locked to that in A and the firing rate is evaluated in Hz in 1 s bins. (C) Bar graph shows average LC firing rate from all animals (n = 4 animals, 51 neurons) across 10 micturition cycles in 5 s bins (normalized z-score). The gray box highlights the bins 30 s prior to micturition-note consistent LC activation at this time). Peak bladder pressure is at time 0 s. (D) Histogram showing the distribution of latencies of LC activation prior to peak micturition pressure for all neurons and across all micturition cycles. (E) Ratemeter records of same cells as in A.
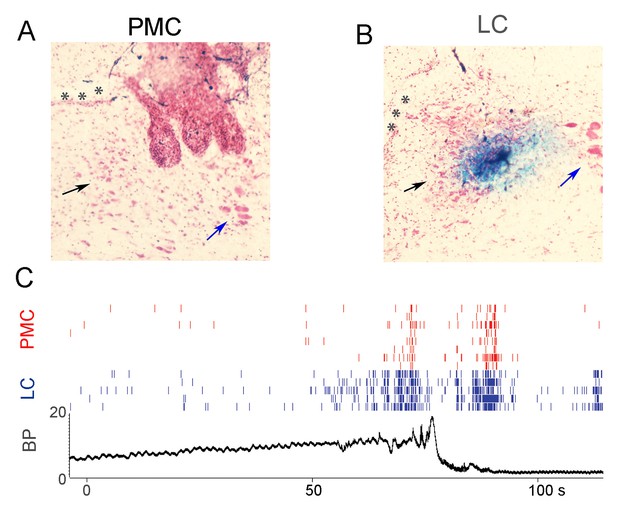
Simultaneous recordings of PMC and LC neuronal firing during micturition.
(A) Histological verification of recording sites in the PMC. The track of the electrode wires can be seen and the most medial track impinges on the lateral part of the PMC (black arrow). Asterisks indicate the ventricle and the blue arrow points to the mesencephalic trigeminal nucleus. (B) Histological verification of the recording site in the LC of the same rat which is apparent as the Prussian blue reaction product. Arrows and asterisks as in A. (C) Spike rasters of multiple BN neurons (red) and LC neurons (blue) recorded from the same rat during a micturition cycle. The lower panel shows bladder pressure recorded simultaneously. Note that LC neuronal activation precedes PMC neuronal activation and both occur prior to peak micturition pressure. Likewise, after bladder pressure returns to baseline, LC neurons become activated and this precedes activation of PMC neurons.
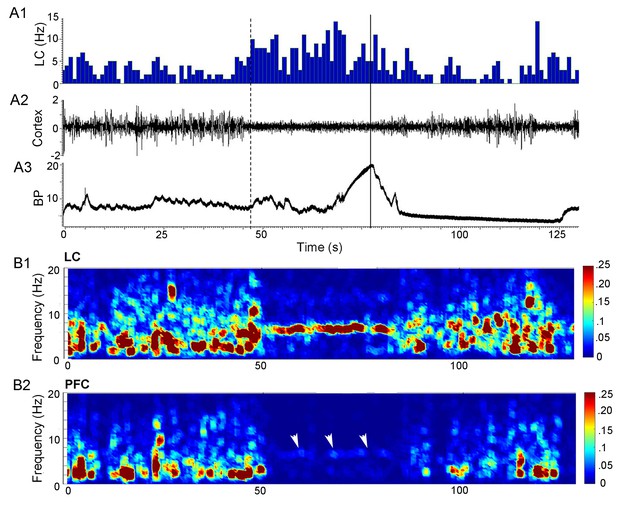
Synchronization between LC and cortical network activity prior to micturition.
(A) A1 shows a ratemeter record of a single LC unit (bin size = 1 s). A2 is the raw cortical LFP trace. A3 shows bladder pressure (BP, mm Hg) with the solid line indicating peak micturition pressure. The dotted line indicates 30 s before peak micturition pressure. Note that LC activation is temporally correlated with cortical desynchronization and both occur just over 30 s prior to peak micturition pressure. (B) Heat maps showing power in different frequency bands (0–20 Hz, y-axis) with respect to time in LC (B1) and mPFC (B2) from same rat as shown in A. The abscissae indicate time (s) and are aligned to correspond to abscissae in A. The LC LFP shows a prominent theta rhythm prior to peak micturition pressure that corresponds to cortical desynchronization. White arrowheads in B2 point to a dim band in the theta frequency range of the mPFC LFP.
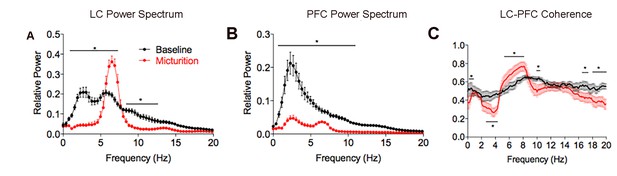
Mean power spectral density plots of LC and mPFC local field potentials and LC-mPFC coherence at different times preceding micturition.
Red lines indicate the mean power (A,B) or coherence (C) determined 30 s before peak micturition pressure (red) and the black lines are the same for the preceding 30 s period. Means are from eight micturition cycles from three rats as described in the text. A two-way repeated measures ANOVA with frequency as the repeated measure indicated significant differences between the two time periods for LC power (F(1,742)=221, p<0.0001), PFC power (F(1,742)=671, p<0.0001 and LC-PFC coherence (F(1,2940) = 121, p<0.001).
Tables
Burst analysis of PMC neurons*
https://doi.org/10.7554/eLife.29917.006| Interval | # Bursts† | #spikes/burst | ISI (ms) | Duration (ms) |
|---|---|---|---|---|
| Pre-micturition | 0.3 ± 0.1 | 4.9 ± 0.2 | 54 ± 2 | 263 ± 14 |
| Intermicturition Interval | 12.3 ± 1.9 | 12.4 ± 1.2 | 38 ± 1 | 432 ± 35 |
| Post-micturition | 2.7 ± 0.5 | 18.4 ± 2.7 | 40 ± 3 | 606 ± 66 |
-
*values are means ± SEM determined from 36 cells from 3 rats.
†Mean #bursts/cell/micturition cycle
-
Pre-micturition = 20 s before micturition
Intermicturition interval = 120 s after first and 120 s before the following micturition
-
Post-micturition = 20 s after micturition
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.29917.012





