Identification and functional characterization of muscle satellite cells in Drosophila
Figures
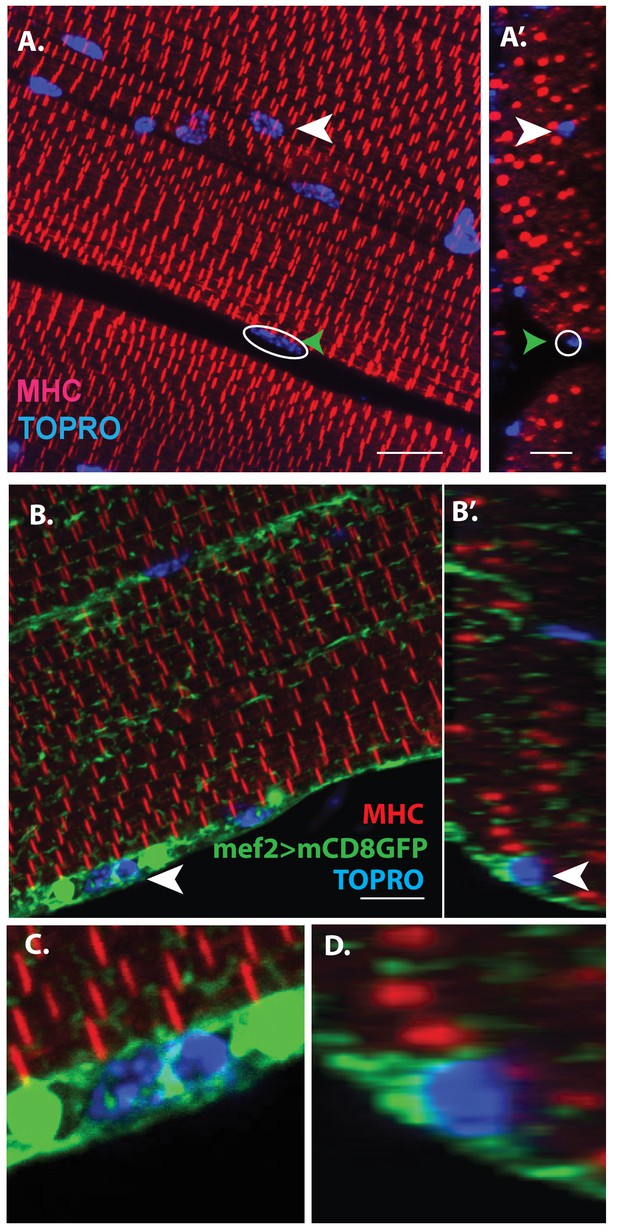
Unfused muscle associated cells are present at the surface of adult flight muscles.
(A) Dorsal longitudinal muscles (DLMs) stained for Myosin Heavy Chain (MHC) (red) to delineate muscle fibrils and TOPRO (Blue) marking nuclei. White arrowhead marks one example nucleus, surrounded by MHC-labeled myofibrils showing it is inside a myofiber. Green arrowhead, white circle, marks one example nucleus located at the peripheral surface of MHC labeled myofiber. (A’) Orthogonal view of (A). (B) DLMs stained for Dmef2 Gal4 > UAS mCD8::GFP marking muscle membrane (green) MHC (red) and TOPRO (blue). Unfused nuclei are enveloped in GFP-labeled membrane (white arrowhead), B’ Orthogonal view of (B) at the muscle fiber surface. (C, D) Magnified views of nucleus indicated in (A’) and (B’), respectively. N = 15. Scale bar 10 μm.
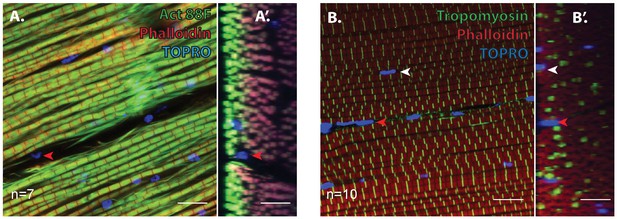
Unfused muscle-associated cells at the surface of adult flight muscles are excluded by Act88F and Tropomyosin inside myofibers.
(A) DLMs stained for Actin88F (green) to delineate muscle fibrils (Phalloidin, Red) and TOPRO (Blue) marking nuclei. Red arrowhead marks one example nucleus, between two DLM myofibers (marked by Act88F) showing through an XY axis view and the orthogonal [YZ axis view (A’]) that the nucleus is outside the myofibers. n = 7. (B) DLMs stained for Tropomyosin (green) to delineate muscle fibrils (Phalloidin, Red) and TOPRO (Blue) marking nuclei. White arrowhead marks one example nucleus, surrounded by tropomyosin myofibrils showing it is inside a myofiber. Red arrowhead marks one example nucleus, between two DLM myofibers. (B’) Orthogonal view of (B) n = 10 Scalebars=10 um.
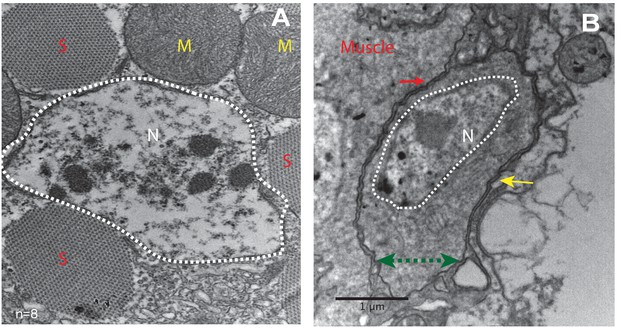
Unfused muscle-associated cells have ultrastructural features of satellite cells.
(A, B) Transmission electron micrographs of adult flight muscle. (A) Nuclei inside DLM fibers are large round structures surrounded by nuclear membranes (white dotted lines). A. Distinct sarcomeres in the cytoplasm of the muscle syncytium (marked as S) and mitochondria (marked as M). (B) Mononucleate cell apposed to mature muscle surface. Cell membrane (marked by a green double-headed arrow) seen distinctly apposed to mature muscle membrane (red arrow) and beneath the basement membrane (yellow arrow) of the muscle fiber. Organelles and wedged shaped nucleus (white dotted line) are visible in the cytoplasm of this cell. N = 8 Scale bar 1 μm.
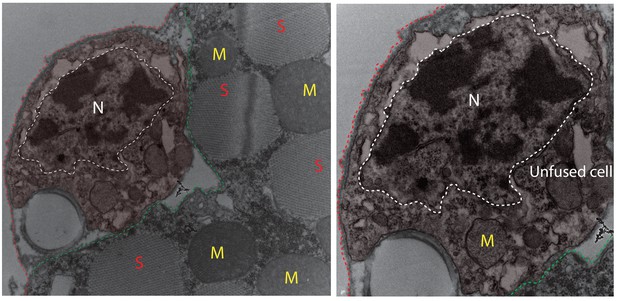
Electron micrograph of unfused mononucleate cell at muscle surface.
Left panel: Mononucleate cell outside mature DLM fiber. Cell boundary marked with colored dotted lines; green apposed to mature muscle surface; red away from the muscle fiber. ‘N’ surrounded by white dotted line marks the cell’s nucleus. Red ‘S’s denote sarcomeric arrays in mature DLM fiber; Yellow ‘M’s mark mitochondria inside fiber DLM mature. Right panel: Higher magnification of the same mononucleate cell in left panel. ‘N’ surrounded by white dotted line marks the cell’s nucleus. Yellow ‘M’ marks the nucleus of the unfused cell.
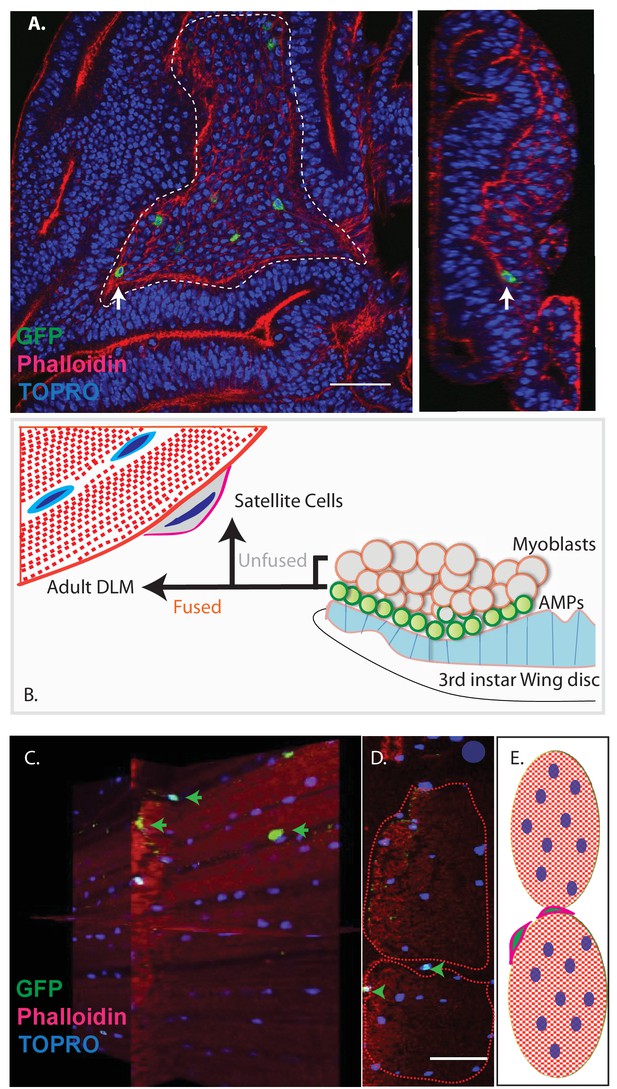
Unfused cells of the AMP lineage persist in adult muscle.
(A) Single-cell MARCM clones of AMP lineage (mef2-Gal4 driver) induced in the third instar (120AEL) and recovered from a single 15 m heat shock at 37°C, clones from the notum of the wing disc (induced in the late third instar (120AEL). A labeled single cell clone (green) is indicated by a white arrow. Right panel shows the same cell (white arrow) in orthogonal view. The AMP cell lies in close proximity to wing disc epithelium. Phalloidin (red) marks F-actin and TOPRO (blue) marks all the nuclei. (B). Simplified schematic describing lineal origin of adult DLM fibers and satellite cells. AMPs (green circles) on the third instar wing disc notum give rise to myoblasts (beige circles) located distal to the epithelium. Cells from the AMP lineage either fuse to muscle templates and give rise to adult DLMs or remain unfused as mononucleate cells closely apposed to the DLM surface. (C). MARCM clones with mef2-Gal4 driving UAS mCD8::GFP induced in the third instar (~120 hr AEL) and recovered in the adults stage. 3D reconstruction of adult muscle with mononucleate GFP-labeled cells (green arrows) located on the surface of mature DLM fibers. Phalloidin (red) marks F-actin and TOPRO (blue) marks all the nuclei. (D) Orthogonal view of the same preparation as in (C). mef2-Gal4-labeled mononucleate MARCM clones (GFP positive) indicated with green arrowheads clearly seen on the surface of adult DLMs. (E) Simplified schematic of (D) Red checkered ovals containing blue ovals indicate mature DLMs. Cells with red membranes and green nuclei represent satellite cells. Scale bars in (A, C, D) 50 μm. N = 12.
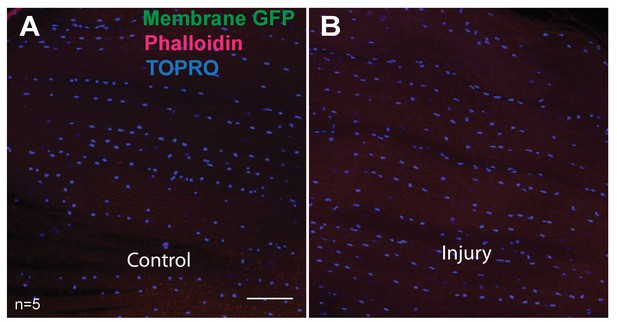
DLMs showing absence of hemocytes.
e33c-Gal4 driver (specific for hemocytes) used to drive UAS mCD8::GFP expression. Muscles are stained for GFP (anti-GFP, green), F-actin (Phalloidin, red) and TOPRO (blue, marks all nuclei). In both control (A) and injured (B) no GFP-labeled cells were seen. Scalebar = 50 μm.
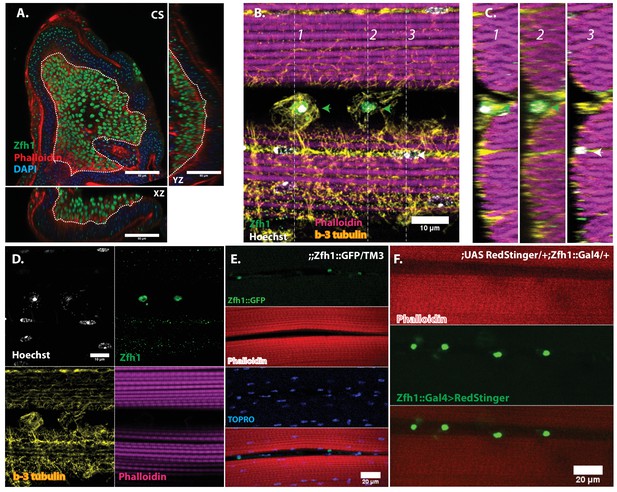
In adult muscle Zfh1 is a specific marker for unfused satellite cells.
(A) Zfh1 immunolabeling (green) of third instar wing disc notum of wild-type flies. Zfh1 expression can be seen in myoblast nuclei located on the disc epithelium revealed by Phalloidin staining (red), as seen in XZ and YZ orthogonal views. TOPRO stains all nuclei (blue). Scale bar 50 um. N = 10. (B) Zfh1 and β−3-tubulin co-immunolabeling of adult DLMs in wild-type flies. Zfh1 expressing nuclei (green) co-stained with Hoechst (white) are located between DLM fibers labeled with Phalloidin (magenta). Two Zfh1 expressing nuclei are marked with green arrowheads. Nuclei inside DLMs do not express Zfh1 (one example indicated with white arrowhead). The cell boundaries of the DLMs and the Zfh1 expressing cells are delimited by β−3-tubulin; the Zfh1 expressing cell with its cytoskeleton is clearly separate from the adjacent DLM fibers. N = 25. (C) Three orthogonal views of the same preparation as in (B) taken at planes 1, 2 and 3 (dotted lines in B) document the positions of Zfh1 expressing cells outside the muscle fiber (green arrows). Their position contrasts with that of the fused Zfh1-negative DLM nuclei near the surface, one of which is indicated (white arrow). N = 15. (D) Same preparation as in (B) with montage showing the individual confocal channels for Hoechst staining (top left), Zfh1 immunolabeling (top right), β−3-tubulin immunolabeling (bottom left) and Phalloidin staining (bottom right). Scale bar 10 um. (E) Expression of GFP-tagged Zfh1 protein (green) in adult muscle of Zfh1::GFP/TM3 flies co-stained with Phalloidin (red) and TOPRO (blue). Top three panels show individual confocal channels; bottom panel is a superposition of the individual channels. Zfh1 protein expression is limited to unfused cells and is not seen inside muscle fibers. Scale bar 20 um. N = 15. (F) Expression of Zfh1-Gal4 (green) in adult muscle of UAS-RedStinger/+; Zfh1:Gal4/+flies co-labeled with Phalloidin (red). Top two panels show individual confocal channels; bottom panel is a superposition of the individual channels. Zfh1-Gal4 expression is limited to unfused cells and is not seen inside muscle fibers. N = 15. Scale bar 20 um.
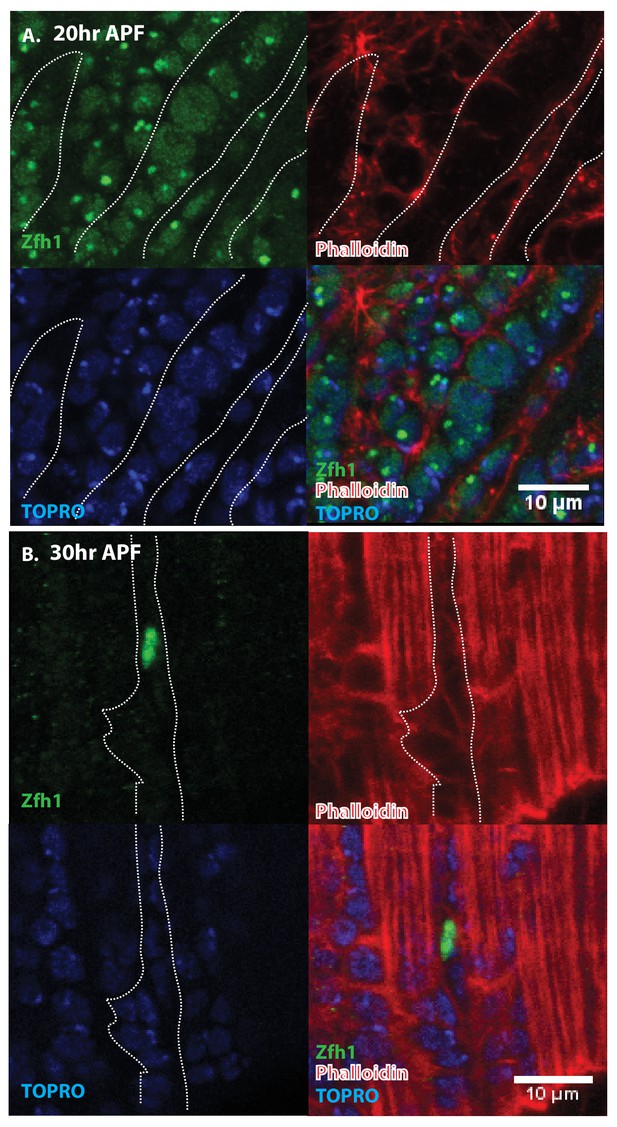
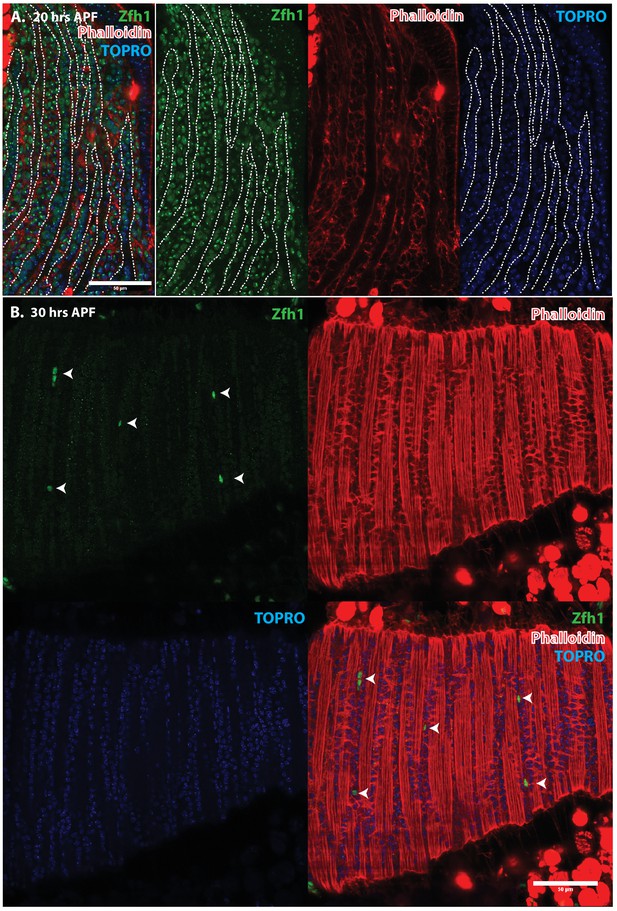
Pattern of Zfh1 expression in AMP lineal cells at 20 hr and 30 hr APF.
Zfh1 immunolabeling (green) of unfused myoblasts and of myoblasts that have fused with DLM templates, co-labeled with Phalloidin (red) and TOPRO (blue). Zfh1 is expressed in all AMP lineal myoblasts at 20 hr APF but is restricted to a small set of unfused AMP lineal cells at 30 hr APF. (A) At 20 hr APF, Zfh1 expression is seen in all unfused myoblast nuclei and in all nuclei inside the DLM templates (outlined by white dotted line). N = 8. (B) At 30 hr APF, Zfh1 expression is limited to a few nuclei located in between DLM templates and is no longer seen in fused nuclei. Single example shown here. N = 10. Scale bars 10 um.
Pattern of Zfh1 expression in AMP lineal cells at 20 hr and 30 hr APF.
Zfh1 immunolabeling (green) of unfused myoblasts and of myoblasts that have fused with DLM templates, co-labeled with Phalloidin (red) and TOPRO (blue). Zfh1 is expressed in all AMP lineage myoblasts at 20 hr APF but is restricted to a small set of unfused AMP lineage cells at 30 hr APF. (A, B) As in Figure 5 but at lower magnification with images showing whole templates at respective time points. Scale bars 50 μm.
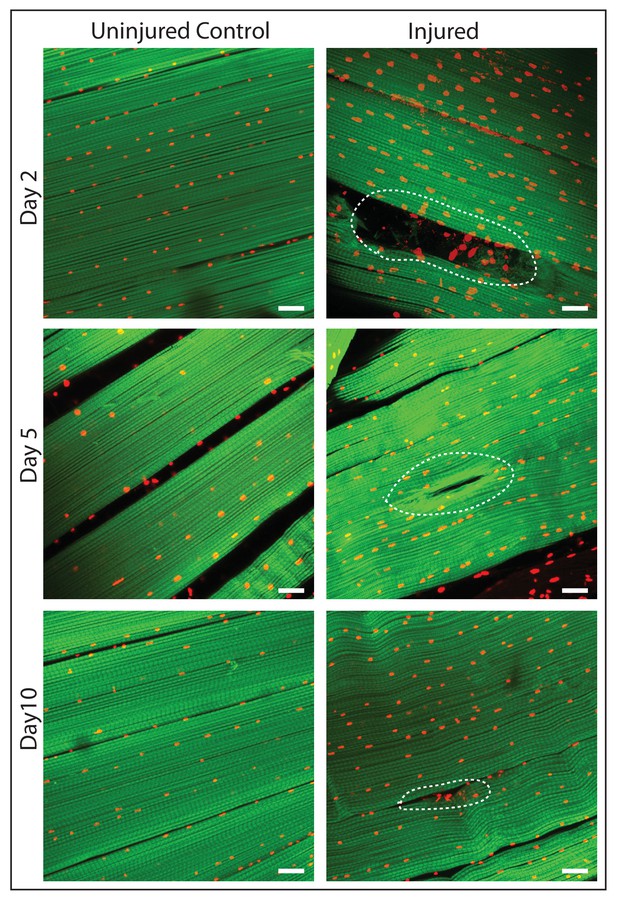
DLM fibers regenerate following induced physical damage.
Representative images of injured flight muscles (right) and time matched controls (left) at day 2, 5 and 10 after localized stab injury. Adult DLMs stained with Phalloidin (green) and TOPRO (red). At day 2 following injury, breaks in actin filaments, and corresponding disruptions in distribution of nuclei at the site of the injury wound (indicated by white dotted line) are seen. At day 5 following injury, the wound is reduced in size and the actin filament arrangement and myonuclei distribution is more has recovered. At day 10 following injury, regeneration is virtually complete and only small remnants of the wound are apparent. N = 10/group per time point. Scale bar 15 um.
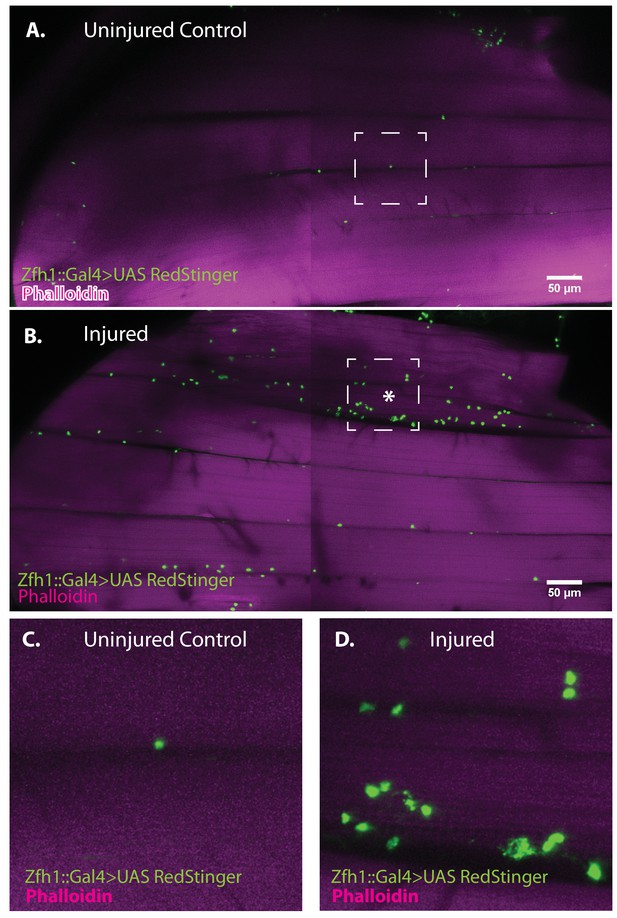
Zfh1-positive satellite cells located between DLMs proliferate in response to physical injury.
(A) Uninjured control DLMs. Zfh1-Gal4 driving UAS RedStinger in adult DLMs. A small number of Zfh1-Gal4-positive mononucleate satellite cells (green) are located between DLM fibers. Anti-DsRed (green) co-stained with Phalloidin (magenta). Scale bar 50 μm. (B) Injured DLMs. Zfh1-Gal4 driving UAS RedStinger in adult DLMs injured by stab wound (* denotes injured fiber). At 24 hr after stab wound, numerous Zfh1-Gal4-positive mononucleate satellite cells (green) are seen between DLM fibers at the site of injury but also away from the site of injury. Anti-DsRed (green) co-stained with Phalloidin (magenta). Scale bar 50 μm. (C) Single Zfh1-Gal4-labeled nucleus located between uninjured DLM fibers in area delineated by white square in A. (D) Multiple doublets and a few singlets of Zfh1-Gal4-positive nuclei located near site of injury in area delineated by white square in (B). N = 15 Scale bar 50 μm.
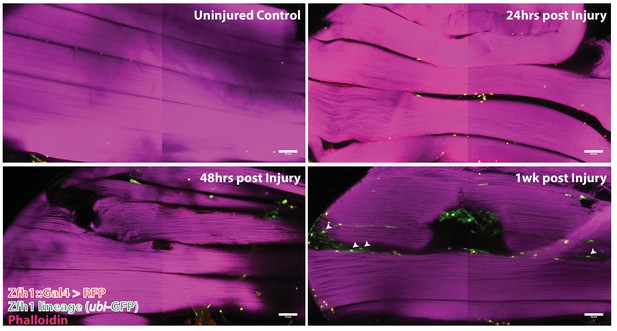
Following muscle injury, satellite cell lineal progeny localize to the surface and the interior of DLM fibers.
Localization of satellite cell lineal progeny examined with Zfh1-Gal4 driven G-trace labeling in uninjured control and in DLM muscles at 24 hr, 48 hr and 1 week after injury. In all cases, G-trace was induced in the adult stage and in the injured animals, 24 hr before infliction of a stab wound. In the uninjured control, only a few cell nuclei located at the DLM surface are labeled as expected for Zfh1-positive satellite cells (top left). At 24 hr and 48 hr after injury more cell nuclei located at the DLM surface are labeled indicating proliferative expansion of the Zfh1-positive satellite cell lineage (top right, bottom left). At 1 week after injury, labeled cell nuclei are located both at the surface and in the interior of DLM fibers implying that some of the Zfh1-positive satellite cell lineal descendants have now fused with the injured DLMs (bottom right). N = 6 per group Scale bar 50 um.
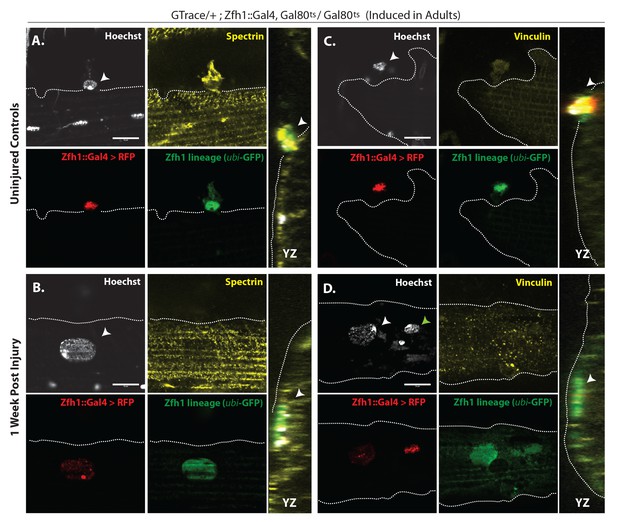
G-trace labeled lineal progeny of Zfh1 expressing satellite cells fuse with damaged DLM fibers after injury.
Localization of satellite cell lineal progeny examined with Zfh1-Gal4 driven G-trace in uninjured controls (A, C) and in experimental animals 1 week after injury (B, D). G-trace was induced in the adult stage and in the injured animals. This induction was 72 hr before infliction of a stab wound. In (A–D), the left four panels are individual confocal channels for Hoechst staining (top left), alpha Spectrin or Vinculin staining (top right), G-trace driven RFP expression (bottom left) and G-trace driven GFP expression (bottom right); the right panel is a superposition of the four channels and viewed from an orthogonal YZ perspective. (A) Control. A single-cell nucleus closely associated with the outside of the DLM surface, as delineated by the alpha Spectrin expression border (dotted line), is both RFP labeled (implying real-time Zfh1 expression) and GFP labeled (implying lineal origin from a Zfh1-positive cell) indicating that it corresponds to a Zfh1-expressing satellite cell. (B) Injured. A single-cell nucleus located within the muscle fiber albeit close to the fiber’s surface, as delineated by the alpha Spectrin expression border (dotted line) is GFP labeled (implying lineal origin from a Zfh1-positive cell) indicating that it corresponds to a satellite cell lineal progeny. The labeled nucleus has a flattened disc-like shape. (C) Control. A single-cell nucleus closely associated with the outside of the DLM surface as is delineated by the Vinculin expression border (dotted line), is both RFP labeled (implying real-time Zfh1 expression) and GFP labeled (implying lineal origin from Zfh1-positive cells) indicating that it corresponds to a Zfh1-expressing satellite cell. (D) Injured. A single-cell nucleus located within the muscle fiber albeit close to the fiber’s surface (white arrow), as delineated by the Vinculin expression border (dotted line) is GFP labeled (implying lineal origin from a Zfh1-positive cell) indicating that it corresponds to a satellite cell lineal progeny. The labeled nucleus has a flattened disc-like shape. Note that the second, apparently adjacent cell nucleus (green arrow) which is RFP labeled is not located within the muscle fiber. N = 8 per group. Scale bars 10 μm.
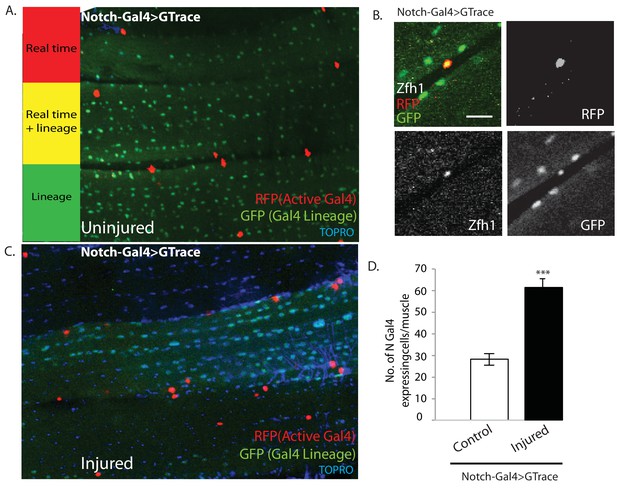
Notch-Gal4 driven G-trace labeling reveals real time Notch expression in muscle satellite cells.
(A) Notch-Gal4 driven G-trace labeling of uninjured adult DLM co-stained by TOPRO. The cell nuclei located on the surface of the DLM fibers are RFP positive (red, anti-RFP labeled) indicating that they correspond to satellite cells that are actively expressing Notch. In contrast, the numerous myonuclei within the muscle fiber are GFP positive (green, anti-GFP labeled) confirming the fact that they are lineal descendants of Notch expressing AMPs. (B) Notch-Gal4 driven G-trace labeling of uninjured adult DLM co-labeled by Zfh1 immunostaining. The cell nuclei located on the surface of the DLM fibers show strong expression of RFP indicating real-time Notch-Gal4 expression and of Zfh1 indicating that they are satellite cells. The top left panel is a superposition of individual channels for Zfh1, RFP, and GFP expression; the remaining panels are the corresponding single channels. (C) Notch-Gal4 driven G-trace labeling of adult DLM 24 hr after injury, co-stained by TOPRO. The cell nuclei located on the surface of the injured DLM fibers have increased in number but are still RFP positive (red, anti-RFP labeled) indicating real-time expression of Notch-Gal4 in these nuclei of the expanded satellite cell lineage. As in (A), myonuclei within the muscle fiber are GFP positive (green, anti-GFP labeled). (D) Quantification of the number of real-time Notch-Gal4 expressing nuclei in control versus injured muscle fibers in these G-trace experiments. Twice as many RFP-positive cells are observed in injured versus control DLMs. n = 10 Data presented are mean ± standard error Student's t test: p-value<0.001 ***. Scale bar 10 μm.
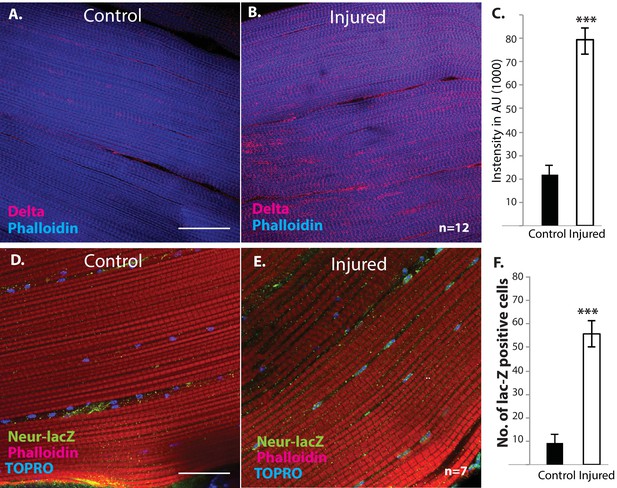
Delta and Neuralized are upregulated in injured muscle fibers.
(A, B) Delta-GFP (anti-GFP, red) expression in DLMs co-labeled with Phalloidin (blue) in control (A) versus injured (B) muscle fibers reveals a marked upregulation of Delta-GFP expression upon injury. (C) Quantification of signal intensity of Delta-GFP in control versus injured DLM fibers; injured muscles show significant upregulation of Delta expression in comparison to uninjured muscle (quantification in arbitrary intensity units). n = 12 Data presented are mean ± standard error. Student's t test: p-value<0.001 ***. (D, E) Neuralized-LacZ expression (green, anti-LacZ immunolabeling) co-labeled by Phalloidin (red) in control versus injured muscle fibers. In comparison to controls (D), injured muscles show elevated Neuralized-lacZ levels in myonuclei, some of which are indicated by white arrows in (E). (F) Quantitation of Neuralized-LacZ expression in control versus injured muscle. For quantification the number of lac-Z-positive nuclei were counted. n = 7. Data presented are mean ± standard error. Student's t test: p-value<0.001 ***. Scale bars 30 μm.
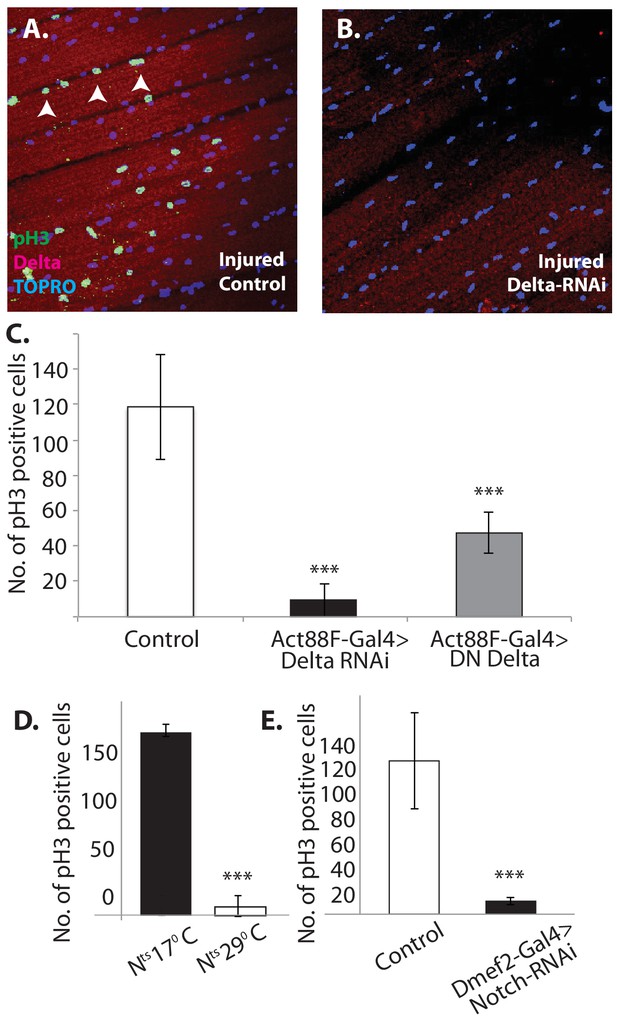
Notch-Delta signaling is required for satellite cell proliferative activity in injured muscle.
(A) Injured control muscle. Mitotic activity assayed by PH-3 expression (green, anti-PH-3 immunolabeling) in DLMs co-labeled for Delta expression (red, anti-Delta immunolabeling) and TOPRO. Numerous satellite cells (three indicated by white arrows) are PH-3-positive indicative of the mitotic activity required for injury-induced expansion of the satellite cell population. (B) Injured muscle with adult-specific Delta downregulation (via Act88F-Gal4, TubGal80ts driving UAS Delta RNAi). Mitotic activity of satellite cells assayed by PH-3 expression as in (A) is absent. (C) Quantification of the number of PH-3 expressing cells in control versus Delta downregulated flies; Delta downregulation is achieved by targeted Delta-RNAi knockdown as well as by targeted dominant negative Delta (DN Delta) expression. n = 9 Data presented are mean ± standard error. Student's t test: p-value<0.001***. (D) Quantification of PH-3 labeled cells in injured muscle of Notch temperature sensitive allele flies at permissive (17°C) versus restrictive temperature (29°C). n = 12. Data presented are mean ± standard error. Student's t test: p-value<0.001***. (E) Quantification of mitotically active PH-3-labeled satellite cells in control versus Notch downregulated flies. n = 12. Data presented are mean ± standard error. Student's t test: p-value<0.001***.
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.30107.018





