Sensory Neurons: A new target for G protein signaling
Many of the cells in our body communicate by releasing small molecules that bind to receptors on the surface of target cells. These molecules include hormones and, in the case of nerve cells, neurotransmitters. Signal transduction pathways then relay the information from the receptor to inside the cell and either activate or inhibit ‘effector’ proteins that cause the cells to respond appropriately. Heterotrimeric G proteins – protein complexes that consist of three different subunits named α, β and γ – provide one such pathway, and work with cell surface receptors called G protein-coupled receptors (GPCRs; Figure 1A).
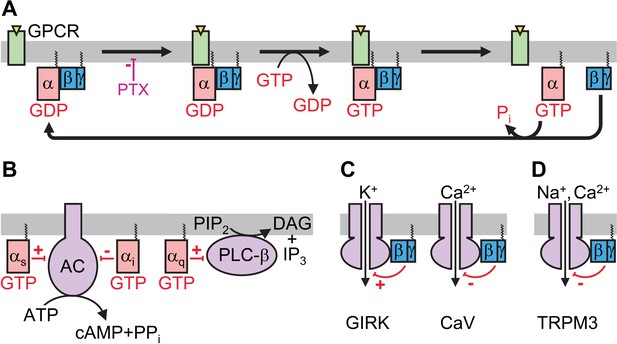
Simplified schematics of heterotrimeric G protein signaling pathways.
(A) Functional cycle of a heterotrimeric G protein. In the resting state (left), heterotrimeric G proteins consist of α, β and γ subunits bound together. The α subunit is bound to a molecule of GDP, and the complex is anchored to the intracellular surface of the cell membrane. When a G protein-coupled receptor (GPCR) is activated by an agonist molecule binding to its extracellular surface, the heterotrimeric complex can interact with the cytosolic surface of the GPCR. Pertussis toxin (PTX) can inhibit this interaction. The molecule of GDP bound to the α subunit is released and a molecule of GTP binds in its place, causing the heterotrimer to dissociate into Gα-GTP and Gβγ subunits. Upon the breakdown of GTP to form GDP and phosphate (Pi), the heterotrimer reforms. (B) Signal transduction through the Gα subunit. Some classes of Gα activate (αs) or inhibit (αi) adenylyl cyclase (AC), the enzyme that produces the second messenger cyclic AMP (cAMP) by removing the terminal pyrophosphate (PPi) from ATP. Others (αq) activate the enzyme phospholipase C-β (PLC-β), which cleaves an important component of the plasma membrane's inner leaflet called phosphatidylinositol bisphosphate (PIP2), converting it into diacylglycerol (DAG) and inositol trisphosphate (IP3). (C) Signal transduction through the Gβγ subunit. Gβγ activates G-protein-gated inward rectifier K+ (GIRK) channels (left) but inhibits voltage-gated calcium ion channels (CaV; right). (D) TRPM3 channels are inhibited by direct binding of Gβγ.
The structure and mechanism of heterotrimeric G proteins has been studied at atomic resolution (Oldham and Hamm, 2008). In the resting state the α subunit (Gα) binds to a molecule called GDP and is tightly associated with the β and γ subunits, forming a heterotrimer. When the complex interacts with an activated GPCR, the molecule of GDP is exchanged for GTP, and the G protein complex dissociates into two parts: Gα-GTP and a stable Gβγ dimer. Both Gα and Gβγ contain ‘anchors’ that keep them attached to the cell membrane, but allow them to diffuse laterally along the membrane to find their target effector proteins. Eventually, Gα breaks down the GTP to form GDP, and Gα-GDP associates with Gβγ to reform the heterotrimer.
In the ‘conventional’ mode of signal transduction (Gilman, 1987) Gα-GTP activates or inhibits a target enzyme, depending on which class of α subunit is involved (Figure 1B). For example, an inhibitory α subunit (Gαi) inhibits the enzyme that produces a chemical messenger called cyclic AMP (or cAMP). Heterotrimeric G proteins may also regulate ion channels within the membrane via a different pathway. For instance, the GIRK channels (which are responsible for slowing the heart rate) are activated by Gβγ directly binding to them (Figure 1C; Logothetis et al., 1987).
Now, in eLife, three groups featuring researchers based at institutes in Germany, the UK, the US and Canada independently present evidence of a new target for GPCR signaling: an ion channel called Transient Receptor Potential Melastatin 3 (TRPM3; Badheka et al., 2017; Quallo et al., 2017; Dembla et al., 2017). Expressed abundantly in sensory neurons, TRPM3 channels help organisms to sense heat and make them more sensitive to pain during inflammation (Vriens et al., 2011). The new reports suggest that the activation of TRPM3 channels is greatly reduced if the channels are also stimulated by any of a variety of GPCRs.
The three studies systematically probed individual steps of the G protein regulatory pathway to dissect its mechanism. First, each of the groups independently show that the inhibition of TRPM3 can be overcome by pre-treatment with pertussis toxin. This toxin prevents the inhibitory Gαi subunit from interacting with the activated GPCR, locking the complex in the resting trimeric state (Figure 1A). Thus, TRPM3 inhibition requires the heterotrimeric G protein to dissociate. But is the inhibiting signal carried through Gαi-GTP or through Gβγ?
The studies accumulate strong evidence showing that the Gα subunit is not involved. Johannes Oberwinkler of Philipps-Universität Marburg and colleagues (including Sandeep Dembla and Marc Behrendt as joint first authors) did not detect any interaction between Gαi and TRPM3. Moreover, they and Tibor Rohacs of New Jersey Medical School and co-workers – who include Doreen Badheka and Yevgen Yudin as joint first authors – show that wild-type inhibitory Gαi subunits do not decrease the activity of TRPM3, and neither can mutant subunits that cannot break down GTP and are therefore permanently active. Furthermore, Talisia Quallo and colleagues at King’s College London show that GPCR-mediated TRPM3 inhibition is unaffected by an inhibitor that selectively acts upon the inhibitory Gαi subunit. Dembla et al. and Badheka et al. also show that altering the concentration of the chemical messenger cAMP (which is decreased by the activity of inhibitory Gαi subunits) has no effect on either the activity or inhibition of TRPM3.
On the other hand, all evidence points to a role for Gβγ in signaling to TRPM3. Badheka et al. and Dembla et al. both show that TRPM3 activity is strongly inhibited by the overexpression of Gβγ, whereas the overexpression of engineered proteins that bind to Gβγ (and so prevent it from interacting with TRPM3) eliminates GPCR-mediated TRPM3 inhibition. Co-immunoprecipitation experiments demonstrate a direct interaction between Gβγ and TRPM3. Finally, Badheka et al. show that the flow of ions through TRPM3 channels is strongly and reversibly inhibited when the cell membrane is flushed with purified Gβγ, but not with Gα or boiled Gβγ. These elegant studies thus reveal that Gβγ inhibits TRPM3 by directly binding to the channel (Figure 1D).
Interesting mechanistic questions remain. Not all Gα subunits are inhibitory and it is unclear whether Gβγ-mediated TRPM3 regulation also occurs with heterotrimers containing other Gα subunits. Like GIRK channels, TRPM3 channels require a molecule called PIP2 in the membrane in order to open (Badheka et al., 2015; Tóth et al., 2015). Because some other Gα subunits (arbitrarily named Gαq subunits) lead to the localized depletion of PIP2 (Figure 1B), heterotrimers containing these subunits do not activate GIRK (Wang et al., 2014; Figure 1C). However, they should enhance inhibition of TRPM3 through GPCRs. Indeed, artificially expressing TRPM3 in human embryonic kidney cells enabled Badheka et al. to show that TRPM3 is readily inhibited through co-expressed Gαq-linked receptors even when the concentration of PIP2 inside cells is buffered. This demonstrates that Gβγ released from Gαq-containing heterotrimers can also inhibit TRPM3. However it remains to be established whether Gαq-linked GPCRs (or any other GPCRs for that matter) contribute to this process in any native cell.
Finally, in vivo experiments by all three groups highlight the practical relevance of the uncovered pathway for pain signaling in the peripheral nervous system: molecules that bind to and activate two types of GPCRs – the GABA-B and μ opioid receptors – significantly reduce TRPM3-dependent pain. Of note, the strongest peripheral painkillers currently available activate the μ opioid receptor, but cause severe adverse effects in the brain such as addiction, tolerance or respiratory depression. The new findings suggest that peripheral pain might be better treated by drugs that inhibit TRPM3 directly.
References
-
Transient receptor potential melastatin 3 is a phosphoinositide-dependent ion channelThe Journal of General Physiology 146:65–77.https://doi.org/10.1085/jgp.201411336
-
G proteins: transducers of receptor-generated signalsAnnual Review of Biochemistry 56:615–649.https://doi.org/10.1146/annurev.bi.56.070187.003151
-
Heterotrimeric G protein activation by G-protein-coupled receptorsNature Reviews Molecular Cell Biology 9:60–71.https://doi.org/10.1038/nrm2299
-
Regulation of the transient receptor potential channel TRPM3 by phosphoinositidesThe Journal of General Physiology 146:51–63.https://doi.org/10.1085/jgp.201411339
Article and author information
Author details
Publication history
Copyright
© 2017, Csanády
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 2,255
- views
-
- 207
- downloads
-
- 10
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Neuroscience
Transient receptor potential melastatin 3 (TRPM3) channels are activated by heat, and chemical ligands such as pregnenolone sulphate (PregS) and CIM0216. Here, we show that activation of receptors coupled to heterotrimeric Gi/o proteins inhibits TRPM3 channels. This inhibition was alleviated by co-expression of proteins that bind the βγ subunits of heterotrimeric G-proteins (Gβγ). Co-expression of Gβγ, but not constitutively active Gαi or Gαo, inhibited TRPM3 currents. TRPM3 co-immunoprecipitated with Gβ, and purified Gβγ proteins applied to excised inside-out patches inhibited TRPM3 currents, indicating a direct effect. Baclofen and somatostatin, agonists of Gi-coupled receptors, inhibited Ca2+ signals induced by PregS and CIM0216 in mouse dorsal root ganglion (DRG) neurons. The GABAB receptor agonist baclofen also inhibited inward currents induced by CIM0216 in DRG neurons, and nocifensive responses elicited by this TRPM3 agonist in mice. Our data uncover a novel signaling mechanism regulating TRPM3 channels.
-
- Neuroscience
Opioids, agonists of µ-opioid receptors (µORs), are the strongest pain killers clinically available. Their action includes a strong central component, which also causes important adverse effects. However, µORs are also found on the peripheral endings of nociceptors and their activation there produces meaningful analgesia. The cellular mechanisms downstream of peripheral µORs are not well understood. Here, we show in neurons of murine dorsal root ganglia that pro-nociceptive TRPM3 channels, present in the peripheral parts of nociceptors, are strongly inhibited by µOR activation, much more than other TRP channels in the same compartment, like TRPV1 and TRPA1. Inhibition of TRPM3 channels occurs via a short signaling cascade involving Gβγ proteins, which form a complex with TRPM3. Accordingly, activation of peripheral µORs in vivo strongly attenuates TRPM3-dependent pain. Our data establish TRPM3 inhibition as important consequence of peripheral µOR activation indicating that pharmacologically antagonizing TRPM3 may be a useful analgesic strategy.






