Dephosphorylation of the NPR2 guanylyl cyclase contributes to inhibition of bone growth by fibroblast growth factor
Figures
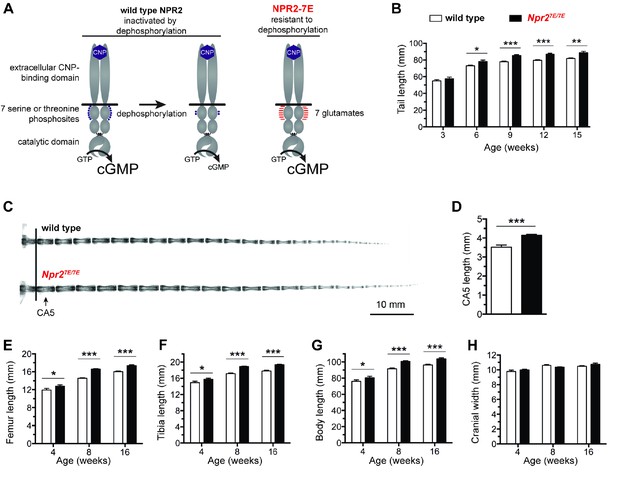
Mutation of the seven regulatory serines and threonines of NPR2 to glutamates increases bone length.
(A) Regulation of NPR2 activity by the phosphorylation state of the seven juxtamembrane serines and threonines on each NPR2 monomer. Purple dots represent phosphates on these serines and threonines. Red lines represent glutamates that are substituted for the serines and threonines. (B) Npr27E/7E mice have longer tails. Measurements were made from 17 to 19 live mice of each genotype. (C) X-rays of representative tails from mice euthanized at 18 weeks of age. (D) Increased CA5 vertebra length in Npr27E/7E mice compared with wild type, determined from x-ray measurements of tails at 18 weeks of age (10–13 mice of each genotype). (E,F) Longer femurs (E) and tibias (F) in Npr27E/7E vs wild type mice. (G) Increased body length in Npr27E/7E vs wild type mice. (H) No difference in cranial width comparing Npr27E/7E and wild type mice. For E–H, each bar indicates measurements from 10 to 31 mice that were euthanized at the indicated ages. All graphs show mean ±s.e.m. Data were analyzed by unpaired t-tests, with the Holm-Sidak correction for multiple comparisons where appropriate. T-tests rather than ANOVA were used because we were only interested in comparisons between genotypes at a given age, rather than comparisons across ages. *p≤0.05; **p≤0.01; ***p≤0.001.
-
Figure 1—source data 1
Numerical data for Figure 1B and D–H, listing individual measurments used to calculate the means and standard errors in the corresponding graphs.
Additional statistical information is also provided.
- https://doi.org/10.7554/eLife.31343.004
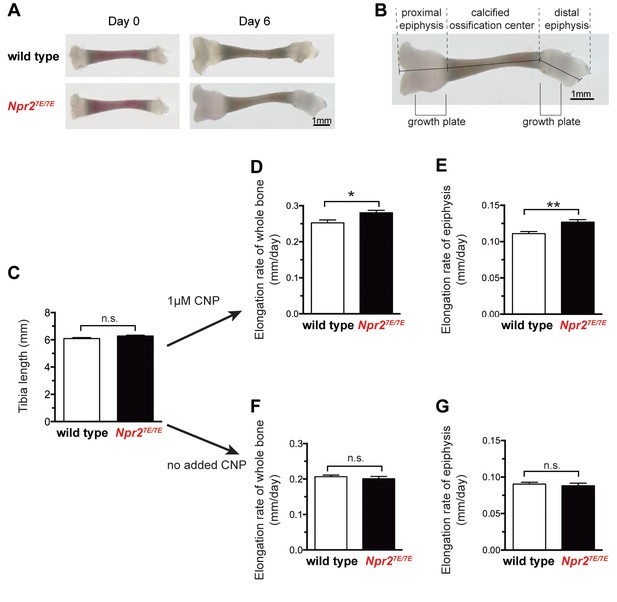
Npr27E/7E mice have longer bones due to a direct effect on the bone.
(A) Representative wild type and Npr27E/7E tibia from 3 to 4 day old mice, cultured in vitro for 6 days in the presence of 1 μM CNP. (B) Diagram showing the methods of measurement, and the position of the growth plates. Graphs C,D, and F depict measurements of the sum of the three indicated lengths: the proximal epiphysis (left), the calcified ossification center, and the distal epiphysis (right). Graphs E and G depict measurements of the length of the proximal epiphysis only. For graphs D-G, the elongation rate was determined by subtracting the length at day 0 from the length at day 6, and dividing by 6 days. (C) Tibia length immediately after dissection. 44 wild type and 79 Npr27E/7E bones were measured. (D,E) Rates of bone elongation in the presence of 1 μM CNP. (D) shows the elongation rate of the entire bone, and E shows the elongation rate of the proximal epiphysis. 21 wild type and 41 Npr27E/7E bones were measured. (F,G) Rates of bone elongation in the absence of added CNP. (F) shows the elongation rate of the entire bone, and G shows the elongation rate of the proximal epiphysis. 23 wild type and 38 Npr27E/7E bones were measured. Data were analyzed by unpaired t-tests. *p≤0.05; **p≤0.01.
-
Figure 2—source data 1
Numerical data for Figure 2C–G, listing individual measurments used to calculate the means and standard errors in the corresponding graphs.
Additional statistical information is also provided.
- https://doi.org/10.7554/eLife.31343.006
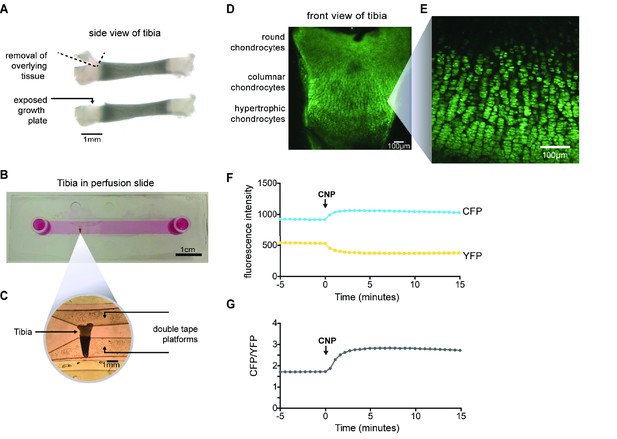
Imaging of CNP-stimulated cGMP production in chondrocytes within intact growth plates.
(A) Preparation of a tibia from a newborn mouse expressing the cGi500 sensor for cGMP. (B) Tibia in a perfusion slide in which medium could be flowed across the growth plate surface while imaging. (C) Higher magnification image of the tibia in the perfusion slide. (D) Confocal image of fluorescence of the entire growth plate. (E) Higher magnification image of the columnar/prehypertrophic region that was used for measurements of cGMP (from a different tibia from that shown in D). (F,G) Time courses of CFP and YFP emission intensities and the CFP/YFP ratio, before and after perfusion of 100 nM CNP.
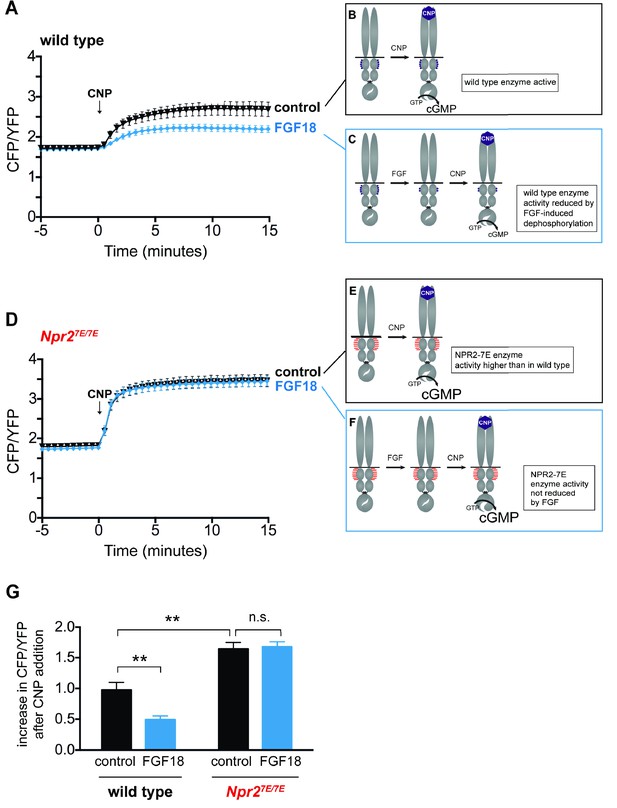
FGF reduces NPR2 guanylyl cyclase activity in growth plate chondrocytes from wild type mice, but not in those from Npr27E/7E mice.
Experiments were performed using tibias dissected from newborn mice (day 0–1) and used for imaging over the next 2 days. (A) Time course of CFP/YFP intensity ratios after 100 nM CNP was perfused across a wild type growth plate, comparing tibias that were preincubated for 80–140 min with a control solution (1 µg/ml heparin) (n = 11) or with FGF18 (0.5 μg/ml FGF18 +1 µg/ml heparin) (n = 14). CNP was present for the remainder of the recording period. An increase in the CFP/YFP ratio in response to CNP indicates cGMP production by NPR2. This and other graphs show mean ±s.e.m. (B,C) Schematic diagrams depicting the dephosphorylation of NPR2 in response to FGF, as demonstrated in rat chondrosarcoma cells (Robinson et al., 2017), and the effect on NPR2 guanylyl cyclase activity, in wild type tibia. Without FGF treatment, the seven regulatory serines and threonines on each monomer are mostly phosphorylated (depicted by five purple dots). After FGF treatment, the regulatory sites are mostly dephosphorylated (depicted by two purple dots). (D) Time course of CFP/YFP intensity ratios after 100 nM CNP was perfused across an Npr27E/7E growth plate, comparing tibias that were preincubated for 80–140 min with a control solution (1 µg/ml heparin) (n = 10) or with FGF18 (0.5 μg/ml FGF18 +1 µg/ml heparin) (n = 11). (E,F) Schematic diagrams depicting NPR2 with seven glutamates on each monomer (indicated by seven red lines) and the lack of effect of FGF on NPR2 enzyme activity. (G) Increases in CFP/YFP ratios after CNP perfusion, comparing data from wild type tibias with and without FGF pretreatment (from A) and data from Npr27E/7E tibias with and without FGF pretreatment (from D). Values were determined by subtracting the mean CFP/YFP ratio 0–5 min before CNP perfusion from the mean ratio 10–15 min after CNP perfusion. Data were analyzed by unpaired t-tests, with the Holm-Sidak correction for multiple comparisons. T-tests rather than ANOVA were used because we were only interested in a subset of comparisons that tested specific hypotheses. **p≤0.01.
-
Figure 4—source data 1
Numerical data for Figures 4A,D,G and Figure 4—figure supplement 1A and B, listing individual measurments used to calculate the means and standard errors in the corresponding graphs.
Additional statistical information is also provided.
- https://doi.org/10.7554/eLife.31343.011
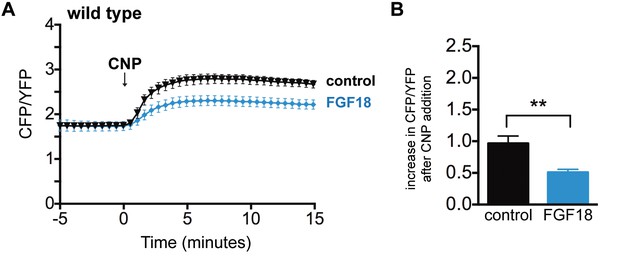
Inhibition of NPR2 activity by a 30–50 min preincubation with FGF.
(A) Time course of CFP/YFP intensity ratios after 100 nM CNP was perfused across a wild type growth plate, comparing tibias that were preincubated for 30–50 min with a control solution (1 µg/ml heparin) (n = 8) or with FGF18 (0.5 μg/ml FGF18 + 1 µg/ml heparin) (n = 8). (B) Increases in CFP/YFP ratios after CNP perfusion, comparing data from wild type tibias with and without FGF pretreatment (from A). Values were determined by subtracting the mean CFP/YFP ratio 0–5 min before CNP perfusion from the mean ratio 10–15 min after CNP perfusion. Data were analyzed by unpaired t-tests. **p≤0.01.
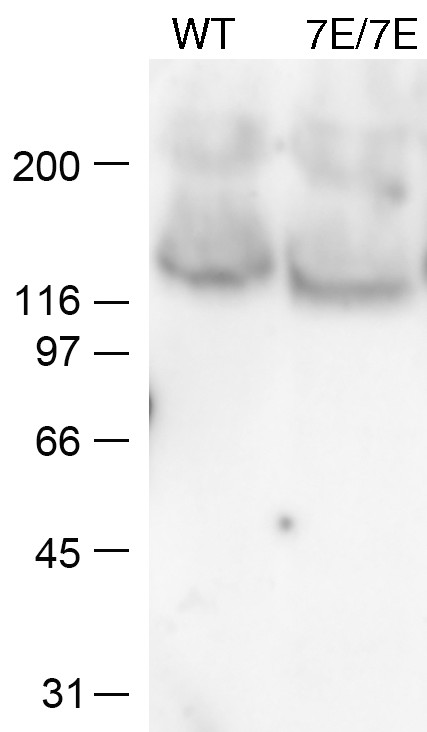
Western blot showing that wild type and Npr27E/7E epiphyses contain similar amounts of NPR2 protein (25 μg total protein loaded per lane).
Similar results were obtained in another independent experiment, confirming our previous finding that ovarian follicles from wild type and Npr27E/7E mice contain similar amounts of NPR2 protein (Shuhaibar et al., 2016).
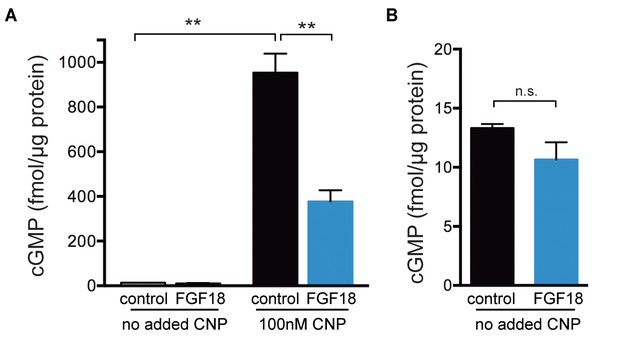
ELISA measurements of cGMP content of epiphyses of tibias from wild type newborn mice.
Tibias were collected and cultured as described for the cGi500 measurements. The proximal epiphyses were then slit to expose the growth plate (see Figure 3A) and the slit tibias were preincubated in a well of medium containing 1 µg/ml heparin with or without FGF-18 (0.5 μg/ml) for 80 min. After the preincubation ± FGF, the epiphysis regions were cut off and prepared for cGMP ELISA measurement as described in the Materials and Methods. Where indicated, the preincubation ±FGF was followed by a 15 min incubation in the same medium, but also including 100 nM CNP, mimicking the conditions used for the cGi500 measurements. (A) cGMP content of the epiphyses normalized to protein content. The graph shows the mean ±s.e.m. of measurements from three separate experiments. (B) The values from A for the no added CNP condition are replotted with an expanded y-axis. Data were analyzed by unpaired t-tests, with the Holm-Sidak correction for multiple comparisons. **p≤0.01.
-
Figure 5—source data 1
Numerical data for Figure 5A,B, listing individual measurments used to calculate the means and standard errors in the corresponding graphs.
Additional statistical information is also provided.
- https://doi.org/10.7554/eLife.31343.013
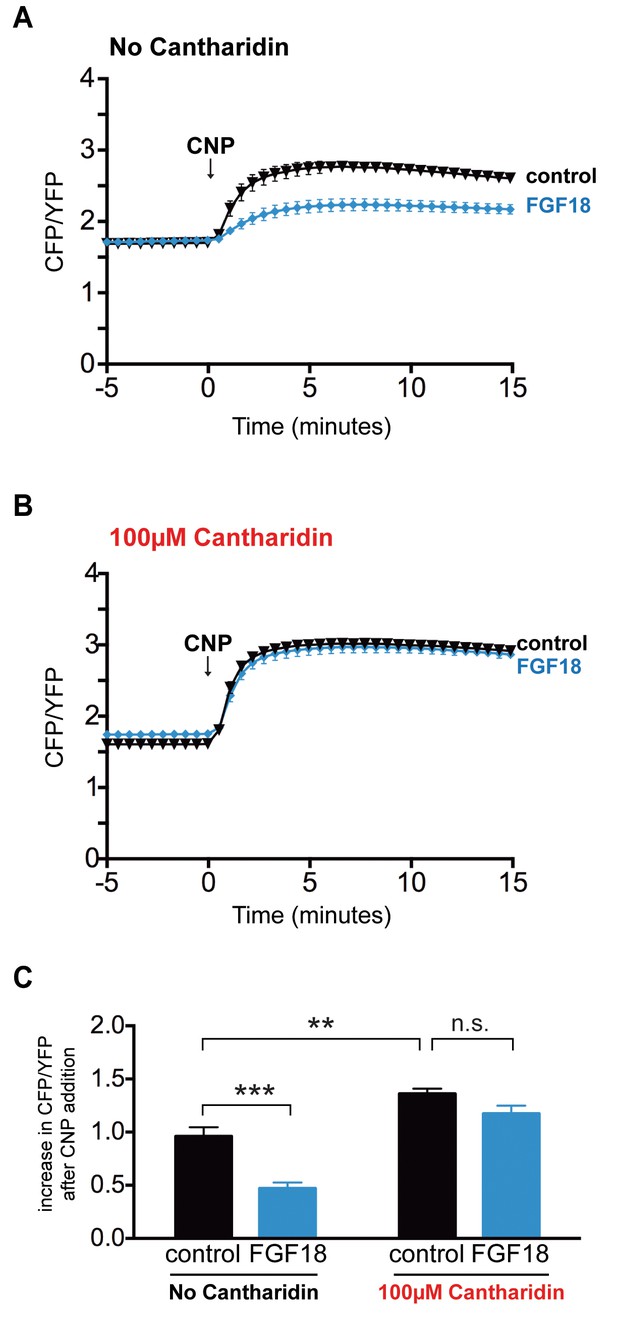
FGF does not reduce NPR2 guanylyl cyclase activity in growth plate chondrocytes of tibia that were pre-incubated with the PPP family phosphatase inhibitor cantharidin.
Experiments were performed using tibias from newborn mice (day 0–1) and used for experiments over the next 1–2 days. (A,B) Time courses of CFP/YFP intensity ratios after 100 nM CNP was perfused across wild type growth plates, comparing tibias that were pre-incubated for 60 min with cantharidin (100 μM + 0.2% DMSO) or a control solution (0.2% DMSO), followed by a 80–120 min incubation with FGF-18 (0.5 μg/ml +1 μg/ml heparin) or a control solution (1 μg/ml heparin). (A) No cantharidin (0.2% DMSO only) followed by FGF-18 (n = 10) or a control solution (n = 6). (B) 100 μM cantharidin followed by FGF-18 (n = 10) or a control solution (n = 8). (C) Increases in CFP/YFP ratios after CNP perfusion, comparing data from tibias with or without cantharidin and FGF-18 pre-treatments (data from A and B). Values were determined by subtracting the mean CFP/YFP ratio 0–5 min before CNP perfusion from the mean ratio 10–15 min after CNP perfusion. Data were analyzed by unpaired t-tests, with the Holm-Sidak correction for multiple comparisons. **p≤0.01; ***p≤0.001.
-
Figure 6—source data 1
Numerical data for Figure 6A–C, listing individual measurments used to calculate the means and standard errors in the corresponding graphs.
Additional statistical information is also provided.
- https://doi.org/10.7554/eLife.31343.015
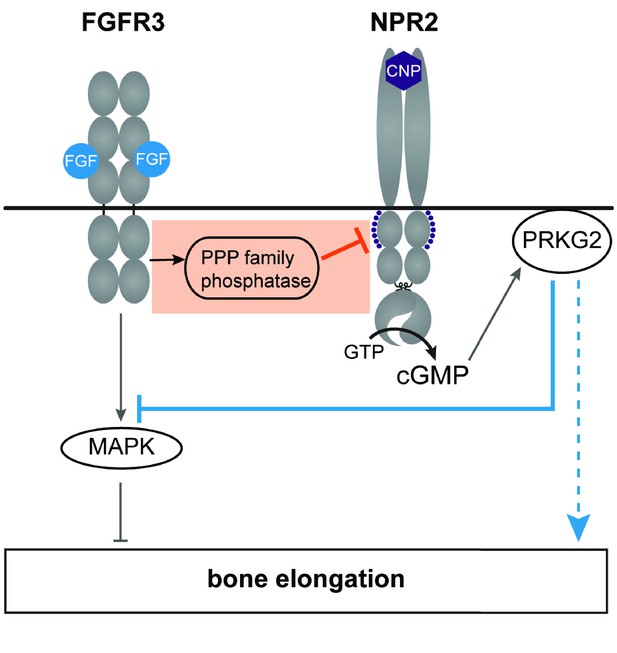
A working model of the crosstalk between NPR2 and FGFR3 signaling pathways in regulating bone elongation, emphasizing the dephosphorylation and inactivation of NPR2 in response to FGFR3 signaling (orange box).
This diagram shows only some aspects of FGFR3 signaling in the growth plate; see Karuppaiah et al. (2016) and Ornitz and Legeai-Mallet (2017) for current models showing other components as well. Our findings indicate that FGF activation of its receptor FGFR3 acts by way of a PPP family phosphatase to decrease the phosphorylation and activity of the NPR2 guanylyl cyclase, thus reducing production of cGMP. Cyclic GMP increases bone growth by activating the cGMP-dependent protein kinase PRKG2. FGFR3 also acts by way of the mitogen-activated protein kinase (MAPK) to oppose bone elongation. One consequence of PRKG2 kinase activation is to inhibit MAPK (solid blue line), with the net effect of increasing bone elongation. PRKG2 may act by other pathways as well, as depicted by the dotted blue line. See main text for references.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| genetic reagent (M. musculus) | Npr2-7E | PMID: 26522847 | ||
| genetic reagent (M. musculus) | cGi500 | PMID: 23801067 | ||
| antibody | Anti-NPR2 | Ter-Avetisyan et al. (2014) |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.31343.017





