A randomized feasibility trial comparing four antimalarial drug regimens to induce Plasmodium falciparum gametocytemia in the controlled human malaria infection model
Figures
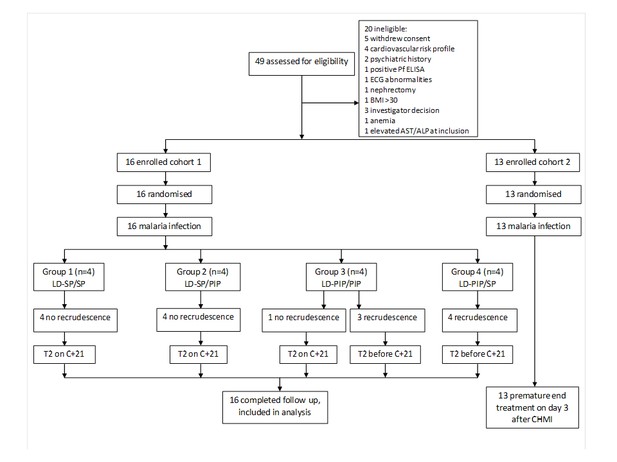
Trial profile.
ECG = electrocardiography, BMI = body mass index, AST = aspartate aminotransferase, ALP = alkaline phosphatase
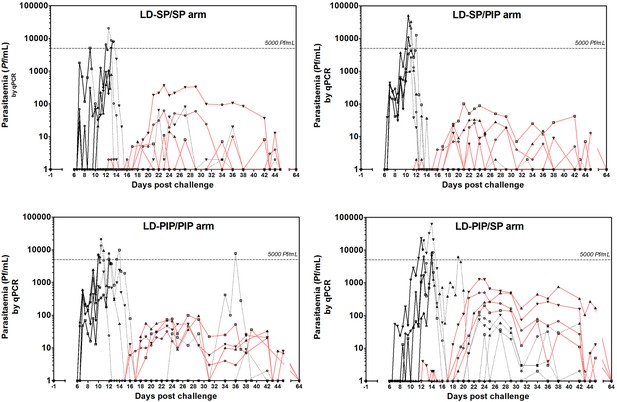
Asexual parasitemia and gametocytemia.
Black line represents 18S qPCR asexual parasitemia. Black dotted-line represents 18S qPCR after treatment 1. Red line represents Pfs25 qRT-PCR gametocytemia.
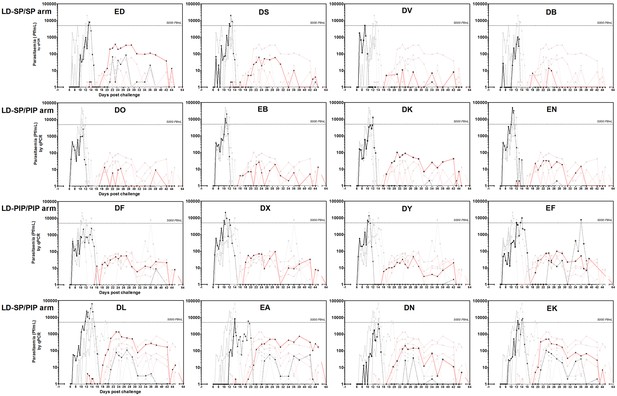
Asexual parasitemia and gametocytemia per study participant.
Black line represents 18S qPCR asexual parasitemia. Black dotted-line represents 18S qPCR after treatment 1. Red line represents Pfs25 qRT-PCR gametocytemia. Grey lines represent individual PCR curves of other participants of the same group.
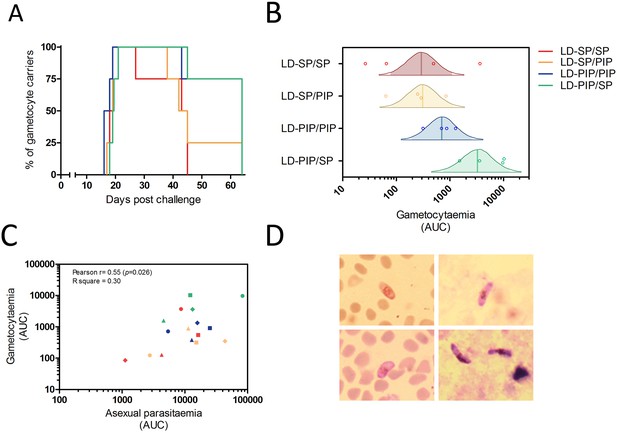
Gametocyte kinetics between study arms.
(A) Percentage gametocyte carriers between study arms (B) Estimated mean area under the curve for concentration of gametocytes per arm (Bayesian framework). The shaded area of each density curve represents the middle 95% percentiles (i.e. 2.5th to 97.5th percentiles) of the estimated mean AUC for a study arm; the density curve itself spans the middle 99% percentiles of the posterior; the posterior mean is indicated by the vertical solid line within each density plot. (C) Association of area under the curves of asexual parasitemia and gametocytemia. The different plotting shapes are the individual participants per group. (D) Thin- and thick- blood smears of concentrated gametocytes after magnetic cell sorting of blood samples from two individuals from LD-PIP/SP arm.
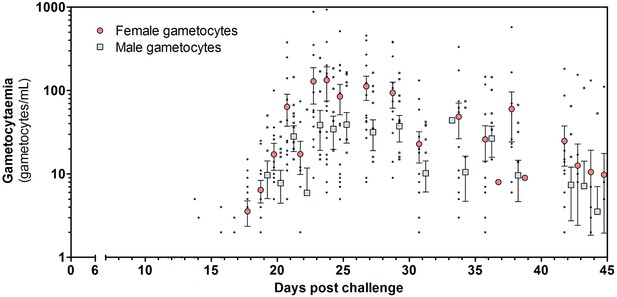
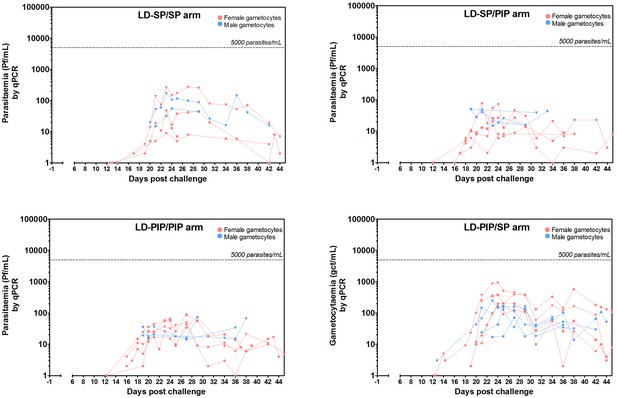
Total female and male gametocyte density of all participants.
Dots represent individual gametocyte data. Circles and squares represent mean and error (SEM) of gametocytes per timepoint.
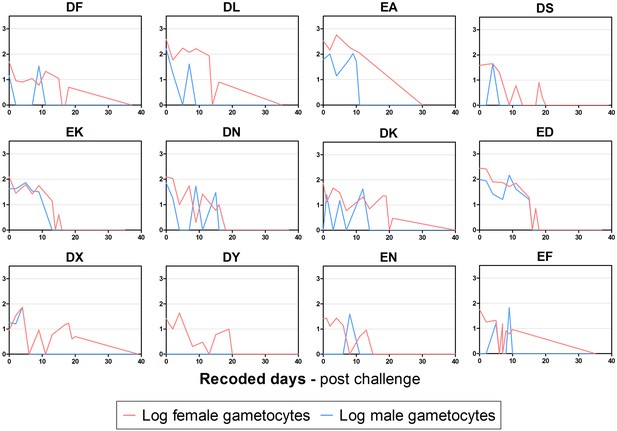
Female and male gametocyte clearance dynamics per participant included in analysis.
Curves are log gametocytes/mL. Recoded days are the days of gametocyte observations from 12 days after the last detection of asexual parasites until the end of study.
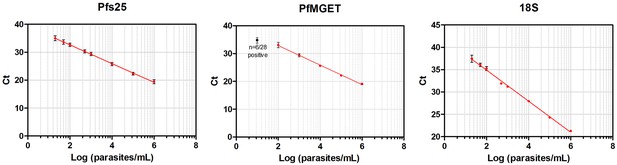
Standard curves of qRT-PCR and qPCR.
Standard curves (Mean, SD) obtained using 10-fold dilutions of cultured gametocytes. The highest concentration was enumerated by two independent expert microscopists. The mean and standard deviation of 54, 28, 72 replicates of the standard curve during the study was determined for the Pfs 25-, PfMGET, and 18S target genes, respectively. For PfMGET, six points starting from 106 pure male gametocytes/mL were measured. 101 was positive in 6/28 replicates (black dot).
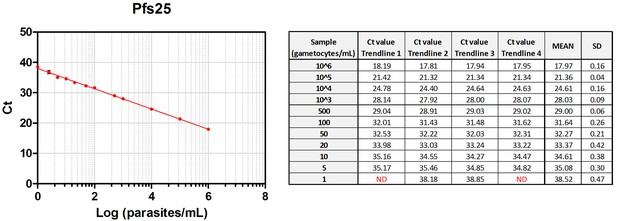
Standard curves of Pfs25 qRT-PCR – low-density trendlines.
Standard curves (Mean, SD) obtained using serial dilutions of cultured gametocytes including low-density trendlines to determine the limit of detection (LOD) and limit of quantification (LOQ) of the Pfs25 qRT-PCR.
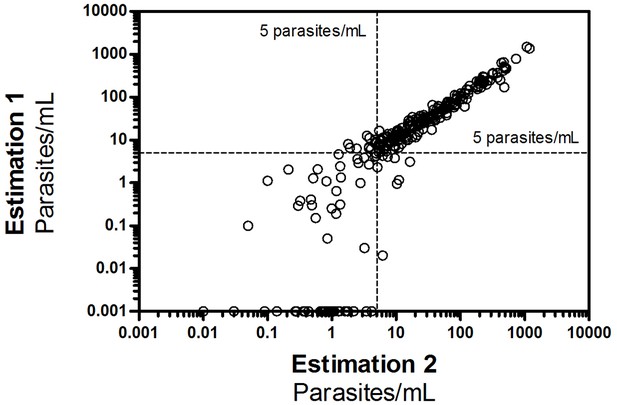
Correlation of duplo Pfs25 qRT-PCR measurements in all study samples.
All duplo- estimation data points of the study participants as measured by Pfs25 qRT PCR. All samples with ≥5 parasites/mL were duplo positive (190/190, 100%), and showed a correlation coefficient R2 of 0.94. Variation of samples < 5 parasites/mL was considerably larger and positivity could not be reliably estimated with 35/75 (47%) of samples that were positive in at least one qRT-PCR being single positives (R2 of 0.46).
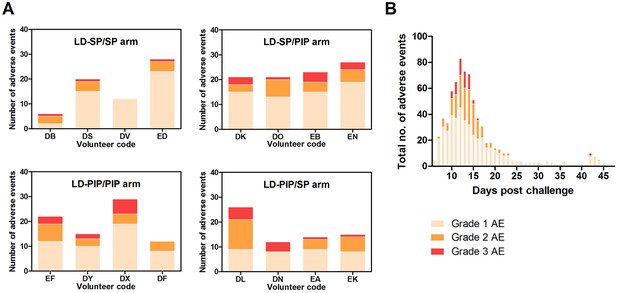
Adverse events.
(A) Adverse events per study arm (B) Total no. of adverse events and time course.
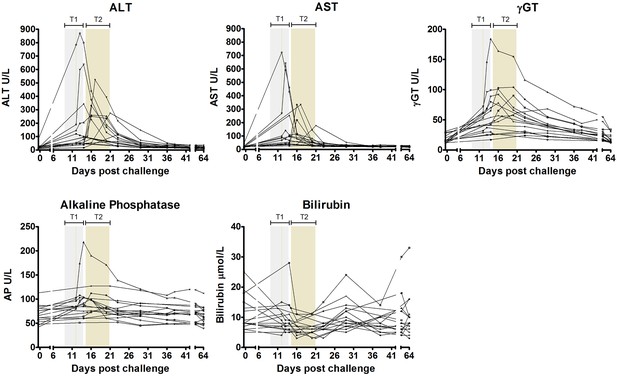
Liver function test derangements.
ALT = alanine aminotransferase, AST = aspartate aminotransferase, γGT = gamma glutamyl transferase, ALP = alkaline phosphatase, T1 = Treatment 1, T2 = Treatment 2.
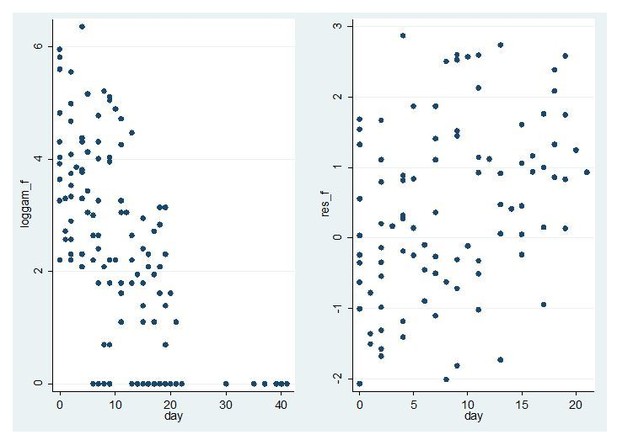
Model fit for CHMI-trans data female gametocytes.
Log female gametocyte densities at different days of follow-up (left) and residuals of non-zero female log gametocyte densities as a function of time of follow-up (right).
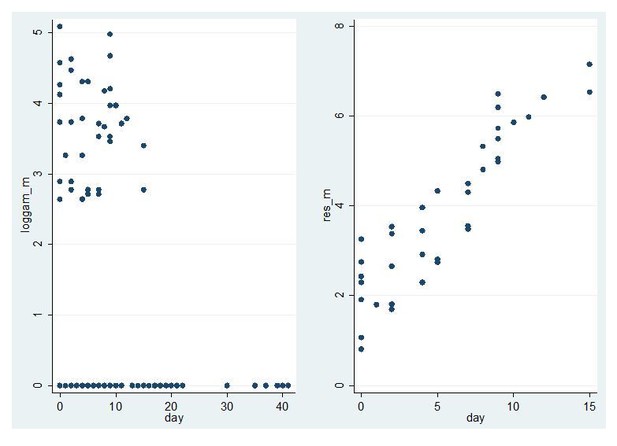
Model fit for CHMI-trans data male gametocytes.
Log male gametocyte densities at different days of follow-up (left) and residuals of non-zero male log gametocyte densities as a function of time of follow-up (right).
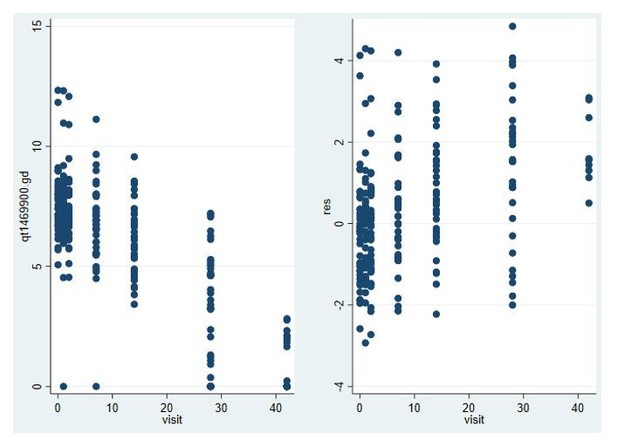
Model fit for male gametocytes in trial in Malian gametocyte carriers treated with non-gametocytocidal drugs dihydroartemisinin-piperaquine or amodiaquine plus sulfadoxine-pyrimethamine (NCT02831023).
Log male gametocyte densities at different days of follow-up (left) and residuals of non-zero male log gametocyte densities as a function of time of follow-up (right).
Tables
Baseline characteristics of the participants included in analysis.
| LD-SP/SP | LD-SP/PIP | LD-PIP/PIP | LD-PIP/SP | ||
|---|---|---|---|---|---|
| No. subjects | n = 4 | n = 4 | n = 4 | n = 4 | |
| Treatment 1 (T1) | Sulfadoxine-pyrimethamine 500 mg/25 mg | Sulfadoxine-pyrimethamine 500 mg/25 mg | Piperaquine 480 mg | Piperaquine 480 mg | |
| Treatment 2 (T2) | Sulfadoxine-pyrimethamine 1000 mg/50 mg | Piperaquine 960 mg | Piperaquine 960 mg | Sulfadoxine-pyrimethamine 1000 mg/50 mg | |
| Sex | |||||
| Male | n (%) | 2 (50%) | 0 (0%) | 1 (25%) | 1 (25%) |
| Female | n (%) | 2 (50%) | 4 (100%) | 3 (75%) | 3 (75%) |
| Age | Mean (range) | 24.5 (21–29) | 24 (21–28) | 21.5 (20–24) | 22.5 (20–27) |
| BMI (kg/m2) | Mean (range) | 21 (18–23) | 22 (19–25) | 24.5 (21–27) | 26.5 (24–29) |
-
Table 1—source data 1
Source data for Table 1.
- https://cdn.elifesciences.org/articles/31549/elife-31549-table1-data1-v2.xlsx
Treatment and parasitological data per study group.
| LD-SP/SP | LD-SP/PIP | LD-PIP/PIP | LD-PIP/SP | ||
|---|---|---|---|---|---|
| Time to T1 (days) | Median (range) | 13 (9.3–12.8) | 10.8 (0.8–11.8) | 10.3 (10.3–12.3) | 12.8 (12.3–14.3) |
| Time between T1-T2 (days) | Median (range) | 9.1 (7.7–11.7) | 10 (9.2–10.2) | 4.7 (2–10.7) | 2.5 (1.5–5.0) |
| Area under the curve (AUC)* | Median (range) | ||||
| Asexual | 6490 (1120–16337) | 13280 (2773–43777) | 14347 (5408–24898) | 12747 (4572–82973) | |
| Sexual | 280 (27–3640) | 271 (64–848) | 784 (316–1274) | 6624 (1515–10244) | |
| Peak parasite density (Pf/mL) | Median (range) | 6467 (1050–20261) | 16376 (2590–50210) | 11603 (2408–21565) | 8491 (3976–63113) |
| Peak gametocyte density (gct/mL) | Median (range) | 38 (11–368) | 30 (13–101) | 83 (46–99) | 627 (199–1285) |
| Day of gametocyte detection after infection (days) | Mean (SD) | 18.3 (1.0) | 18.5 (1.0) | 17.3 (1.5) | 19.4 (1.3) |
| Time to gametocyte detection relative to first asexual parasites† (days) | Mean (SD) | 10.5 (1.3) | 11.5 (1.0) | 10.1 (1.3) | 10.1 (1.2) |
| Proportion of days gametocyte positive (%)‡ | Mean (SD) | 27.4 (6.7) | 35.9 (7.6) | 51.4 (7.9) | 48.3 (8.1) |
| Duration gametocytemia§ (days) | Median (range) | 7.5 (1–24) | 6 (2–14) | 17 (12–25) | 24.5 (17–25) |
-
*The area under the curve (AUC) represents the total parasite exposure over time (asexual- or sexual parasite load).
†Time to gametocyte detection is calculated as the day of the detection of gametocytes (≥5 gct/mL) minus the day of first peak asexual parsitaemia.
-
‡The proportion of gametocyte positive days is calculated as all days with ≥5 gct/mL by Pfs25-qRT-PCR divided by all days where Pfs25 qRT-PCR was performed.
§Maximum number of consecutive days of Pfs25 qRT-PCR measured gametocytemia ≥5 gct/mL.
List of adverse events possibly or probably related to the trial.
| Adverse events | Total | LD-SP/SP | LD-SP/PIP | LD-PIP/PIP | LD-PIP/SP | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of subjects | Number of subjects | Number of episodes | Mean duration in days (SD) | Number of subjects | Number of episodes | Mean duration in days (SD) | Number of subjects | Number of episodes | Mean duration in days (SD) | Number of subjects | Number of episodes | Mean duration in days (SD) | |
| Fatigue, Malaise | 16 | 4 | 10 | 3.6 (4.5) | 4 | 15 | 2.0 (3.0) | 4 | 10 | 2.9 (1.1) | 4 | 6 | 6.8 (8.1) |
| Headache | 15 | 3 | 12 | 1.0 (1.5) | 4 | 25 | 1.2 (1.2) | 4 | 17 | 1.3 (1.2) | 4 | 21 | 1.6 (1.4)) |
| Fever | 15 | 4 | 9 | 0.4 (0.4) | 4 | 10 | 0.3 (0.4) | 3 | 11 | 0.4 (0.3) | 4 | 13 | 0.7 (0.4) |
| Nausea | 14 | 4 | 12 | 0.6 (0.8) | 4 | 15 | 1.1 (1.6) | 3 | 8 | 1.2 (1.5) | 3 | 10 | 0.7 (1.0) |
| Chills | 14 | 3 | 4 | 1.7 (1.0)) | 3 | 5 | 1.7 (2.0) | 4 | 10 | 1.2 (1.3) | 4 | 6 | 0.9 (1.1) |
| Myalgia | 11 | 3 | 5 | 3.2 (3.3) | 3 | 9 | 2.1 (1.9) | 3 | 5 | 1.2 (1.0) | 2 | 3 | 2.2 (2.6) |
| Abdominal pain | 10 | 2 | 5 | 0.3 (0.2) | 3 | 3 | 0.6 (0.9) | 2 | 8 | 1.1 (1.3) | 3 | 3 | 1.6 (2.4) |
| Pruritis | 6 | 2 | 3 | 0.6 (0.8) | 2 | 2 | 3.3 (0.5) | 1 | 2 | 0.3 (0.4) | 1 | 1 | 3.6 |
| Athralgia | 5 | 1 | 1 | 2.2 | 2 | 4 | 1.5 (1.8) | 0 | - | - | 2 | 2 | 5.1 (3.6) |
| Diarrhoea | 5 | 1 | 1 | 0.8 | 1 | 1 | 0.1 | 2 | 2 | 1.7 (2.1) | 1 | 1 | 4 |
| Diziness | 3 | 1 | 1 | 0.1 | 0 | - | - | 2 | 5 | 0.5 (0.7) | 0 | - | - |
| Reflux | 2 | 0 | - | - | 2 | 2 | 2.9 (1.8) | 0 | - | - | 0 | - | - |
| Pyrosis | 1 | 0 | - | - | 0 | - | - | 0 | - | - | 1 | 1 | 8.6 |
| Aspecific chest pain | 1 | 1 | 2 | 0.0 (0.0) | 0 | - | - | 0 | - | - | 0 | - | - |
| Syncope | 1 | 0 | - | - | 1 | 1 | 0.0 | 0 | - | - | 0 | - | - |
| Mouth ulcera | 1 | 1 | 1 | 10.0 | 0 | - | - | 0 | - | - | 0 | - | - |
| Grade 3 adverse events | |||||||||||||
| Total | 14 | 3 | 4 | 3 | 4 | ||||||||
| Headache | 8 | 0 | - | - | 2 | 2 | 0.3 (0.2) | 2 | 2 | 0.6 (0.1) | 4 | 4 | 1.1 (1.3) |
| Chills | 6 | 1 | 1 | 0.9 | 2 | 2 | 1.7 (2.0) | 2 | 2 | 0.3 (0.3) | 1 | 1 | 2.2 |
| Nausea | 5 | 1 | 1 | 0.1 | 2 | 3 | 0.3 (0.6) | 1 | 1 | 0.7 | 1 | 1 | 0 |
| Fever | 4 | 0 | - | - | 0 | - | - | 2 | 5 | 0.5 (0.4) | 2 | 5 | 0.7 (0.5) |
| Fatigue, malaise | 4 | 0 | - | - | 3 | 4 | 0.8 (0.4) | 1 | 1 | 2 | 0 | - | - |
| Abdominal pain | 1 | 1 | 1 | 0.5 | 0 | - | - | 0 | - | - | 0 | - | - |
Laboratory abnormalities per study arm.
| LD-SP/SP | LD-SP/PIP | LD-PIP/PIP | LD-PIP/SP | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N (% of total) of grade 1 | N (% of total) of grade 2 | N (% of total) of grade 3 | N (% of total) of grade 1 | N (% of total) of grade 2 | N (% of total) of grade 3 | N (% of total) of grade 1 | N (% of total) of grade 2 | N (% of total) of grade 3 | N (% of total) of grade 1 | N (% of total) of grade 2 | N (% of total) of grade 3 | |
| Any lab. abnormality | 15 (14) | 7 (7) | 2 (2) | 13 (12) | 10 (9 | 3 (3) | 16 (15) | 9 (8) | 2 (2) | 13 (12) | 8 (8) | 8 (8) |
| Decreased hemoglobin | 0 | 0 | 0 | 1 (14) | 2 (29) | 0 | 1 (14) | 1 (14) | 0 | 1 (14) | 1 (14) | 0 |
| Decreased WBC | 1 (8) | 3 (23) | 0 | 1 (8) | 2 (15) | 0 | 1 (8) | 2 (15) | 0 | 1 (8) | 2 (15) | 0 |
| Decreased neutrophils | 3 (23) | 1 (8) | 0 | 2 (15) | 0 | 0 | 3 (23) | 1 (8) | 0 | 3 (23) | 0 | 0 |
| Decreased lymphocytes | 3 (20) | 1 (7) | 0 | 1 (7) | 3 (20) | 0 | 3 (20) | 1 (7) | 0 | 1 (7) | 2 (13) | 0 |
| Decreased platelets | 3 (25) | 0 | 0 | 2 (17) | 0 | 0 | 4 (33) | 0 | 0 | 1 (8) | 2 (17) | 0 |
| Elevated ALT | 2 (13) | 1 (6) | 1 (6) | 2 (13) | 0 | 2 (13) | 1 (6) | 2 (13) | 1 (6) | 0 | 0 | 4 (25) |
| Elevated AST | 1 (7) | 1 (7) | 1 (7) | 2 (13) | 1 (7) | 1 (7) | 1 (7) | 2 (13) | 1 (7) | 0 | 0 | 4 (27) |
| Elevated yGT | 1 (11) | 0 | 0 | 1 (11) | 1 (11) | 0 | 2 (22) | 0 | 0 | 3 (33) | 1 (11) | 0 |
| Elevated ALP | 0 | 0 | 0 | 0 | 1 (33) | 0 | 0 | 0 | 0 | 2 (67) | 0 | 0 |
| Elevated total bilirubin | 1 (50) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1*(50) | 0 | 0 |
| Elevated creatinine | 0 | 0 | 0 | 1 (100) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Elevated BUN | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
-
Number of subjects with the highest grade reported for a laboratory abnormality. Grading based on WHO toxicity grading scale. No grade four abnormalities were reported. Lymphocytes (109/l) were graded based on grade 1: 0.9–0.6; grade 2: 0.3–0.5; grade 3:<0.3.
Liver function tests were graded based on grade 1: 1.1.–2.5X ULN, grade 2: 2.6–5.0x ULN, grade 3:>5.0X ULN. WBC, white blood count; ALT, alanine aminotransferase; AST, aspartate aminotransferase; yGT, glutamyl transpeptidase; ALP, alkaline phosphatase;
-
See Figure 6—figure supplement 1 for a detailed overview of liver function test abnormalities.
BUN, blood urea nitrogen. T1, treatment 1; T2, treatment 2.*Subject showed elevated total bilirubin at baseline.
Additional files
-
Source code 1
R codes used for Bayesian statistical analysis.
- https://cdn.elifesciences.org/articles/31549/elife-31549-code1-v2.zip
-
Source code 2
SAS code used for estimation of gametocyte half-life for gametocytes.
- https://cdn.elifesciences.org/articles/31549/elife-31549-code2-v2.docx
-
Supplementary file 1
Individual data of the participants included in analysis.
- https://cdn.elifesciences.org/articles/31549/elife-31549-supp1-v2.docx
-
Supplementary file 2
Selected P. falciparum gene targets and primers of qRT PCR assays.
- https://cdn.elifesciences.org/articles/31549/elife-31549-supp2-v2.docx
-
Supplementary file 3
Quality parameters of qRT PCR and qPCR.
The table shows for each target: limit of detection (LOD; defined as lowest pathogen concentration with reproducible detection); limit of quantification (LOQ; defined as lowest pathogen concentration where the CV was <5%), slope, efficiency (E), and the coefficient of correlation of combined trendlines (R2).
- https://cdn.elifesciences.org/articles/31549/elife-31549-supp3-v2.docx
-
Supplementary file 4
Clinical trial protocol.
- https://cdn.elifesciences.org/articles/31549/elife-31549-supp4-v2.pdf
-
Reporting standard 1
CONSORT extension for Pilot and Feasibility Trials Checklist.
- https://cdn.elifesciences.org/articles/31549/elife-31549-repstand1-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/31549/elife-31549-transrepform-v2.docx