Small molecule induced oligomerization, clustering and clathrin-independent endocytosis of the dopamine transporter
Figures
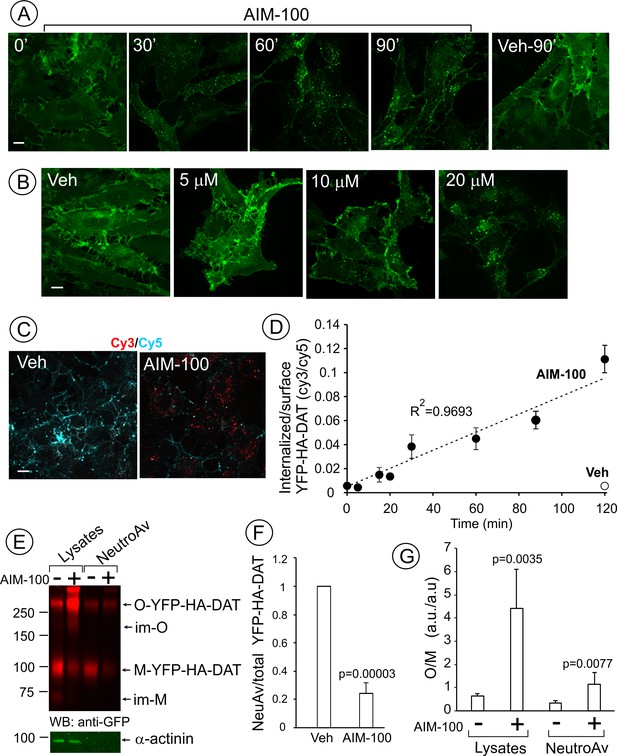
AIM-100 causes accumulation of YFP-HA-DAT in endosomes and enhances DAT oligomerization in PAE cells.
(A) Cells were incubated with vehicle (DMSO) or 20 µM AIM-100 for 0–90 min at 37°C, and 3D images were acquired from fixed cells through the 515 nm channel. Maximal intensity projections of all z-planes of representative images are shown. Scale bar, 10 µm. (B) Cells were incubated with vehicle (veh, DMSO) or 5–20 µM AIM-100 for 90 min at 37°C and fixed. Maximal intensity projections of all z-planes of representative YFP images are shown. Scale bar, 10 µm. (C) Cells were pre-incubated with HA11 for 1 hr at 37°C, and then further incubated with vehicle or 20 µM AIM-100 for 0–120 min at 37°C. After fixation, cultures were stained with secondary anti-mouse antibodies conjugated with Cy5 (surface HA-DAT), permeabilized with Triton X-100 and stained with secondary anti-mouse conjugated with Cy3 (internalized HA-DAT). 3D images were acquired through 488 (YFP, not shown), 561 (Cy3, red) and 640 nm (Cy5, cyan) channels. Individual confocal sections of 120 min time points are shown. (D) Time course of the Cy3/Cy5 ratio was calculated in HA11 uptake experiments exemplified in (C). Results are presented as mean values (±S.D., n = 3). R2, linearity coefficient. (E) Cells were incubated with vehicle or 20 µM AIM-100 for 2 hr at 37°C, and biotinylated at 4°C as described in ‘Methods’. An aliquot of lysates was incubated with NeutroAvidin (NeuAv) to pull-down biotinylated proteins. Lysates and pulldowns were resolved by SDS-PAGE electrophoresis and probed by western blotting with GFP and α-actinin (loading control) antibodies. O, oligomers; M, monomers; im-M, immature monomers; im-O, immature oligomers. (F) Quantification of the fraction of the biotinylated DAT relative to total DAT (lysates) in six independent experiments (mean ±S.D.). P values are ‘AIM-100’ compared to ‘vehicle’. (G) Quantification of the ratio of the amount of >250 kDa species (O, oligomers,) to the amount of 95 kDa YFP-HA-DAT species (M, monomers). Results are presented as mean values (±SD, n = 6). P values are for ‘AIM-100’ compared to ‘vehicle’.
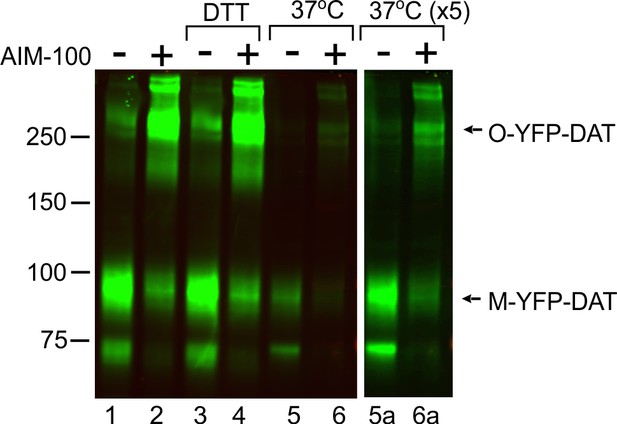
Biochemical properties of SDS-resistant DAT oligomers.
PAE/YFP-DAT cells were incubated with vehicle (DMSO) or 20 µM AIM-100 for 2 hr at 37°C, and lysed in conventional lysis buffer (TGH containing protease and phosphatase inhibitors) (lanes 1, 2, 5, 6, 5a and 6a) or the same TGH containing 10 mM DTT (iodacetamide was omitted) (DTT) (lanes 3 and 4). Samples in lanes 1–4 were denatured in the sample buffer for 5 min at 95°C, whereas samples in lanes 5, 6, 5a and 6a were denatured in the same buffer for 30 min at 37°C. The lysates were analyzed by immunoblotting with the GFP antibody. Because denaturation at 37°C is less efficient, the GFP antibody signal was weaker in lanes 5 and 6 than in other lanes. Therefore, additional image of these lanes, that was acquired using the 5-fold longer acquisition time, is presented (lanes 5a and 6a).
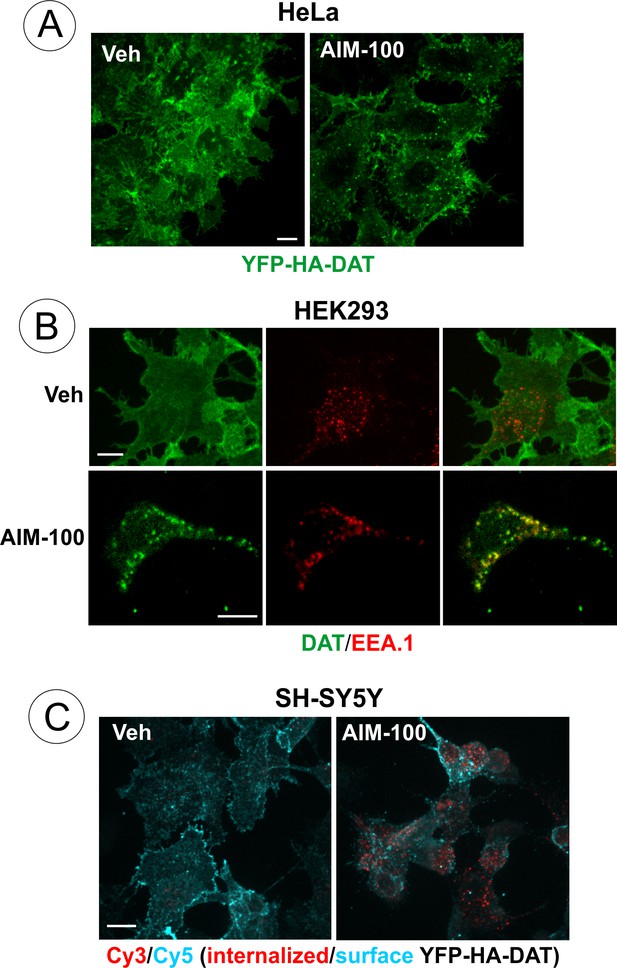
AIM-100-induced DAT endocytosis in HeLa, HEK293 and SH-SY5Y cells.
(A) HeLa cells stably expressing YFP-HA-DAT were incubated with vehicle (DMSO) or 20 µM AIM-100 for 90 min at 37°C, fixed and imaged through the 515 nm channel. Individual confocal sections are shown. (B) HEK293 cells stably expressing DAT were incubated with vehicle (DMSO) or 20 µM AIM-100 for 2 hr at 37°C, fixed, permeabilized and stained with rat monoclonal antibodies to DAT and mouse monoclonal antibody to EEA.1, followed by secondary anti-rat Cy5-conjugated (green) and anti-mouse Cy3-conjugated (red) antibodies. Individual confocal sections are shown. Arrows indicate examples of DAT localization in EEA.1 containing endosomes. (C) SH-SY5Y cells stably expressing YFP-HA-DAT were incubated with HA11 for 30 min at 37°C, and then further incubated with vehicle (DMSO) or 20 µM AIM-100 for 2 hr at 37°C. After fixation, cultures were stained with secondary anti-mouse antibodies conjugated with Cy5 (surface YFP-HA-DAT), permeabilized with Triton X-100 and stained with secondary anti-mouse conjugated with Cy3 (internalized YFP-HA-DAT). 3D images were acquired through 488 (YFP, not shown), 561 (Cy3, red) and 640 nm (Cy5, cyan) channels. Individual confocal sections are shown. Scale bars, 10 µm.
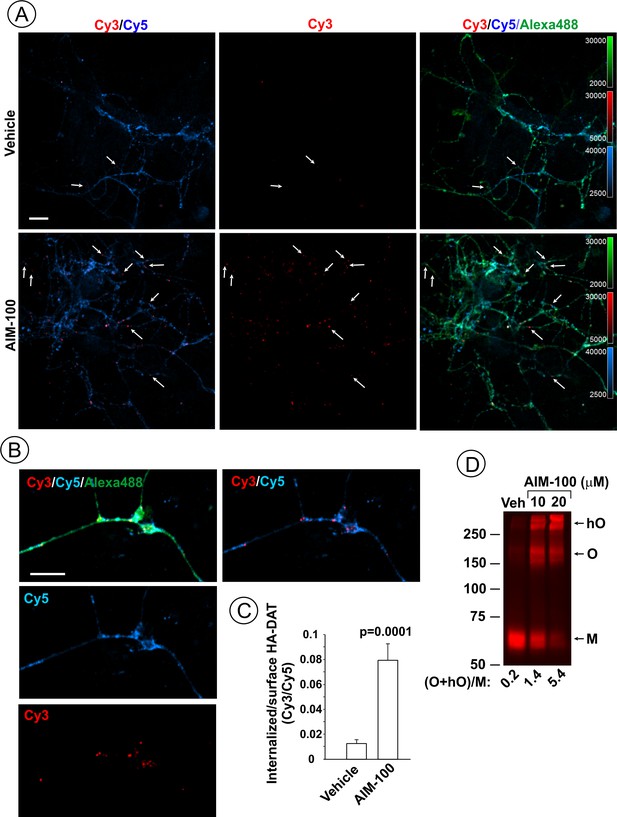
AIM-100 induces endogenous HA-DAT endocytosis and oligomerization in mouse dopaminergic neurons.
(A–C) Cultured post-natal mesencephalic neuronal cultures were pre-incubated with HA11 antibodies for 30 min at 37°C, and then further incubated with vehicle (DMSO) or 10 µM AIM-100 for 2 hr at 37°C. After fixation, cultures were stained with secondary anti-mouse antibodies conjugated with Cy5 (surface HA-DAT), permeabilized with Triton X-100 and incubated with rat-anti-DAT antibody, and then stained with secondary anti-mouse conjugated with Cy3 (internalized HA-DAT) and secondary anti-rat antibody conjugated with Alexa488 (total HA-DAT immunoreactivity). 3D images were acquired through 488 (Alexa488, green), 561 (Cy3, red) and 640 nm (Cy5, blue) channels. Individual confocal sections are presented. (A) Representative images. Arrows point on examples of Cy3 enriched puncta (endosomes) that localize in neuronal processes. (B) Representative images of endosomal HA-DAT (Cy3 fluorescence) in axonal varicosities in AIM-100-treated cells. Scale bars, 10 µm. (C) Quantification of Cy3/Cy5 ratio values from 3D images exemplified in (A). Results are presented as mean values (±S.E.M.; n = 13-15). P value is calculated for ‘AIM-100’ compared to ‘vehicle’. (D) Striatal synaptosomes prepared from adult HA-DAT mice were incubated with vehicle or 10–20 µM AIM-100 for 2 hr at 37°C. Lysates were resolved by electrophoresis and probed by western blotting with DAT antibody. M, monomers, O, oligomers; hO, high-order oligomers. O/M ratios are presented as a mean of the ratio values obtained from two mice.
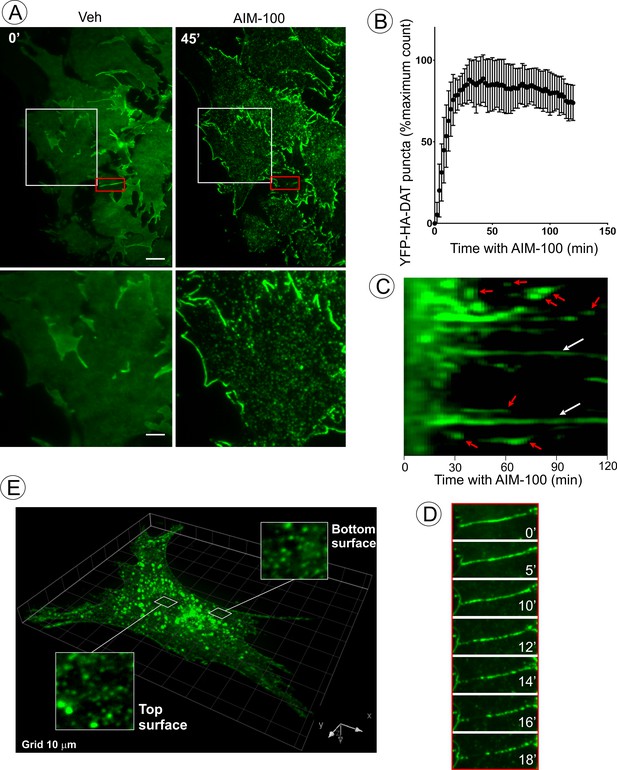
AIM-100 causes formation of DAT nanoclusters on the plasma membrane.
(A) Time-lapse TIR-FM imaging of PAE/YFP-HA-DAT cells. Individual time frames before and 45 min after addition of AIM-100 (20 µM) are shown. (See Figure 3—video 1). Insets below represent high magnification images of the regions marked by white rectangles. Scale bars are 5 µm in images and 2 µm in insets. (B) The number of YFP-HA-DAT puncta in cells treated with AIM-100 was calculated as described in ‘Materials and methods’ in nine time-lapse TIR-FM imaging sequences represented in (A). Mean values (±S.D.) of the percentage of maximum number of puncta per time frame are presented. (C) Representative kymographs time-series of YFP-HA-DAT imaging from randomly-selected region of cells presented in (A). Examples of stable clusters are indicated by white arrows. Examples of shorter-living clusters that may represent vesicle scission events are shown by red arrows. (D) High magnification time-lapse images (0–18 min with AIM-100) of the region marked by red rectangles in (A) demonstrating clustering of YFP-HA-DAT in a filopodium. (E) Cell were incubated with AIM-100 for 45 min as in (A), and 3D stack of confocal images were acquired. Insets show high magnification images of regions of the bottom and top of the cell (above nucleus) indicated by white rectangles.
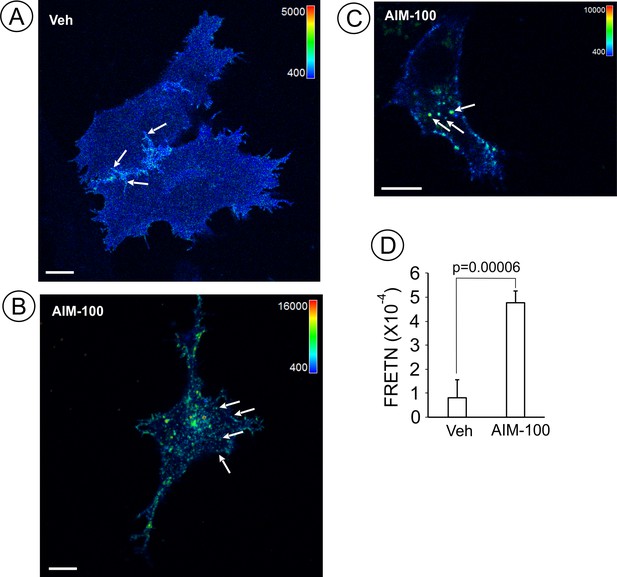
AIM-100 increases FRET between CFP- and YFP-DATs.
PAE cells stably co-expressing CFP-DAT and YFP-DAT were incubated with DMSO (veh) (A) or 20 µM AIM-100 (B–C) for 90 min at 37°C, and FRET imaging was performed at room temperature (20°C). FRETC images were calculated as described in ‘Methods’ and are presented as pseudocolor intensity-modulated images (FRETC/CFP). FRETC image in (B) is through the bottom cell membrane to visualize FRET in plasma membrane clusters, whereas an image in (C) is through the perinuclear area of the cell where many endosomes are located. Note different FRETC intensity scales in images of vehicle- and AIM-100-treated cells, reflecting increased FRETC signals in the latter cells. The intensity scales of the CFP fluorescence are the same in all three images. Arrows show examples of filopodia, plasma membrane clusters and endosomes that were used as sub-regions for calculations of FRETN values in (D). Scale bars, 10 µm. (D) Bar graph represents mean FRETN values of individual cell regions, structures and compartments measured in vehicle- and AIM-100-treated cells (±SEM; n = 21–37). A.l.u.f.i, arbitrary linear units of fluorescence intensity. P values were calculated for ‘AIM-100’ versus ‘vehicle’.
Time-lapse TIR-FM imaging of PAE/YFP-HA-DAT cells.
AIM-100 (20 µM) was added at time point ‘0’. Imaging intervals 2 min. Total time – 120 min.
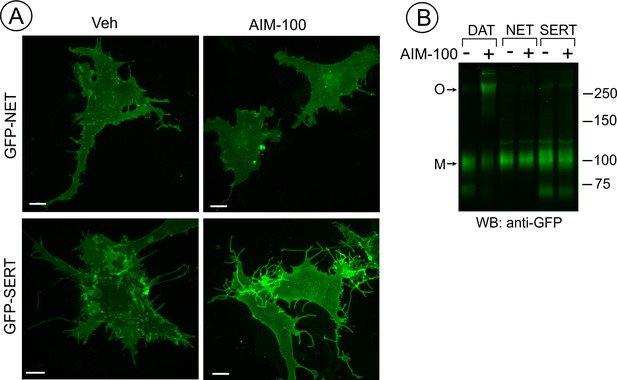
SERT and NET are not endocytosed and oligomerized in AIM-100 treated cells.
(A) PAE cells transiently or stably expressing GFP-SERT or GFP-NET were incubated with vehicle or 20 µM AIM-100 for 2 hr at 37°C. 3D images were acquired from living cells. Maximal intensity projections of z-planes of representative YFP images are shown. Scale bars, 10 µm. (B) PAE/cells stably expressing YFP-DAT, GFP-SERT or GFP-NET were incubated as in (A), and lysates were electrophoresed and probed by western blotting with the GFP antibody. M, monomers; O, oligomers.
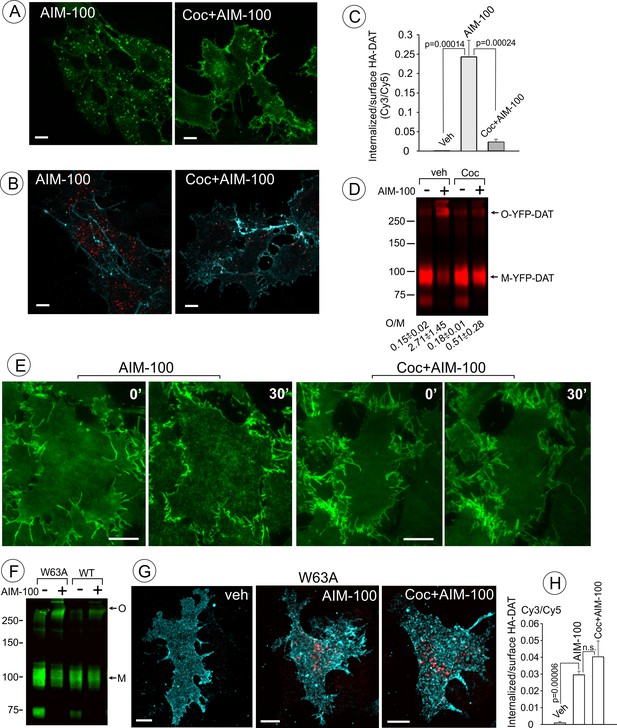
Cocaine inhibits AIM-100-induced endocytosis, oligomerization and clustering of DAT.
(A) PAE/YFP-HA-DAT cells were incubated without or with 4 µM cocaine for 30 min, and further incubated with vehicle (DMSO) or 20 µM AIM-100 for 1.5 hr at 37°C in the same media. 3D images were acquired from living cells through the 515 channel. Individual confocal sections through the middle of the cell are shown. (B) PAE/YFP-HA-DAT cells were incubated with HA11 for 30 min at 37°C, and then incubated with vehicle (PBS) or 10 µM cocaine (coc) for 10 min, and further incubated with vehicle (DMSO) or 20 µM AIM-100 in the presence of vehicle (PBS) or 1 µM cocaine for 2 hr at 37°C. After fixation, cultures were stained with secondary anti-mouse antibodies conjugated with Cy5 (surface HA-DAT), permeabilized with Triton X-100 and stained with secondary anti-mouse conjugated with Cy3 (internalized HA-DAT). 3D images were acquired through 488 (YFP, not shown), 561 (Cy3, red) and 640 nm (Cy5, cyan) channels. Individual confocal sections are presented. (C) Cy3/Cy5 ratios were calculated in experiments exemplified in (B). Results are presented as mean values of the ratio (±S.D.; n = 3-5). (D) PAE/YFP-DAT cells were incubated with vehicle or 10 µM cocaine for 10 min at 37°C, and further incubated with vehicle or 20 µM AIM-100 for 2 hr at 37°C, and lysates were analyzed by immunoblotting with the GFP antibody. Representative experiment is shown. M, monomers; O, oligomers. The mean values of the O/M ratios (±S.D.) (below the blot) were measured in three independent experiments. (E) Time-lapse TIR-FM imaging of PAE/YFP-HA-DAT cells that were pre-incubated or not with 10 µM cocaine for 5 min at 37°C, and then incubated with AIM-100 (20 µM) for 1 hr at 37°C. Individual time frames before (0’) and 30 min after addition of AIM-100 are shown. Scale bars are 10 µm. (F) PAE/YFP-DAT and PAE/W63A-YFP-HA-DAT cells were incubated with DMSO (veh) or AIM-100 as in (D), and lysates were analyzed by western blotting with the GFP antibody. Representative experiment of three independent experiments is shown. M, monomers; O, oligomers. (G) PAE/W63A-YFP-HA-DAT cells were incubated with HA11, cocaine and AIM-100 as in (B). After fixation, cultures were stained with secondary antibodies as in (B). 3D images were acquired through 488 (YFP, not shown), 561 (Cy3, red) and 640 nm (Cy5, cyan) channels. Maximum intensity projections of z-stack of confocal images are presented. Scale bars, 10 µm. (H) Cy3/Cy5 ratios were calculated in experiments exemplified in (E). Results are presented as mean values of the ratio (±SD, n = 4-5). n.s., p=0.146.
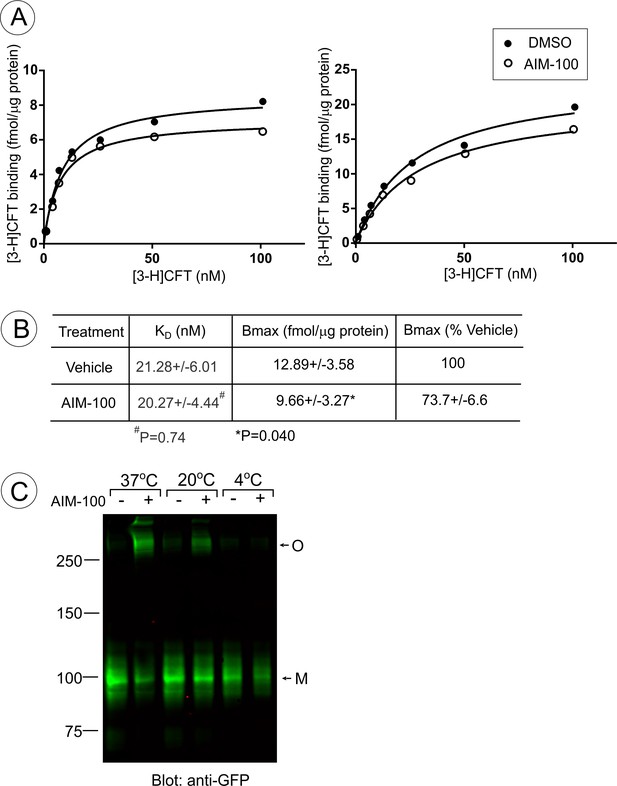
AIM-100 non-competitively inhibits CFT binding.
(A and B) PAE/YFP-DAT cells were incubated with the range of [3-H]CFT concentrations in F12 medium with DMSO (Veh) or 20 µM AIM-100 at 20°C for 30 min as described in ‘Methods’. (A) Examples of the concentration-dependence plots of [3-H]CFT binding. (B) Mean values of KD and Bmax (±S.E.M.) were determined in five independent experiments exemplified in (A). P values were calculated for ‘AIM-100’ versus ‘vehicle’ using paired two-tailed t-test. (C) PAE/YFP-DAT cells were incubated with vehicle (DMSO) or 20 µM AIM-100 for 2 hr at 4°C, 20°C or 37°C, lysed, and YFP-DAT was detected by blotting with the GFP antibody. M, monomers; O, oligomers.
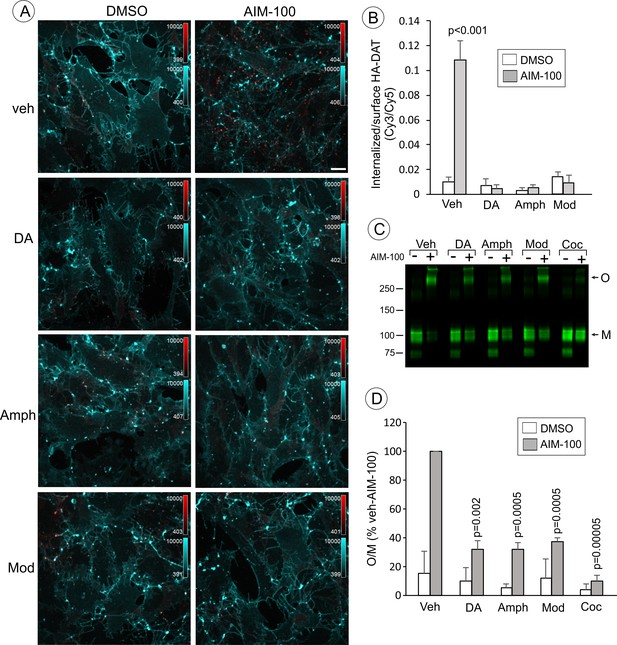
Dopamine, amphetamine and modafinil inhibit AIM-100-induced oligomerization and endocytosis of DAT.
(A) PAE/YFP-HA-DAT cells were incubated with HA11 for 30 min at 37°C, and then incubated with vehicle (water), 100 µM dopamine (DA), 100 µM amphetamine (Amph) or 20 µM modafinil (Mod) for 15 min. The cells were further incubated with vehicle (DMSO) or 20 µM AIM-100 for 2 hr at 37°C in the same media. After fixation, cultures were stained with secondary anti-mouse antibodies conjugated with Cy5 (surface HA-DAT), permeabilized with Triton X-100 and stained with secondary anti-mouse conjugated with Cy3 (internalized HA-DAT). 3D images were acquired through 488 (YFP, not shown), 561 (Cy3, red) and 640 nm (Cy5, cyan) channels. Maximum intensity projection images are presented. Scale bar. 10 µm. (B) Cy3/Cy5 ratios were calculated in experiments exemplified in (B). Results are presented as mean values of the ratio (±S.D.; n = 4–6). P values are calculated for ‘AIM-100’ against other experimental variants treated with AIM-100 and inhibitors/substrates. (C) PAE/YFP-HA-DAT cells were incubated with vehicle, 100 µM DA, 100 µM Amph, 20 µM Mod or 10 µM cocaine (Coc) for 10 min. The cells were further incubated with DMSO or 20 µM AIM-100 for 2 hr at 37°C in the same media, and lysates were analyzed by immunoblotting with the GFP antibody. Representative experiment is shown. M, monomers; O, oligomers. (D) The mean values of the O/M ratio (±S.D.) were calculated in three independent experiments exemplified in (C), and expressed as percent of the O/M value determined in cells incubated with AIM-100 but not with inhibitors or substrates (‘veh-AIM-100’). P values are calculated for cells treated with AIM-100 and inhibitors/substrates versus ‘veh-AIM-100’.
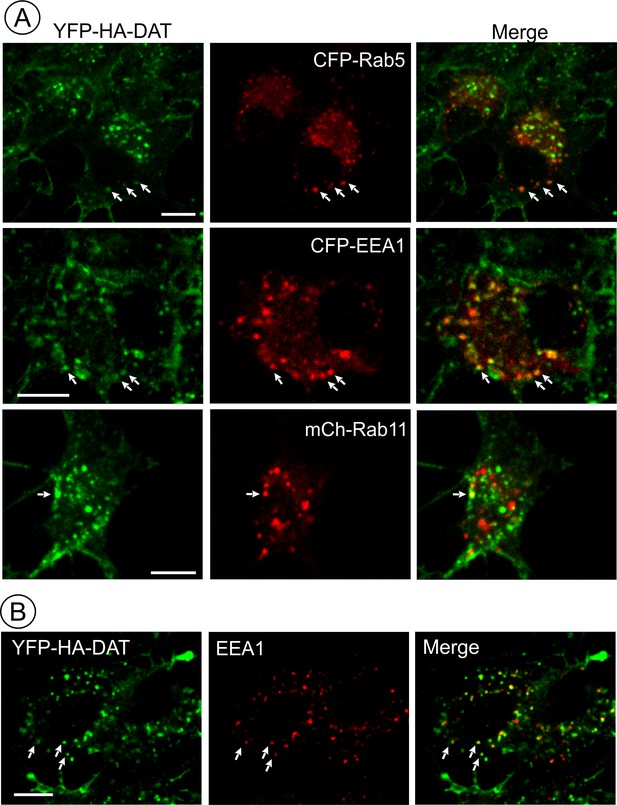
AIM-100 induced accumulation of YFP-HA-DAT in early but not recycling endosomes.
(A) PAE/YFP-HA-DAT cells were transfected with CFP-Rab5, CFP-EEA.1, or mCherry (mCh)-Rab11, and incubated with 20 µM AIM-100 for 1.5 hr (CFP-Rab5 and CFP-EEA.1) or 2 hr (mCherry-Rab11) at 37°C. 3D images were acquired from fixed cells through 445 (CFP, red), 515 (YFP, green) and 561 nm (mCherry, red) channels. Individual confocal sections are presented for all except mCherry-Rab7 images where maximum intensity projection of z-stack of confocal images are presented to better demonstrate low extent of colocalization. (B) PAE/YFP-HA-DAT cells were incubated with 20 µM AIM-100 for 1.5 hr at 37°C, fixed and stained with the EEA.1 antibody followed by Cy3-conjugated secondary. 3D images were acquired through 515 (YFP, green) and 561 nm (Cy3, red) channels. Individual confocal sections are presented. Scale bars, 10 µm.
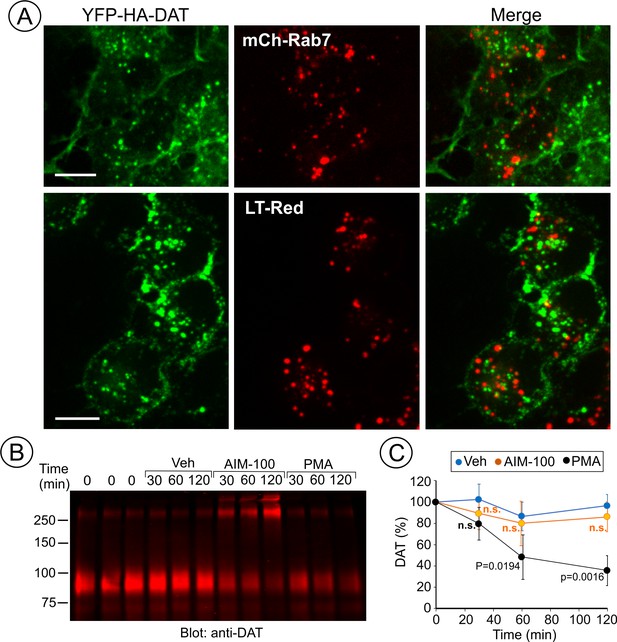
AIM-100 does not target DAT to late endosomes and lysosomes for degradation.
(A) PAE/YFP-HA-DAT cells were transfected with mCherry-Rab7 (mCh-Rab7), or preincubated with LysoTrackerRed (LT-Red) for 5 min, and then incubated with 20 µM AIM-100 for 90 min at 37°C. 3D images were acquired through 515 (YFP, green) and 561 nm (mCherry or LT-Red, red) filter channels from fixed (mCh-Rab7) or living cells (LT-Red). Maximum intensity projections of z-stack of confocal images are presented. Scale bars, 10 µm. (B) PAE/YFP-HA-DAT (western blot is shown) and PAE/YFP-DAT cells were pretreated with 50 µM cycloheximide and further maintained with this inhibitor during 0–120 min incubation with vehicle, 20 µM AIM-100 or 1 µM PMA. Cell lysates were probed for DAT. (C) On the right, the amount of YFP-HA-DAT and YFP-DAT (sum of oligomers and monomers) was quantified. Results are presented as mean values (-/+SD, n = 3) of percent to the amount of DAT at time ‘0’. P values are calculated versus ‘vehicle’.
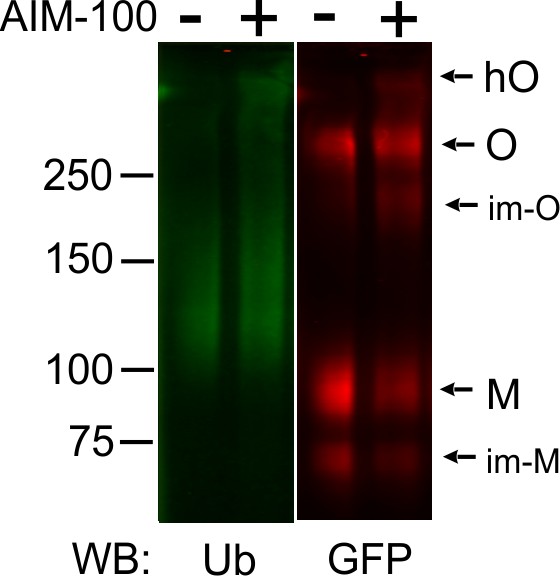
AIM-100 does not induce DAT ubiquitination.
PAE/YFP-HA-DAT cells grown in 12-well plates were incubated with vehicle (DMSO) or 20 µM AIM-100 for 2 hr at 37°C, lysed, and YFP-HA-DAT was immunoprecipitated using the DAT antibody. Immunoprecipitates were resolved by SDS-PAGE electrophoresis and probed by western blotting with ubiquitin (Ub) and GFP antibodies. O, oligomers; hO, high-order oligomers; M, monomers; im-M, immature monomers; im-O, immature oligomers.
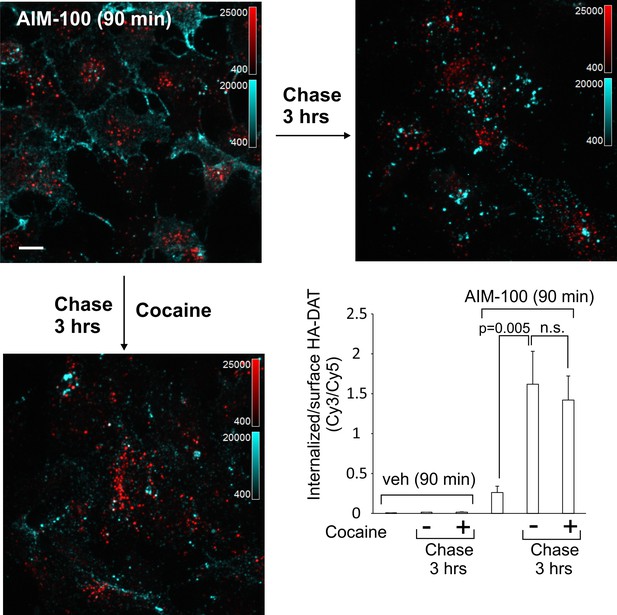
DAT internalized in AIM-100-treated cells is not recycled.
PAE/YFP-HA-DAT cells were incubated with HA11 for 45 min at 37°C, washed and further incubated with vehicle or AIM-100 for 90 min at 37°C. Cells were fixed, or further incubated in F12 medium with or without 5 µM cocaine for 3 hr at 37°C before fixation. After fixation, cultures were stained with secondary anti-mouse antibodies conjugated with Cy5 (surface HA-DAT), permeabilized with Triton X-100 and stained with secondary anti-mouse conjugated with Cy3 (internalized HA-DAT). 3D images were acquired through 488 (YFP, not shown), 561 (Cy3, red) and 640 nm (Cy5, cyan) channels. Maximum intensity projections of z-stack of confocal images are presented. Scale bars, 10 µm. Cy3/Cy5 ratios were calculated, and results are presented as mean values of the ratio (±SEM; n = 7-10). n.s., p=0.19.
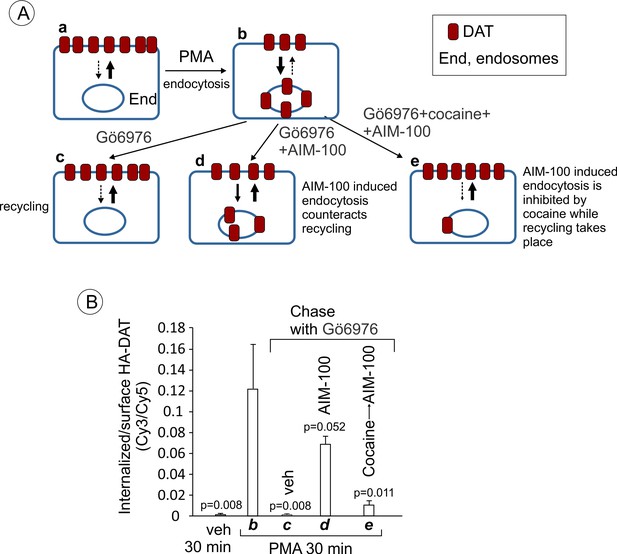
YFP-HA-DAT is recycled after PMA-induced internalization in the presence of AIM-100.
(A) Schematics of the recycling assay and the interpretation of the results of such assay in (B). Cells are incubated with DMSO (veh) (a) or PMA at 37°C for 30 min to internalize a fraction of DAT (b). At this point, most internalized DAT is in early endosomes, and thus is still capable of recycling (Sorkina et al., 2003; Sorkina et al., 2005). The cells were then chase-incubated in F12 medium with Gö6976 for 3 hr to block PKC activity and allow PKC-independent DAT trafficking (c). The chase incubation of PMA-treated cells with Gö6976 at 37°C results in the gradual re-distribution of DAT from endosomes to the plasma membrane (recycling), where it is accumulated in ruffles, cell edges and filopodia. In the presence of AIM-100 during the chase incubation, DAT continues to internalize in an AIM-100-depenent manner (d). If PMA- and Gö6976 treated cells are first preincubated with cocaine to prevent AIM-100-induced internalization of DAT and then chase-incubated with AIM-100, DAT translocated to the plasma membrane from endosomes, suggesting that AIM-100 does not inhibit the recycling of DAT from endosomes in this type of experiments. Widths of arrows roughly correspond to the predicted rate of internalization and recycling. Punctate arrows indicate a minimal rate. (B) PAE/YFP-HA-DAT cells were incubated with HA11 antibodies for 45 min at 37°C. Cells were then incubated with vehicle (DMSO) or 1 µM PMA at 37°C for 30 min (corresponding to A–b). The cells were then incubated in F12 medium with 5 µM Gö6976 for 30 min, followed by 2.5 hr at 37°C with 5 µM Gö6976 alone (A–c), together with 20 µM AIM-100 (A–d), or 10 µM cocaine and AIM-100 (30 min pre-incubation with cocaine and then AIM-100 for 2.5 hr) (A–e). After fixation, cultures were stained with secondary anti-mouse antibodies conjugated with Cy5 (surface HA-DAT), permeabilized with Triton X-100 and stained with secondary anti-mouse conjugated with Cy3 (internalized HA-DAT). 3D images were acquired through 488 (YFP), 561 (Cy3) and 640 nm (Cy5) channels. Cy3/Cy5 ratios were calculated, and results are presented as mean values of the ratio (±SEM; n = 3-6). All P values are calculated in comparison with condition ‘b’ (cells treated with PMA for 30 min).
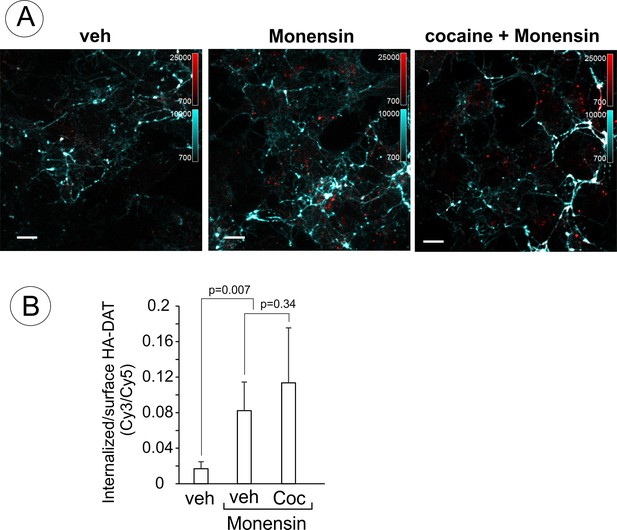
Endosomal accumulation of DAT in the presence of monensin is not inhibited by cocaine.
(A) PAE/YFP-HA-DAT cells were incubated with HA11 for 30 min at 37°C, and then incubated in F12 media with vesicle (water) or 10 µM cocaine for 10 min at 37°C. The cells were further incubated with vehicle (DMSO) or 25 µM monensin with or without 1 µM cocaine for 60 min at 37°C. After fixation, cultures were stained and imaged through 488 (YFP, not shown), 561 (Cy3, red) and 640 nm (Cy5, cyan) channels as in Figure 9. Individual confocal sections are shown. Scale bars, 10 µm. (B) The Cy3/Cy5 (intenalized/surface YFP-HA-DAT) ratio was calculated in HA11 uptake experiments exemplified in (C). Results are presented as mean values (±SD, n = 5-6).
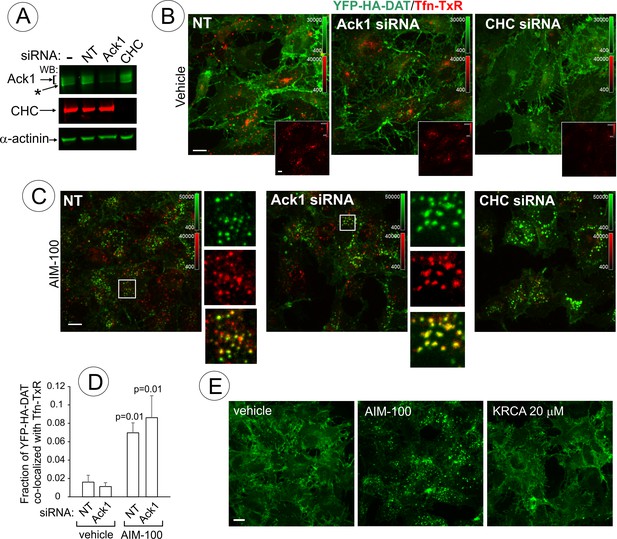
AIM-100-induced DAT endocytosis is independent of Ack1 and clathrin.
(A-D) PAE/YFP-HA-DAT cells were transfected twice with non-targeting (NT), clathrin heavy chain (CHC) or Ack1 siRNAs. After 3–5 days, the cells were lysed and tested for the efficiency of knock-downs (A) or used for microscopy imaging (B-D). Asterisk in (A) indicates non-specific band recognized by Ack1 antibodies. (B) Cells were incubated with vehicle (DMSO) for 2 hr at 37°C in the presence of 5 µg/ml Tfn-TxR. 3D images were acquired through 488 nm (YFP) and 561 nm (TxR) channels. Merged images of maximal intensity projections of 3D images are shown. Insets show corresponding Tfn-TxR images to better demonstrate inhibition of Tfn-TxR internalization in CHC-depleted cells. Corresponding full-size images are presented in Figure 10—figure supplement 1A. (C) Cells incubated with 20 µM AIM-100 for 2 hr at 37°C in the presence of 5 µg/ml Tfn-TxR, were imaged as in (B). Merged images of YFP-HA-DAT and Tfn-TxR are presented. Insets show high magnification of the regions marked by white rectangle to demonstrate co-localization of Tfn-TxR and YFP-HA-DAT. (D) Quantification of co-localization of YFP-HA-DAT with Tfn-TxR in experiments represented in (B) and (C). Results are presented as mean values of the fraction of YFP-HA-DAT co-localized with Tfn-TxR (±SD; n = 3-7). P values are ‘AIM-100’ compared to ‘vehicle’. (E) Cells were incubated with 20 µM AIM-100, vehicle (DMSO) or 20 µM KRCA-0008 for 2 hr at 37°C, fixed, and 3D images of YFP were acquired. Maximal intensity projection images are presented. Scale bars, 10 µm.
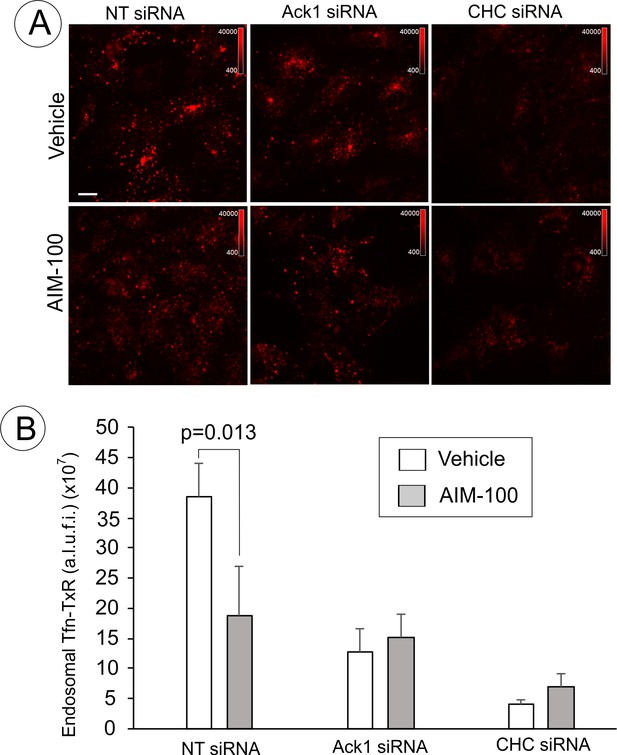
Endocytosis of Tfn-TxR in the presence of AIM-100 in cells depleted of Ack1 or CHC.
(A) Single-channel images of Tfn-TxR (561 filter channel; maximal intensity projections of 3D images) corresponding to the merged images shown in Figure 10B and C are presented. Scale bars, 10 µm. (B) Quantification of the amount of vesicular (endosomal) Tfn-TxR in 3D images from the experiment presented in (A). Bars represent mean value of arbitrary linear units of fluorescence intensity (a.l.u.f.i.) of Tfn-TxR (±SD; n = 3–4). P value is calculated for ‘vehicle’ versus ‘AIM-100’. The experiment is representative of at least five independent experiments.
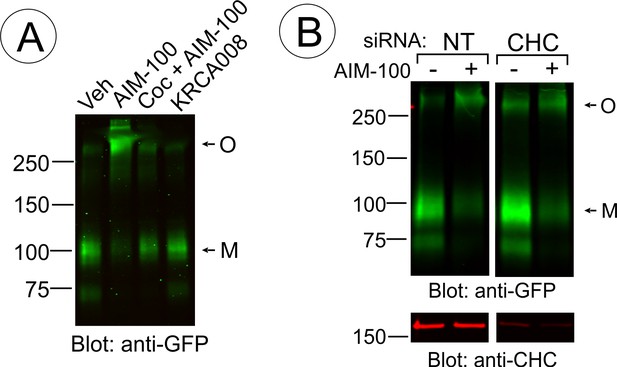
AIM-100-induced DAT oligomerization is not induced by KRCA-0008 and not affected by CHC knockdown.
(A) PAE/YFP-HA-DAT cells were incubated with vehicle (DMSO), 20 µM AIM-100, 10 µM cocaine plus 20 µM AIM-100 or 20 µM KRCA-0008 for 2 hr at 37°C, lysed, and YFP-HA-DAT was detected by blotting with the GFP antibody. M, monomers; O, oligomers. (B) PAE/YFP-HA-DAT cells were transfected twice with non-targeting (NT) or clathrin heavy chain (CHC). After 5 days, the cells were incubated with DMSO or AIM-100 as in (A), lysed, and probed for DAT oligomerization (GFP antibody) and the efficiency of knock-downs (CHC antibody). All lanes are from the same blot. M, monomers; O, oligomers.
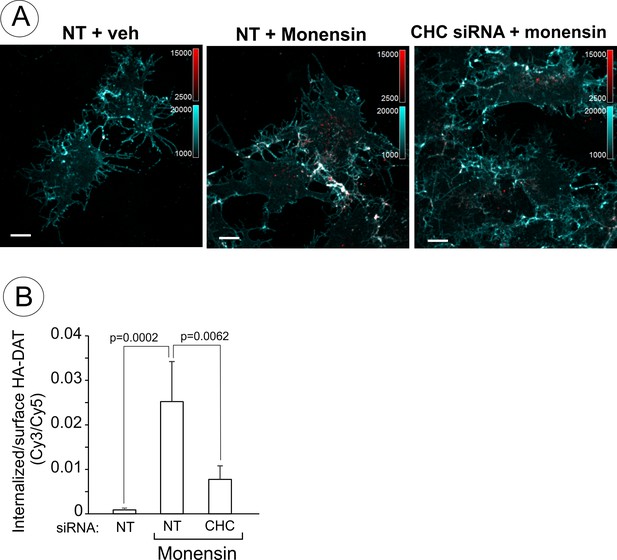
Endosomal accumulation of DAT in the presence of monensin is partically clathrin-dependent.
(A) PAE/YFP-HA-DAT cells were transfected with non-targeting (NT) or clathrin heavy chain (CHC) siRNAs. After 3 days, the cells were incubated with HA11 for 30 min at 37°C, and then further incubated with vehicle (DMSO) or 25 µM monensin for 60 min at 37°C. After fixation, cultures were stained with secondary anti-mouse antibodies conjugated with Cy5 (surface YFP-HA-DAT), permeabilized with Triton X-100 and stained with secondary anti-mouse conjugated with Cy3 (internalized YFP-HA-DAT). 3D images were acquired through 488 (YFP, not shown), 561 (Cy3, red) and 640 nm (Cy5, cyan) channels. Individual confocal sections are shown. Scale bars, 10 µm. (B) The Cy3/Cy5 ratio was calculated in HA11 uptake experiments exemplified in (A). Results are presented as mean values (±SD, n = 5-6).
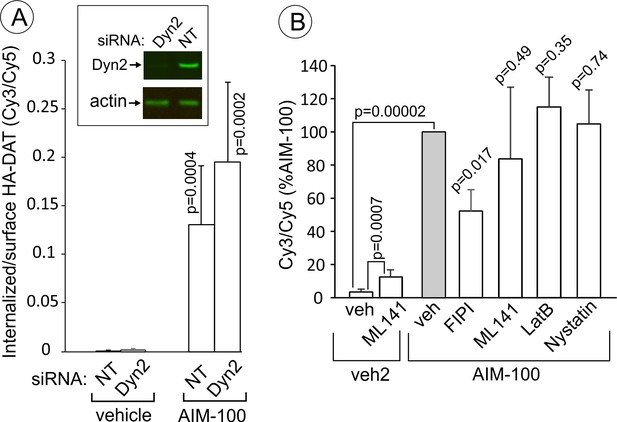
AIM-100-induced DAT endocytosis is independent on dynamin-2, actin cytoskeleton, cdc42 and cholesterol-rich lipid rafts.
(A) PAE/YFP-HA-DAT cells were transfected twice with non-targeting (NT) or dynamin-2 (Dyn2) siRNAs. After 3–5 days, the cells were lysed and tested for the efficiency of knock-downs or for microscopy imaging. Cells were incubated with HA11 for 45 min at 37°C, and then incubated with (vehicle) or 20 µM AIM-100 for 2 hr at 37°C. After fixation, cultures were stained with secondary anti-mouse antibodies conjugated with Cy5 (surface HA-DAT), permeabilized with Triton X-100 and stained with secondary anti-mouse conjugated with Cy3 (internalized HA-DAT). 3D images were acquired through 488 (YFP), 561 (Cy3) and 640 nm (Cy5) channels. Cy3/Cy5 ratios were calculated, and the results are presented as mean values of the ratio (±SD, n = 5-6). P values are for ‘AIM-100’ compared to ‘vehicle’. (B) Cells were incubated with HA11 for 1 hr at 37°C, and then incubated with vehicles corresponding to ML141 (10 µM), FIPI (1 µM), Latranculin B (LatB, 0.4 µM) or nystatin (25 µM) for 15 min at 37°C. Cells were then incubated with (DMSO; vehicle-2) or 20 µM AIM-100 for 2 hr at 37°C in the presence of inhibitors. After fixation, cultures were stained and imaged as in (A). Cy3/Cy5 ratios were calculated, and the results are presented as mean values of the ratio (±SD, n = 6) relative to this value in vehicle-pretreated/AIM-100-treated cells (grey bar). P values are for ‘AIM-100 plus an inhibitor’ versus ‘AIM-100 plus vehicle’, unless indicated otherwise on the graph.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Dopamine trasporter (Homo sapiens) | human DAT | n/a | Q01959 | |
| Dopamine transporter (Mus musculus) | mouse DAT | n/a | Q61327 | |
| Genetic reagent | Mouse transgenic knock-in HA-DAT | Rao et al., 2012 (available from Jackson lab) | B6.Cg-Sklca3tm1.1Asor/J; Stock #029381 | |
| Recombinant DNA reagent | YFP-HA-DAT | Sorkina et al., 2006 | Addgene #90244 | |
| Recombinant DNA reagent | W63A/YFP-HA-DAT | Sorkina et al., 2009 | Addgene #90946 | |
| Recombinant DNA reagent | YFP-DAT | Sorkina et al., 2003 | Addgene #90228 | |
| Antibody | HA11 (mouse monoclonal) | BioLegend | clone 16B12 (MMS101P) RRID:AB_291261 | |
| Antibody | DAT (rat monoclonal) | EMD Millipore | MAB369; RRID:AB_2190413 | |
| Antibody | ubiquitin (rabbit polyclonal) | Sigma-Aldrich | U5379; RRID:AB_477667 | |
| Antibody | EEA1 (mouse monoclonal) | BD Biosciences | BDB610457; RRID:AB_397830 | |
| Antibody | CHC (rabbit polyclonal) | Abcam | ab21679; RRID:AB_2083165 | |
| Antibody | GFP (rabbit polyclonal) | Abcam | ab290; RRID:AB_303395 | |
| Antibody | Ack1 (mouse monoclonal) | Santa Cruz | A-11; sc-28336; RRID:AB_626629 | |
| Antibody | dynamin-2 (rabbit polyclonal) | ABR ((Thermo Fisher Scientific)) | Cat # PA1-661; RRID:AB_2293040 | |
| Antibody | GFP (mouse monoclonal) | Clontech | JL8; Cat# 632380; RRID:AB_10013427 | |
| Antibody | Cy5 (donkey-anti-mouse) | Jackson Immuno Research | 715-175-151; RRID:AB_2340820) | 1:50 in antibody uptake assay |
| Antibody | Cy3 (donkey anti-mouse) | Jackson Immuno Research | 715-165-151; RRID:AB_2315777 | 1:500 in antibody uptake assay |
| Antibody | Alexa488 (donkey anti-rat) | Jackson Immuno Research | 712-545-153; RRID:AB_2340684 | 1:500 |
| Antibody | IRDye-800 (goat anti mouse) | LI-COR | 926–32210; RRID:AB_621842 | 1:20000 |
| Antibody | IRDye-680 (goat anti mouse) | LI-COR | 926–32220; RRID:AB_621840 | 1:20000 |
| Chemical compound, drug | Tfn-TxR (transferrin-Texas Red) | Invitrogen | Cat #T2875 | |
| Chemical compound, drug | LT-Red (LysoTrackerRed) | Invitrogen | Cat# L7528 | |
| Chemical compound, drug | AIM-100 | EMD MilliporeSigma | Cat#104833 | |
| Chemical compound, drug | KRCA-0008 | EMD MilliporeSigma | Cat# SML1304 | |
| Chemical compound, drug | PMA | EMD MilliporeSigma | Cat# P8139 | |
| Chemical compound, drug | Go6976 | EMD MilliporeSigma | Cat# 365250 | |
| Chemical compound, drug | Latranculin B | EMD MilliporeSigma | 428020 | |
| Transfected construct | siRNA to Ack1 | Integrated DNA Technologies Inc. | Cat# 87793753; 87793756 | |
| Transfected construct | siRNA to CHC | Thermo Fisher Scientific | Sorkina et al., 2005 | |
| Cell line (human) | SH-SY5Y | ATCC | RRID:CVCL_0019 | |
| Cell line (human) | HEK293T | ATCC | RRID:CVCL_0063 | |
| Cell line (human) | HeLa | ATCC | RRID:CVCL_0030 | |
| Cell line (porcine) | PAE | Westermark et al., 1990 | PMID: 2153283 | |
| Software | SlideBook6 | Intelligent Imaging Innovations, Inc. | ||
| Software | Odyssey Application Software 3.0 | Li-COR, Inc. |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.32293.024





