Pupillometry reveals perceptual differences that are tightly linked to autistic traits in typical adults
Figures
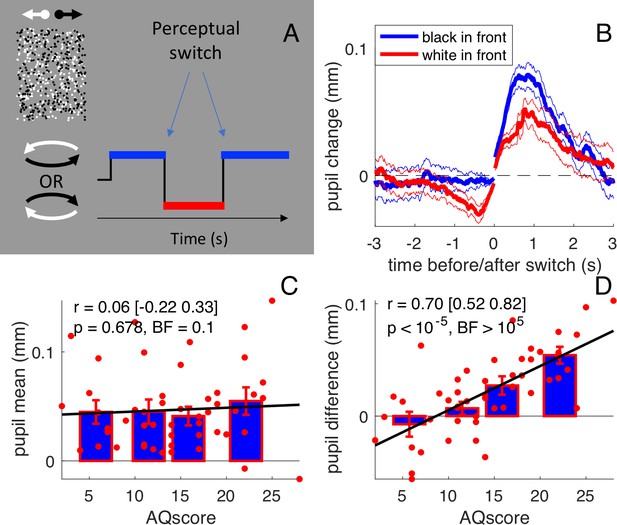
Stimulus and results of the main experiment: free viewing of the bistable cylinder.
(A) Participants (N = 50) viewed two overlapping fields of dots, which created the illusion of a cylinder rotating in depth with ambiguous rotation direction. Perception oscillated between black or white dots in front: there were two types of perceptual phases. (B) Pupil size recordings were aligned to the switch of each type of perceptual phase (corresponding to zero time), from which the average pupil size in the 150 ms immediately following or preceding the switch was subtracted. Pupils dilated after the switch and constricted before it, to a different extent depending on whether perception switched to anticlockwise (implying that the black dots in front) or clockwise (white dots in front). Thick lines give averages and thin lines show 1 s.e.m. across subjects. (C) Pupil dilation after the switch (‘general pupil dilation’ averaged across both types of perceptual phases, over the 0:1 s interval) does not correlate with AQ scores. Text insets report Pearson’s Rho values, the 95% confidence interval, and associated p-values and Bayes Factor (conventionally, the latter value indicates strong evidence in favor of the null hypothesis when smaller than 0.3, and strong evidence in favor of the alternative hypothesis when larger than 3). Bars show averages and s.e.m. for the quartiles of the AQ scores distribution and thick lines show the linear fit through the data. (D) The difference between pupil traces during the two types of perceptual phases (averaged over the intervals −1:0 and 0:1 s), that is the ‘luminance-dependent’ pupil modulation, correlates with subjects’ AQ score. All conventions as in C.
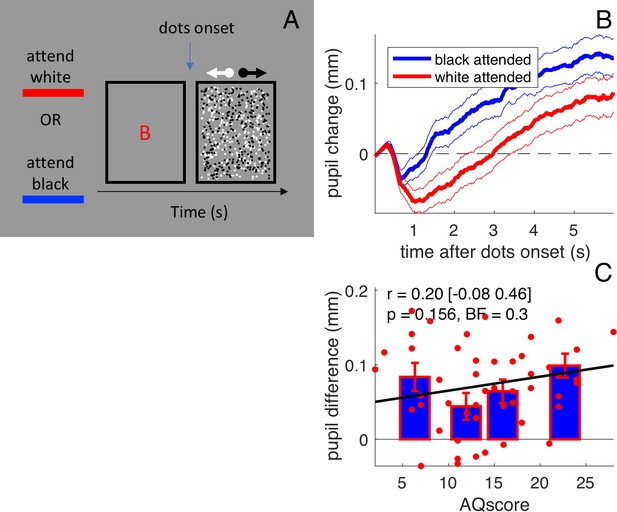
Stimulus and results of forcing attention to one surface.
(A) Participants (N=50) viewed the same two overlapping fields of dots as in the main experiment, but we presented them briefly (6 sec) and preceded them with a cue letter that instructed subjects to which surface they should attend. (B) Pupil size recordings were aligned to the stimulus onset and the average pupil size in the preceding 150 ms was subtracted. Pupils dilated over the course of the stimulus, to a different extent depending on which surface was cued (more dilated when the dark surface was attended). Thick lines give averages and thin lines show 1 s.e.m. across subjects. (C) The pupil difference between trials where the black or the white surface was attended (averaged over the [1:3]s interval) does not correlate with AQ scores. All conventions as in Figure 1.
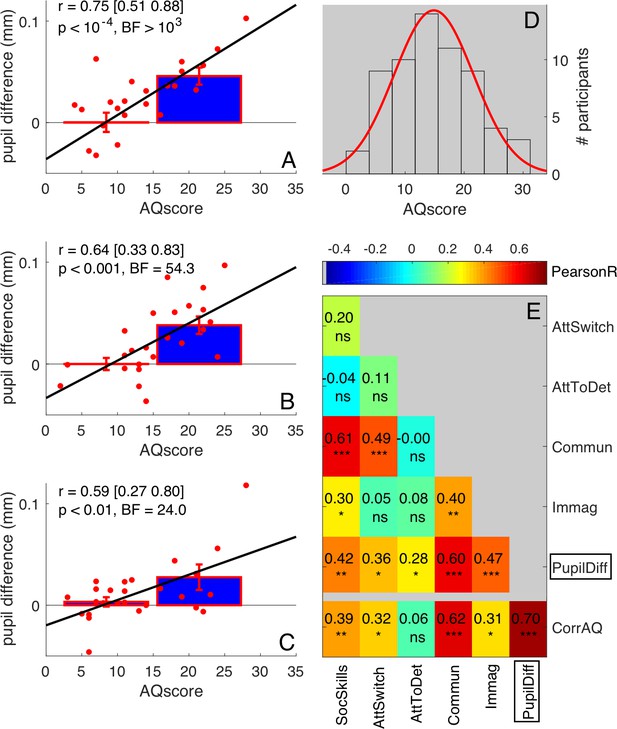
Robustness of correlations between pupil difference and AQ scores.
(A–C) Correlations of the signed amplitude of pupil difference (or ‘luminance-dependent’ pupil modulation) with AQ scores separately for our three self-replications: two sessions of the main experiment tested on different subsamples of participants (A-B, 25 participants each, pooled in Figure 1C of the main text) and the ‘swapped motion direction’ experiment (C, 26 participants). Bars show averages and s.e.m. for participants with AQ score below and above the median AQ score in our pool, and continuous lines show the best fit linear function across results from all participants (red dots). Text insets give Pearson’s correlation coefficients and associated p-values. (D) Distribution of AQ scores in our sample of participants, with best fit Gaussian function. (E) Correlation matrix between the pupil difference in the ‘main’ experiment (N = 50, pooling across panels A and B, as done in Figure 1C of the main text) and each AQ subscale. The bottom row shows correlations with the corrected total AQ: between the pupil difference and the total AQ, or between each subscale and the sum of the other four subscales. Pearson’s r values are colour coded and given by the text with associated significance values (*p<0.05, **p<0.01, **p<0.001, ns non-significant).
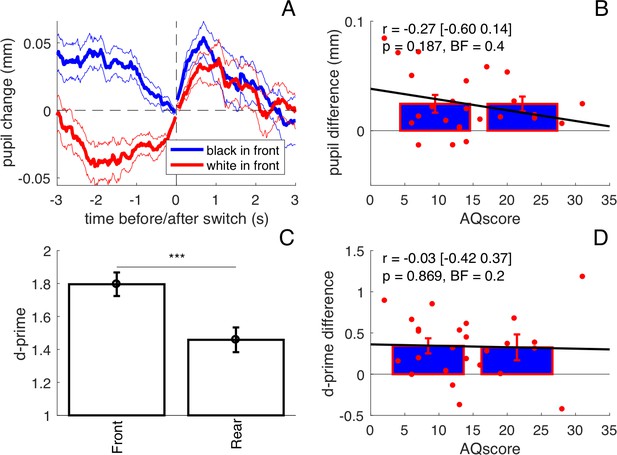
Results from the ‘double task’ experiment (N = 25).
(A) Average pupil traces when the black or the white dots were seen on the foreground (same format as Figure 1B in the main text). (B) Lack of correlation between pupil difference values in the [−1:1]s interval and AQ scores. Bars show averages and s.e.m. for participants with AQ score below and above the median AQ score in our pool, and continuous lines show the best fit linear function across results from all participants (red dots). Text insets give Pearson’s correlation coefficients, the 95% confidence interval, associated p-values and Bayes Factor. (C) Sensitivity to speed increments occurring in the foreground and background surface, measured by d-prime and averaged across participants; error bars show s.e.m.; stars show the results of a paired t-test comparing the two sets of d-prime values (***p<0.001). (D) The sensitivity difference between foreground and background does not correlate with AQ. Same conventions as in panel B.
Videos
the bistable cylinder
https://doi.org/10.7554/eLife.32399.005Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.32399.008





