Proteolytic processing of palmitoylated Hedgehog peptides specifies the 3-4 intervein region of the Drosophila wing
Figures
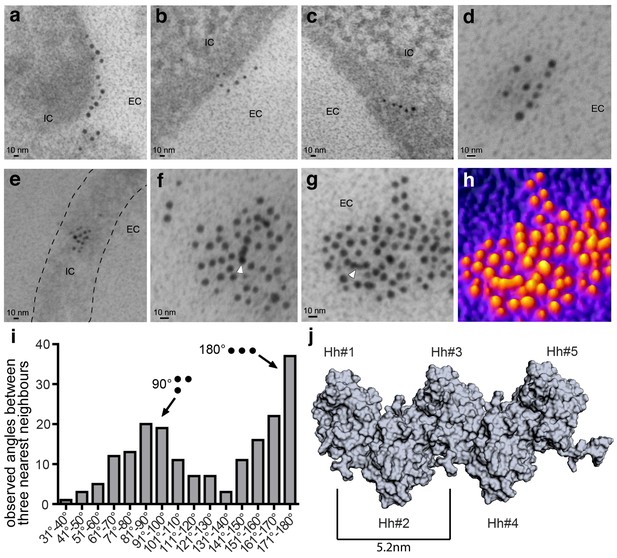
Immuno-TEM analysis of Shh clusters at the Bosc23 cell surface (a–g). α-Shh immunogold labeling suggests that Shh forms linear arrangements (EC: extracellular; IC: intracellular).
Shh and Hh acyltransferase were coexpressed in Bosc23 cells and cell-surface-associated clusters visualized by two independent α-Shh antibodies and 5 nm and 10 nm immunogold-labeled secondary antibodies. Note Shh clusters on cellular extensions (dashed line in e) and continuous immunogold labeling in cell-surface aggregates (f,g, white arrowheads) consistent with linear Shh clustering. (h) 3D heat map conversion of the cell-surface cluster shown in g. Bright yellow indicates 5 nm and 10 nm gold, and dark colors represent the background. (i) Quantification of angular distributions of the three most adjacent gold particles within clusters confirm non-random Shh clustering. 187 angles in 20 individual cell-surface clusters were analyzed. (j) Hh pentamer model. Hh monomers form extended zigzag chains (Ohlig et al., 2011).
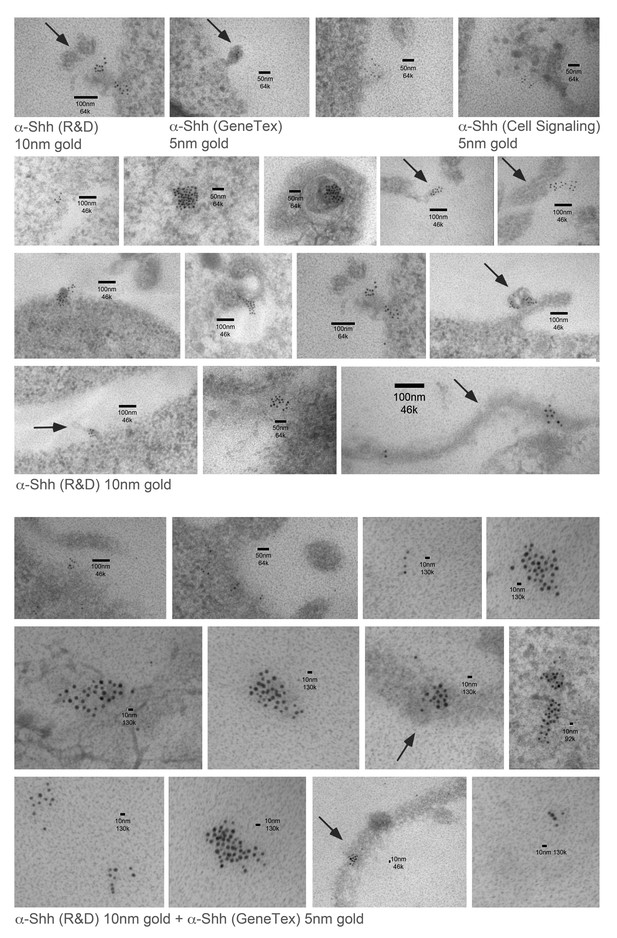
Immuno-TEM examples of Shh clusters at the Bosc23 cell surface.
Top: Three different anti-Shh primary antibodies detect local Shh clusters at the cell surface. Shh/Hh acyltransferase were coexpressed in Bosc23 cells and cell-surface-associated clusters were visualized by immunogold-labeled secondary antibodies. Center: Examples of Shh clusters detected by α-Shh (R and D). Bottom: Examples of Shh clusters detected by combined immunostaining. Size bars and magnifications are shown. Note the presence of membrane-tethered Shh chains on cellular extensions that possibly represent filopodia (arrows).
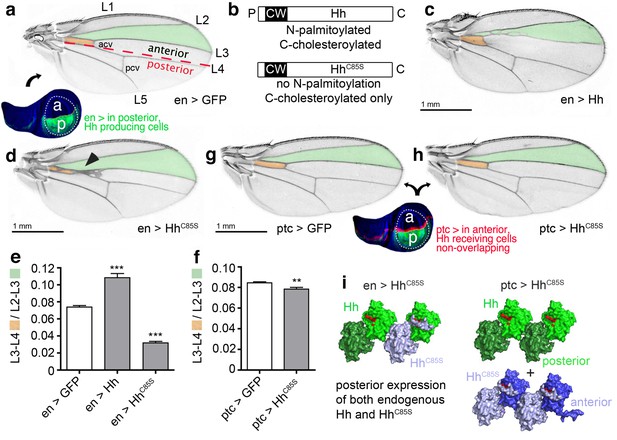
HhC85S expressed in the posterior wing disc compartment, but not in the anterior compartment, dominantly represses the formation of Hh-dependent wing structures (a) Drosophila third-instar wing disc and adult wing.
An en >GFP control wing disc and wing are shown. The posterior en-expression domain of the wing disc is labeled in green. Adult wings are shown with anterior up and proximal left. Longitudinal veins L1-L5, the anterior cross vein (acv) and the posterior cross vein (pcv) are marked. The anterior (a)/posterior (p) compartment border is shown as a red dashed line. (b) Schematic of transgenic constructs. P: palmitate, C: cholesterol, CW: Cardin-Weintraub motif. (c) Hh overexpression under en-control (en >Hh) expands the anterior L3-L4 intervein region. (d) en >HhC85S: L3 and L4 appose proximally; the acv is lost. (e,f) To quantify Hh activity, the most proximal L3-L4 areas highlighted in orange were determined and the values obtained divided by the L2-L3 areas. ***p≤0.001, **p≤0.01, n = 20. (g,h) Ptc-controlled GFP and HhC85S expression in the anterior (Hh-receiving) wing disc compartment at the a/p border (red stripe) do not impair wing development. (i) Left: Proposed mixed composition of morphogen clusters upon transgenic HhC85S (gray) expression in the posterior compartment that simultaneously produces the wild-type morphogen (green). Here, the N-terminal peptide of one molecule in the chain blocks the Ptc-receptor-binding site (red) of the adjacent molecule in the chain. Shh crystal lattice interactions (pdb: 3m1n) are shown to illustrate a possible cluster structure. Right: HhC85S expression in the anterior compartment (under ptc control) prevents the assembly of mixed morphogen clusters, leaving endogenous Hh function unimpaired.
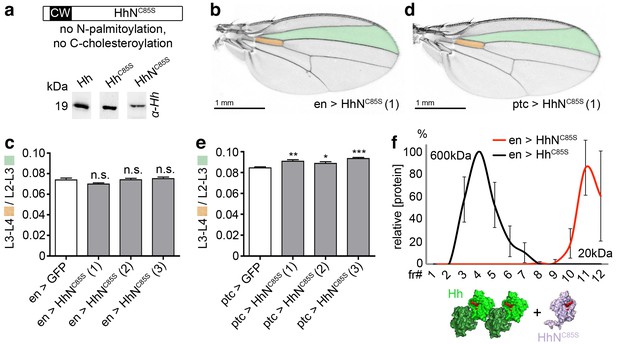
Unlipidated monomeric HhNC85S does not repress the formation of Hh-dependent wing structures.
(a) Schematic of HhNC85S and compared expression of recombinant Hh, HhC85S and HhNC85S in Drosophila S2 cells. Proteins were detected by α-Hh antibodies. (b) Unimpaired Drosophila wing formation upon en-controlled HhNC85S overexpression at 25°C. A representative result from one of four independently derived HhNC85S fly lines is shown. (c) Relative L3-L4/L2-L3 intervein ratios of three independent HhNC85S fly lines (1-3) are expressed as a quantitative readout for Hh patterning activity. ***p≤0.001, **p≤0.01, *p≤0.05, n.s. (not significant): p>0.05, n = 20. (d) Drosophila wing formation upon ptc-controlled HhNC85S overexpression at 25°C. (e) Relative L3-L4/L2-L3 intervein ratios of three independent HhNC85S fly lines (1-3). ***p≤0.001, **p≤0.01, *p≤0.05, n.s. (not significant): p>0.05, n = 20. (f) Gel filtration analysis of HhC85S and HhNC85S expressed in Drosophila larvae under en-control. Multimeric HhC85S eluted in fractions 3–8, corresponding to molecular weights of 70 kDa-600 kDa, as expected. By contrast, HhNC85S eluted in fractions 10–12 (corresponding to 19 kDa monomers). Elution profiles are expressed relative to the highest protein amounts detected, which were set to 100%. n = 3. Bottom: Proposed generation of endogenous Hh clusters (green) at the posterior cell surface that are devoid of monomeric soluble HhNC85S.
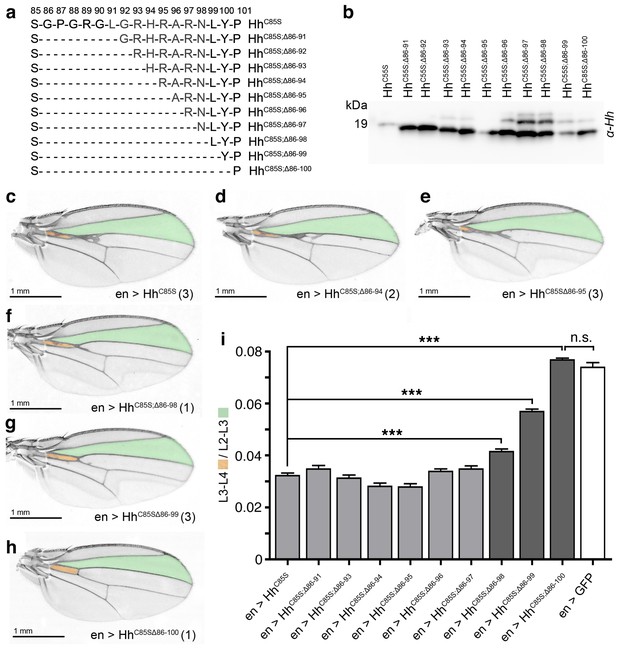
N-terminal truncation of palmitoylation-deficient HhC85S rescues wing formation.
(a) All truncated proteins also lacked the N-terminal cysteine, preventing Hh palmitoylation (Hardy and Resh, 2012). Residues #93–97: CW motif. (b) All proteins were expressed and secreted from S2 cells, as determined by immunoblotting. (c–h) En-regulated overexpression of HhC85S and N-terminally truncated proteins (HhC85S;Δ). Unaffected wing development despite en-regulated expression of unpalmitoylated HhC85S;Δ86-100 (h). (i) Quantification of wings shown in c-h. En-regulated GFP and HhC85S expressions served as positive and negative controls, respectively. Pooled analysis of three transgenic fly lines, each derived from an independent injection. en >HhC85S: 0.032 ± 0.001, en >HhC85S;Δ86-91: 0.035 ± 0.001 (p=0.1375), en >HhC85S;Δ86-93: 0.031 ± 0.001 (p=0.5458), en >HhC85S;Δ86-94: 0.028 ± 0.001 (p=0.0134), en >HhC85S;Δ86-95: 0.028 ± 0.001 (p=0.001), en >HhC85S;Δ86-96: 0.034 ± 0.001 (p=0.25), en >HhC85S;Δ86-97: 0.035 ± 0.001 (p=0.117), en >HhC85S;Δ86-98: 0.041 ± 0.001 (p<0.0001), en >HhC85S;Δ86-99: 0.057 ± 0.001 (p<0.0001); en >HhC85S;Δ86-100: 0.076 ± 0.0007 (p=0.0001), en >GFP: 0.074 ± 0.002. ***p≤0.001, n.s. (not significant): p>0.05, n = 60 (n = 20 per line), all flies developed at 25°C.
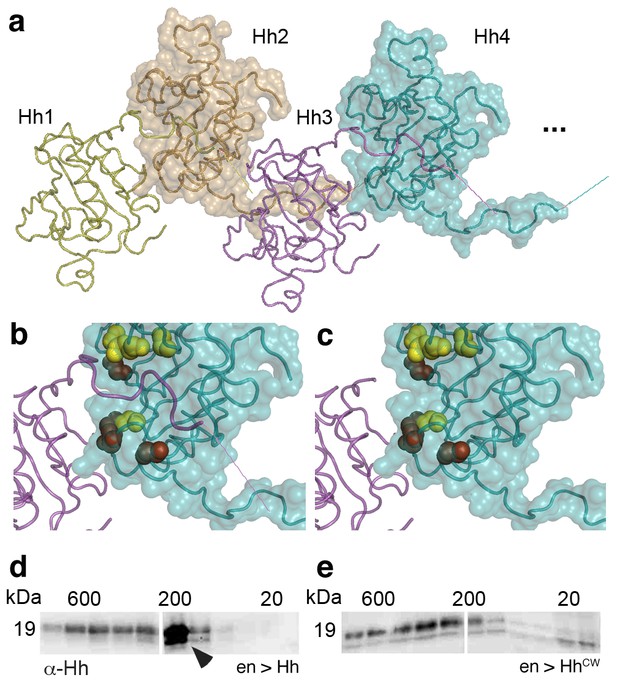
Modeled linear Drosophila Hh clusters, using pdb 2IBG and pdb 3M1N as templates.
(a) Predicted intermolecular Drosophila Hh interactions, resulting in linear zigzag chains. The molecular surface of every other Hh molecule is shown to demonstrate intermolecular interactions between N-terminal palmitoylated peptides and the adjacent molecules in the chain. Modeled N-terminal palmitate is shown as a line (pointing to the right). (b) Close-up of Hh N-terminal interactions with the predicted Ptc binding site of the adjacent molecule in the cluster. Yellow spheres denote Drosophila Hh residues corresponding to Shh residues that interact with Ptc (Drosophila Hh H193, H194, H200, H240) (Bosanac et al., 2009). Red spheres denote residues corresponding to Shh amino acids bound by the Shh inhibitory antibody 5E1 (K105, R147, R213, R238, R239 in Drosophila Hh) (Maun et al., 2010). (c) Based on our model, N-terminal Hh truncation makes these sites accessible in the cluster. (d,e) Top: Gel filtration analysis of transgenic L3 fly larvae expressing Hh or a HhCW variant with a mutated Hh cleavage site under en-control in vivo. Note the presence of a truncated Hh fraction (representing the situation in c) in larvae expressing ectopic wild-type Hh (arrowhead). Top bands in d,e represent the situation shown in b, bottom bands the situation shown in c.
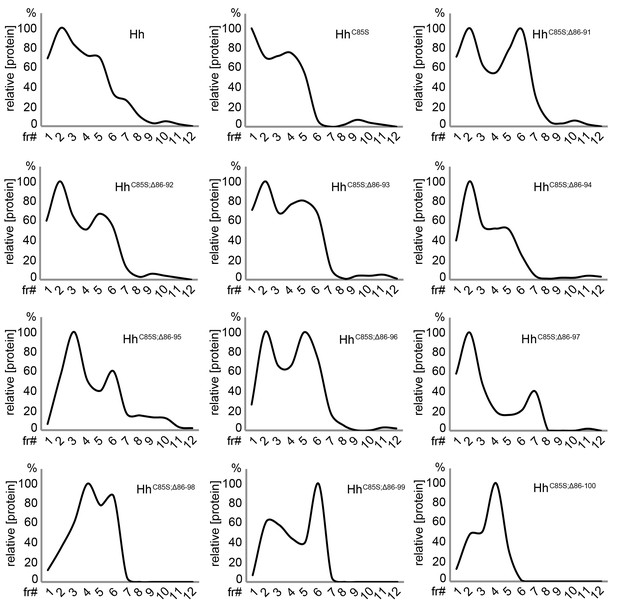
Unimpaired multimerization of N-terminally truncated Hh variants.
Gel filtration analysis of Hh, HhC85S, and N-terminally truncated variants (HhC85S;Δ86-91 - HhC85S;Δ86-100) expressed in Drosophila S2 cells under actin-control. Soluble Hhs were detected in the form of ‘large’ (>600 kDa, fractions 1–2) and ‘smaller’ (100 kDa-600 kDa, fractions 3–6) clusters. Elution profiles are expressed relative to the highest protein amounts in a given fraction, which was set to 100%.
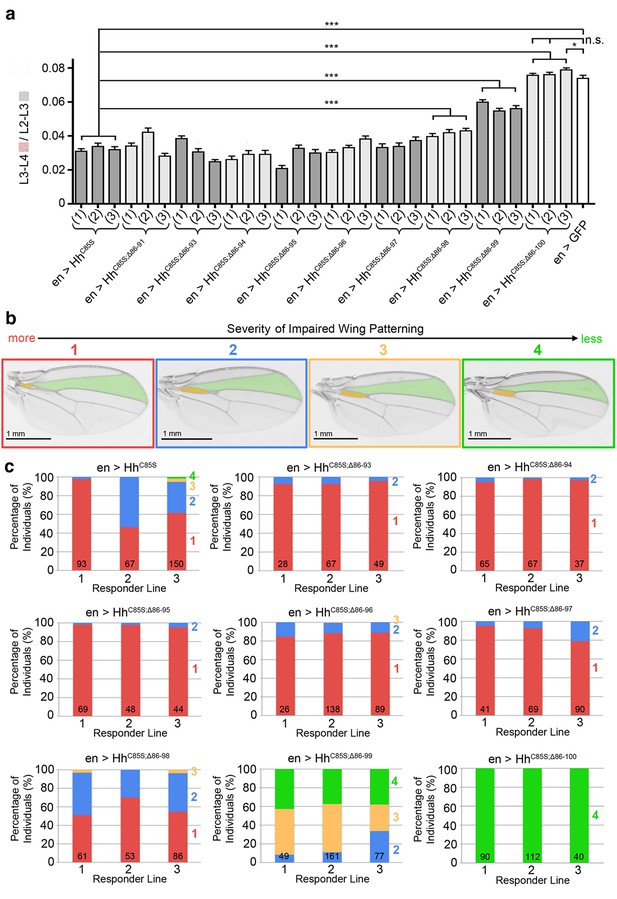
Graded variable wing defects as a consequence of full-length and N-terminally truncated HhC85S expression in the wing disc.
(a) Analysis of three transgenic fly lines, each derived from an independent injection of cDNA encoding the same unpalmitoylated N-truncated construct. En-regulated GFP and HhC85S expressions served as positive and negative controls, respectively. en>HhC85S (1-3): 0.032 ± 0.001, en>HhC85S;Δ86-91 (1-3): 0.035 ± 0.001 (p=0.1375), en>HhC85S;Δ86-93 (1-3): 0.031 ± 0.001 (p=0.5458), en>HhC85S;Δ86-94 (1-3): 0.028 ± 0.001 (p=0.0134), en>HhC85S;Δ86-95 (1-3): 0.028 ± 0.001 (p=0.0085), en>HhC85S;Δ86-96 (1-3): 0.034 ± 0.001 (p=0.2503), en>HhC85S;Δ86-97 (1-3): 0.035 ± 0.001 (p=0.1165), en>HhC85S;Δ86-98 (1-3): 0.041 ± 0.001 (p<0.0001), en>HhC85S;Δ86-99 (1-3): 0.057 ± 0.001 (p<0.0001); en>HhC85S;Δ86-100 (1): 0.076 ± 0.001 (p=0.4058), en>HhC85S;Δ86-100 (2): 0.076 ± 0.001 (p=0.3330), en >GFP: 0.074 ± 0.002. ***p≤0.001, **p≤0.01, *p≤0.05, n.s. (not significant): p>0.05, n = 20 (per line), all flies developed at 25°C. (b) Observed wing phenotypes were classified into four distinct grades, ranging from 1 (strongly affected wings, red) to 4 (wild-type wings, green). (c) Variations in wing formation are expressed as relative percentages of observed phenotypes, using the scheme shown in a. Three transgenic fly lines derived from independent injections of each construct were analyzed (1-3); the number of analyzed wings is shown in the bars. Note the phenotypic variations between transgenic lines of the same construct, which we explain as stochastic differences in the relative amounts and distributions of unpalmitoylated transgenic proteins in wild-type Hh clusters. A significant fraction of wings obtained from en>HhC85S;Δ86-99 flies were normal, and all wings from en>HhC85S;Δ86-100 flies were indistinguishable from wild-type wings. This demonstrates that dominant-negative HhC85S protein activities are fully reversed by the additional removal of N-terminal inhibitory peptides.
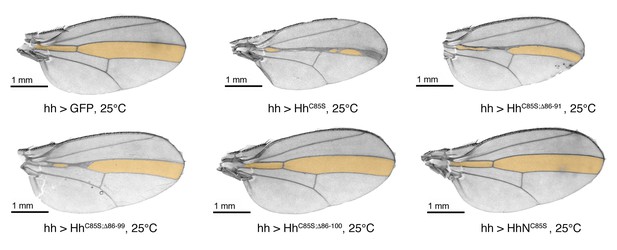
Confirmation that N-truncated HhC85S and HhNC85S do not repress the formation of Hh-dependent wing structures, using the driver line hh-Gal4.
Confirmation of wing phenotypes obtained from en-Gal4-driven transgene expression by the alternative posterior driver line hh-Gal4. In contrast to multimeric, non-palmitoylated HhC85S, hh-Gal4 controlled expression of N-terminally truncated, palmitoylation-deficient HhC85S;Δ86-100 and unlipidated monomeric HhNC85S do not impair the Hh-regulated formation of the L3-L4 intervein region (orange). A hh >GFP control wing is also shown. Anterior is up, proximal is left.
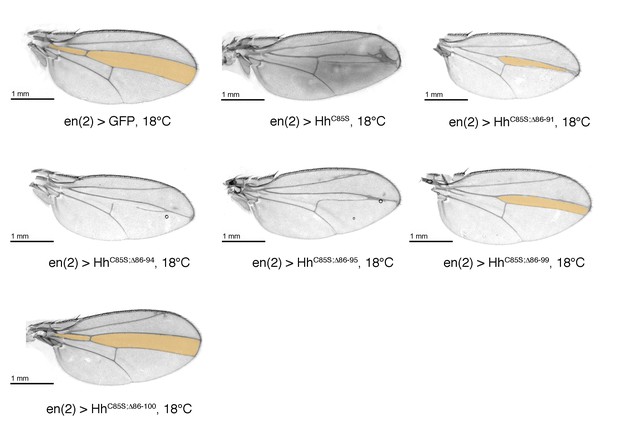
Confirmation that N-truncated HhC85S does not repress the formation of Hh-dependent wing structures, using the driver line en(2)-Gal4.
Confirmation of wing phenotypes using the alternative posterior driver line en(2)-Gal4. As previously shown for en-Gal4 and hh-Gal4 driven transgene expression, non-palmitoylated HhC85S, but not N-terminally truncated, palmitoylation-deficient HhC85S;Δ86-100 reduce the L3-L4 intervein region (orange). An en(2) >GFP control wing is shown. Anterior is up, proximal is left.
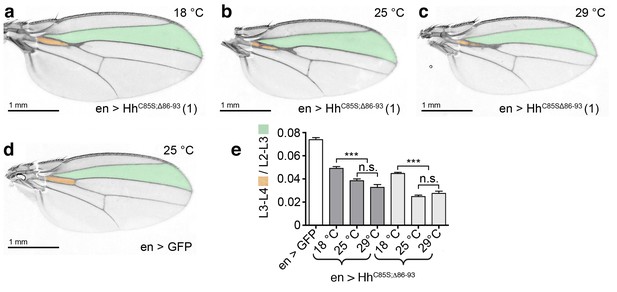
Temperature-dependent dominant-negative HhC85S;Δ86-93 function in the posterior Drosophila wing disc compartment.
(a–c) en-driven overexpression of HhC85S;Δ86-93 in a wild-type background at 18°C, 25°C, and 29°C. Adult Drosophila wings are shown. (d) GFP served as control (wild-type wing). At 25°C and at 29°C, the L3-L4 intervein space of en>HhC85S;Δ86-93 wings narrows if compared with wings developing at 18°C. In addition, wings get smaller. (e) Quantification of wild-type Hh patterning activity. Two transgenic lines were analyzed (brackets). en >GFP: 0.074 ± 0.002, en>HhC85S;Δ86-93 (line 1) at 18°C: 0.049 ± 0.002, at 25°C: 0.038 ± 0.002, and at 29°C: 0.033 ± 0.002. Differences between 25°C and 29°C were insignificant (p=0.06); differences between 18°C and 25°C (p<0.0001) and 29°C (p<0.0001) were significant. en>HhC85S;Δ86-93 (line 3) at 18°C: 0.045 ± 0.001, at 25°C: 0.025 ± 0.001, and at 29°C: 0.028 ± 0.002. Differences between 25°C and 29°C were insignificant (p=0.2450); differences between 18°C and 25°C (p<0.0001) and 29°C (p<0.0001) were again significant. ***p≤0.0001; n.s. (not significant): p>0.05, n = 20.
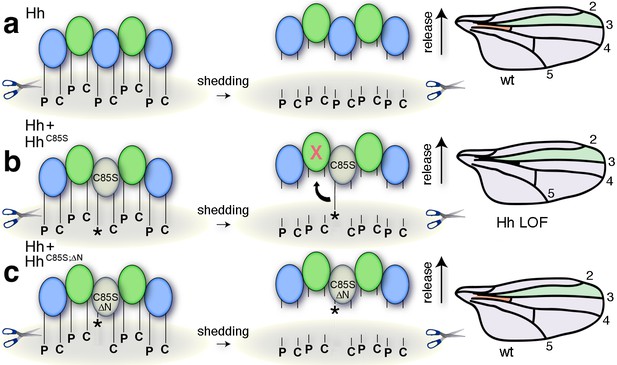
Simplified model for the conversion of membrane-bound Hh into soluble clusters.
Surface-tethered wild-type Hh monomers (colored green and blue for clarity) form multimeric clusters with their extended N- and C-terminal lipidated peptides tethered to the cell membrane. (a) Membrane-proximal proteolytic processing (scissors) removes lipidated membrane anchors and releases Hh clusters from posterior cells. As a consequence, protein concentrations at any position in the responsive (anterior) field correlate with their biological activities (their Ptc-receptor-binding capacities). Because partially processed clusters are not released, the role of both lipids at this point is to control quantitative Hh processing and bioactivation. (b) Unpalmitoylated HhC85S only requires processing of its cholesterylated C-terminus for release: As a consequence, a fraction of wild-type Hh in mixed clusters has its Ptc-receptor-binding sites and bioactivity blocked (indicated by the X) by unprocessed adjacent HhC85S N-termini (asterisk). Signaling at any position in the field is thus reduced, leading to dominant negative wing phenotypes (right). LOF: loss of function. (c) Artificial truncation of unpalmitoylated HhC85S N-termini restores wild-type Hh function. wt: wild type.
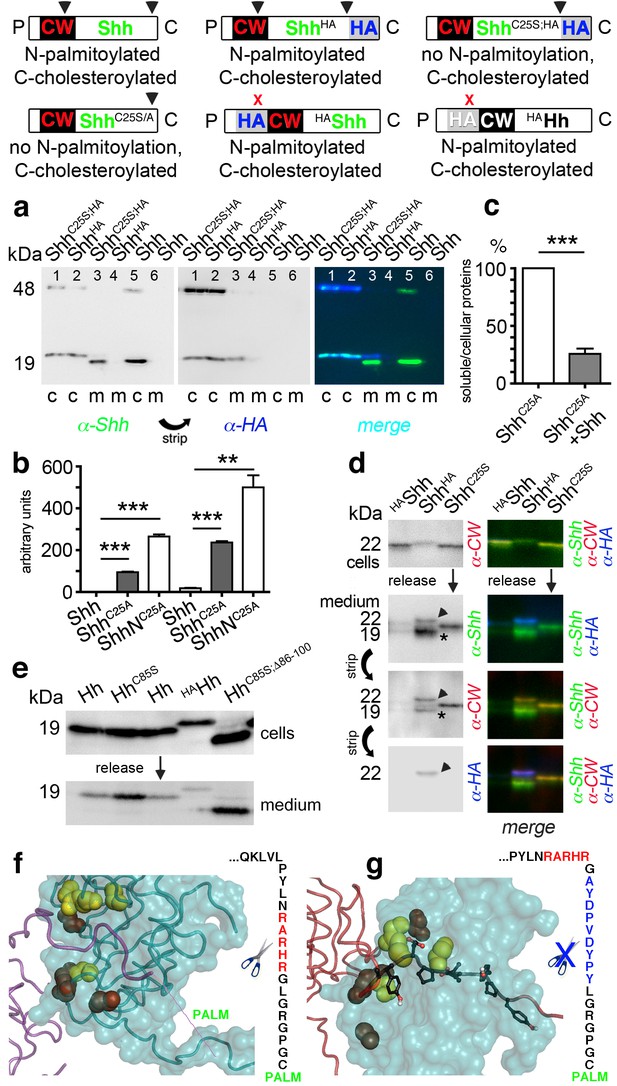
N-palmitoylation controls Hh morphogen release in vitro.
Top: Schematics of transgenes. Arrowheads indicate cleavage sites, the x denotes blocked cleavage. (a) Palmitoylated vertebrate Hh orthologs Shh and ShhHA and non-palmitoylated ShhC25A;HA were expressed in Bosc23 cells, and the proteins in the cellular (c) and corresponding soluble fractions (m) were analyzed by immunoblotting. To better demonstrate protein processing during release, we inverted grayscale blots and colored them (right: green: α-Shh, blue: α-HA). Green signals label untagged or processed Shh, and cyan signals label unprocessed HA-tagged proteins. Higher electrophoretic mobility confirms terminal processing during release. Tagged and untagged palmitoylated proteins are not efficiently released. (b) Compared dual-lipidated, monolipidated and non-lipidated Shh release after 1 hr (left) and 4 hr (right). ***p≤0.0001, **p≤0.001, n = 3. (c) Release of ShhC25A is downregulated 4-fold upon dual-lipidated Shh coexpression (27.7 ± 4.6% if compared to ShhC25A release alone, which was set to 100%, n = 7, ***p≤0.0001). (d) Impaired release of N-terminally HA-tagged HAShh. α-CW antibodies detect the N-terminal CW motif (KRRHPKK). Bright cellular signals in merged blots denote unprocessed proteins (arrowhead), orange signals denote C-processed/N-unprocessed proteins, and green signals confirm the removal of N- and C-terminal peptides (asterisk). Note ShhHA processing at the CW site during release. By contrast, N-terminal processing of ShhC25S is impaired. (e) Immunoblot analysis of recombinant Hh proteins released into media of transfected Drosophila S2 cells (left). Top row: S2 cells express palmitoylated and non-palmitoylated proteins to comparable levels. Bottom row: S2 cells released high levels of unpalmitoylated HhC85S and HhC85S;Δ86-100, and lower levels of palmitoylated Hh, into the media. Only very low levels of HAHh were released in unprocessed form (top band). (f) Intermolecular interactions of Drosophila Hh N-terminal peptides. Right: schematic of the palmitoylated N-terminal ‘stem’ peptide, including basic CW residues (red) serving as the predicted membrane-proximal cleavage site. (g) Modeled insertion of the HA tag upstream of the N-terminal CW motif (located between Hh residues L91 and G92) of Drosophila Hh. This moves the CW motif nine amino acids more distal to the membrane and replaces its previous position with the HA tag (blue, right). Modeled N-terminal palmitate is shown as a zigzag line (pointing to the right). Yellow spheres denote Drosophila Hh residues corresponding to Shh residues that interact with Ptc (Drosophila Hh H193, H194, H200, H240) (Bosanac et al., 2009). Red spheres denote residues corresponding to Shh amino acids bound by the Shh inhibitory antibody 5E1 (K105, R147, R213, R238, R239 in Drosophila Hh) (Maun et al., 2010).
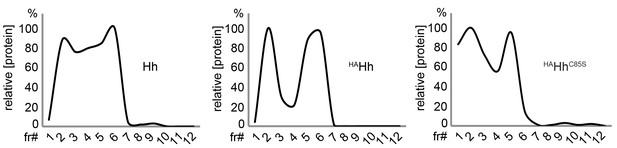
Unimpaired multimerization of HA-tagged Hh.
Gel filtration analysis of Hh, HAHh and non-palmitoylated HAHhC85S expressed in Drosophila S2 cells under actin-control. All soluble Hhs were detected in the form of ‘large’ (>600 kDa, fractions 1–2) and ‘smaller’ (100 kDa-600 kDa, fractions 3–6) clusters. Elution profiles are expressed relative to the highest protein amounts in a given fraction, which was set to 100%.
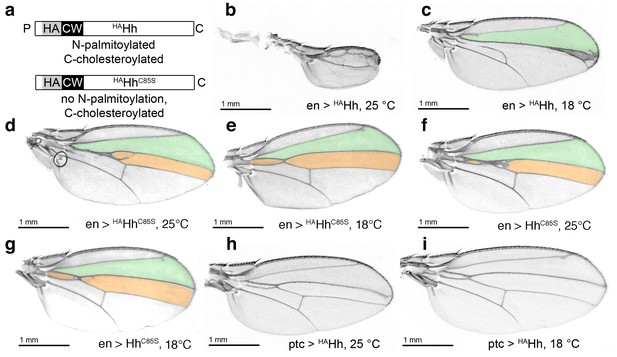
HA tag insertion into the putative N-terminal processing site strongly represses wild-type Hh in vivo.
(a) Schematic of HA-tagged Hh constructs. P: palmitate, C: cholesterol, CW: CW motif, HA: HA tag. (b) At 25°C, most flies die at the late larval/early pupal stage. Wings of the few surviving en >HAHh flies show severe dominant-negative Hh loss of function. This phenotype was observed in four fly lines derived from two HAHh integration events each into VK37, 51C and attP integration sites. (c) At 18°C, more flies develop, and L3 and L4 appose into a large central vein. (d,e) Additional deletion of the palmitate acceptor cysteine (HAHhC85S) largely reverses Hh loss of function. At 25°C, wings show proximally apposed L3-L4 veins, and at 18°C, the anterior crossvein is reduced, as previously observed for non-palmitoylated HhC85S. (f,g) Representative en >HhC85S wing phenotypes are shown. (h,i) By contrast, ptc-controlled HAHh expression in the anterior (Hh-receiving) wing disc compartment at 18°C and 25°C mildly affected wing formation.
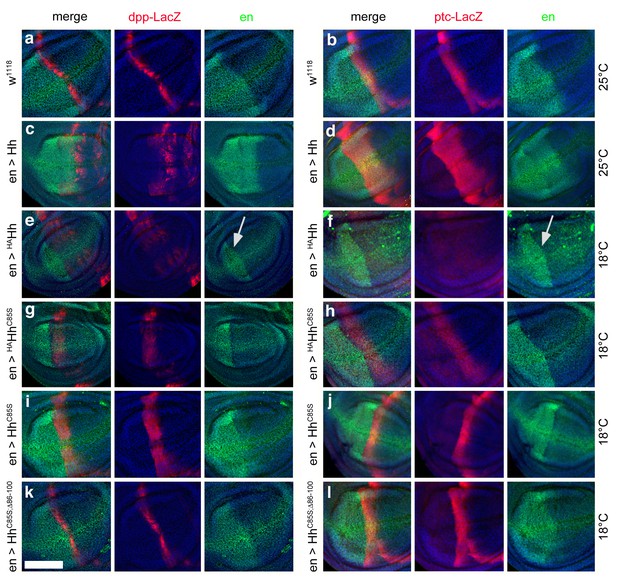
Effect of different Hh variants on en, dpp-LacZ and ptc-LacZ expression in the wing disc (a,b) Dpp-LacZ (a) and ptc-LacZ (b) reporter gene expression at the a/p border in wild-type third-instar discs.
Nuclear β-galactosidase is immunofluorescently labeled (red). Overexpression of CD8-GFP under en-control labels the posterior compartment. Fly larvae developed at 25°C. The left image is a merge. (c,d) Hh overexpression expands dpp-LacZ and ptc-LacZ expression anteriorly. 4D9 α-engrailed/invected (inv) antibodies label the posterior compartment in this and the following panels (green). Fly larvae developed at 25°C. (e,f) En-controlled HAHh overexpression reduced dpp-LacZ expression in the anterior wing disc. Ptc-LacZ expression was always completely abolished, and en/inv expression was restricted to most posterior wing disc regions (arrow). Fly larvae for this and all subsequent analyses developed at 18°C because wing disc growth arrested at 25°C, preventing further analysis. (g,h) Additional deletion of the palmitate membrane anchor increased dpp-LacZ expression and also restored a stripe of weak yet expanded ptc-LacZ expression. The expansion of ptc-LacZ and dpp-LacZ domains beyond wild-type levels may be linked to reduced inv/en expression in anterior target cells (note the unchanged posterior restriction of inv/en-expression). (i,j) En-controlled HhC85S expression leads to comparable dpp-LacZ expression. Ptc-LacZ reporter expression was reverted into more intense and restricted staining, indicating an additional inhibitory effect of the HA-tag. (k,l) Restored wild-type pattern of dpp-LacZ expression and ptc-LacZ expression as a consequence of en-controlled HhC85S;Δ86-100 expression shows that expanded dpp-LacZ expression and reduced ptc-LacZ expression in en >HhC85S and en >HAHhC85S discs were caused by the unprocessed N-terminal peptide. Wing discs are oriented such that anterior is right and dorsal is up; all magnification, camera and processor settings were kept identical. Scale bar: 100 μm.
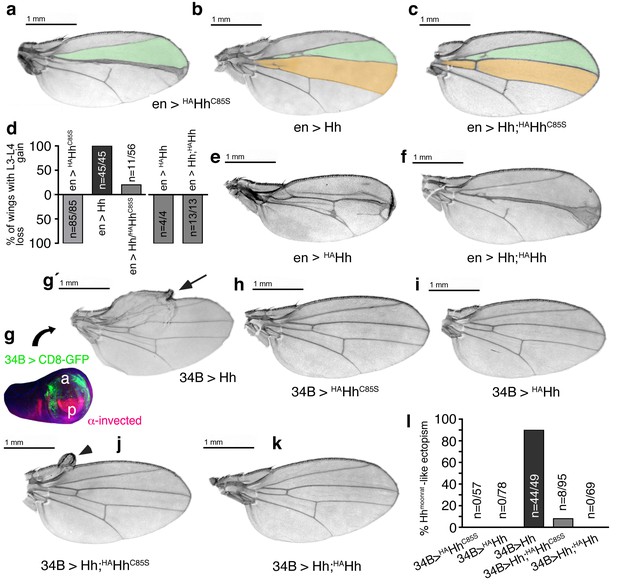
Increased Hh amounts compensate for impaired Ptc binding by unprocessed N-termini, but not for impaired Hh release in vivo.
(a) If expressed from chromosome 3 at 25°C, en>HAHhC85S wings show proximally apposed L3-L4 veins. (b) En >Hh wings show enlargement of L3-L4 intervein area. (c) En >Hh;HAHhC85S coexpression reversed en >HAHhCC85S loss of function at 25°C and about 20% of wings showed Hh gain-of-function. Wing phenotypes are shown and quantifications shown in (d). (e) If expressed from chromosome 3 at 18°C, only 4% of en >HAHh flies hatch and show severe dominant-negative Hh loss-of-function phenotypes. (f) Upon Hh coexpression, at 18°C, 22% flies develop with their L3 and L4 always fused into one central vein. (g,g´) 34B-Gal4 expresses UAS-transgenes at the border of the anterior wing disc (green) that does not overlap with the posterior hh-producing disc compartment (red). 34B > Hh expression at 25°C led to clear anterior overgrowth in 90% of wings. (h,i) 34B > HAHhCC85S or HAHh expression did not impair wing development. This again confirmed biological inactivity of both Hh variants. (j) 34B-controlled Hh;HAHhC85S coexpression partially reversed Hh gain-of-function, reducing ectopic overgrowth to a small fraction of wings (8%). (k) 34B > Hh;HAHh coexpression completely reversed Hh gain-of-function. This confirms cell-autonomous Hh repression by direct HAHh contacts in mixed clusters, as quantified in (l).
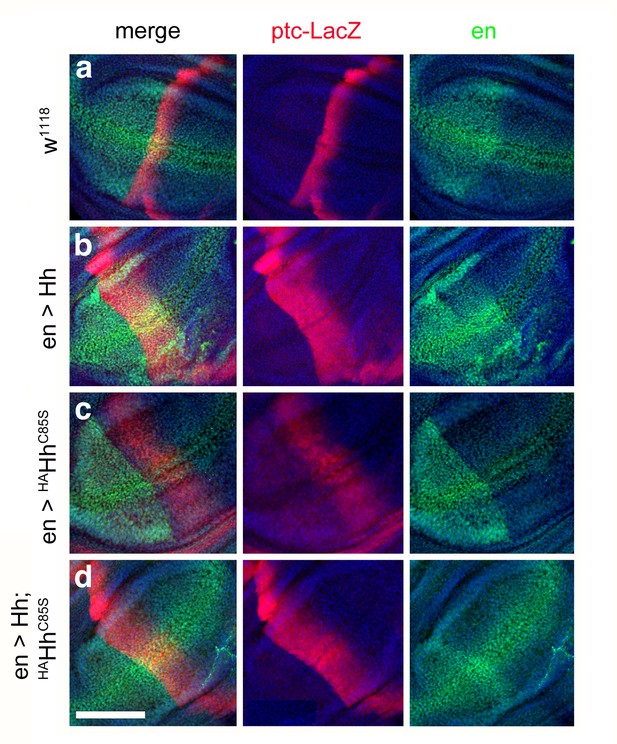
Combined en >Hh;HAHhC85S expression restores ptc-LacZ expression in the wing disc.
(a) Ptc-LacZ reporter gene expression at the a/p border in wild-type third-instar discs. Nuclear β-galactosidase is immunofluorescently labeled (red). 4D9 α-engrailed/invected (inv) antibodies label the posterior compartment in this and the following panels (green). The left image is a merge. Fly larvae developed at 25°C. (b) En-controlled Hh overexpression increased and expanded ptc-LacZ expression anteriorly. (c) En-controlled HAHhC85S overexpression reduced ptc-LacZ expression, as previously shown. (d) En-controlled Hh;HAHhC85S coexpression generated a stripe of intense expanded ptc-LacZ staining, consistent with the gain-of-function phenotype observed in wings. Wing discs are oriented such that anterior is right and dorsal is up; all magnification, camera and processor settings were kept identical. Scale bar: 100 μm.
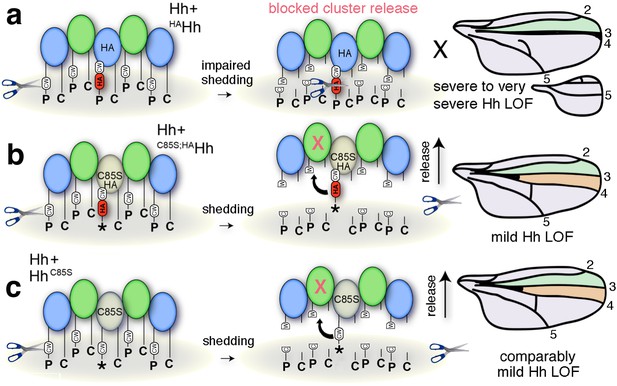
Simplified model for the impaired solubilization of membrane-bound Hh clusters containing HAHh.
(a) HA insertion in the predicted CW sheddase cleavage site strongly impedes wild-type Hh function. We explain this as completely blocked cell-surface release of all Hh morphogens in mixed clusters also containing unprocessed HAHh. The X indicates blocked proteolytic processing. LOF: loss of function. (b) Additional removal of the N-terminal lipid anchor converts severely impaired wing development (and en >HAHh larval lethality at 25°C) into milder phenotypes characteristic for unprocessed yet soluble clusters containing HhC85S (c). This confirms that the N-terminal lipid anchor acts to control quantitative Hh processing and bioactivation during release and that dominant-negative HhC85S function in wing development is not directly caused by the lack of N-palmitate.
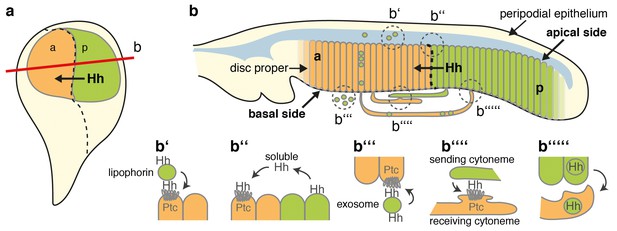
Model of membrane-dependent and membrane-independent Hh protein signaling from posterior producing cells to distant receiving cells and their potential congruency.
(a) Schematic of a third instar wing disc with the Hh-producing posterior compartment labeled in green and the Ptc-receptor-producing anterior compartment labeled in orange. The a/p compartment border crossed by spreading Hh is shown as a dashed line. (b) Line drawing of a vertical section of the wing disc at a site marked with a red line in (a). Two pools of Hh are secreted from posterior columnar cells of the wing imaginal disc by different mechanisms (dashed circles). One pool is released from the apical side of the polar epithelium using lipophorins as hydrophilic carriers (b′) (Panáková et al., 2005) or via proteolytic processing of lipidated Hh membrane anchors, as suggested in this work (b′′). Subsequently, unprocessed lipidated Hh or processed ectodomains diffuse through the fluid-filled peripodial space (labeled blue) to bind to Ptc receptors expressed in the anterior compartment. Another pool of apical cell-surface Hh is internalized and re-secreted apically (D'Angelo et al., 2015) or basolaterally (Callejo et al., 2011) using exosomes (b′′′) (Matusek et al., 2014) or long cellular protrusions, known as cytonemes, as carriers. Cytonemes can extend from posterior Hh-producing cells to deliver Hh to cell surface receptors on receiving anterior cells in their close vicinity, or can meet ‘receiving’ cytonemes extending from more distant anterior cells at defined contact sites (b′′′′) (González-Méndez et al., 2017). Receiving anterior cytonemes that take up Hh from basal subcellular sites of expressing posterior cells for subsequent intracellular transport to the apical pole of anterior epithelial cells have also been described (b′′′′′) (Chen et al., 2017). However, an explanation is needed about how lipidated Hh can ‘switch’ between sending and receiving cytonemes, or relay from vesicular or cytoneme membranes to their receptors. This problem may be solved by proteolytic Hh processing, resulting in its relay between cytonemes or from producing cell membranes to Ptc receptors on receiving anterior cells. We note that the findings presented in this work do not support the alternative possibility, that is, that different transport modes work in parallel, because HA insertion into the N-terminal Hh processing site abolished (most) Hh-dependent patterning of the L3-L4 intervein region in a dominant-negative manner.
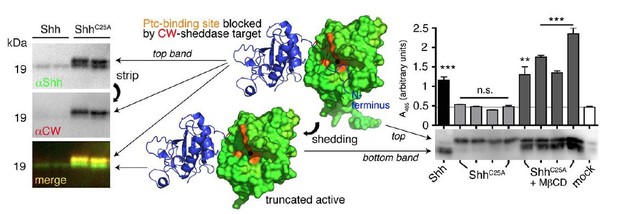
N-terminal Shh processing activates Shh.
Center: Intermolecular interactions observed in the human Shh crystal structure (pdb:3M1N) (17). Analysis of crystal symmetry mates revealed that N-terminal peptides (amino-terminal amino acids 25-45, blue) wrap around the symmetry-related molecule (green) and interact with its zinc-coordination site, which is the Ptc receptor binding site. Zinc is shown as a black sphere, the yellow surface marks the Ptc-receptor binding site, and the putative sheddase target site (called CW motif) is marked in red. Proteolytic processing during release truncates the protein (bottom) and increases its electrophoretic mobility (left). To better demonstrate N-terminal Shh processing during release, we inverted and colored (gray) the scale blots obtained from α-Shh antibody (binds processed and unprocessed Shh) and α-CW antibody (binds the putative cleavage site) incubation. Yellow signals in merged blots thus denote N-unprocessed proteins bound by both antibodies, and green signals confirm the removal of N- terminal peptides (arrow). Complete processing of N-palmitoylated N-termini (Shh) and incomplete processing of non-palmitoylated termini (ShhC25A) is confirmed by differential α-CW-antibody binding. Right: Protein processing converts otherwise inactive ShhC25A into the active morphogen, as indicated by induced Hh-dependent C3H10T1/2 reporter cell differentiation into osteoblasts. *** denotes statistical significance (p<0.0001), **denotes statistical significance (p<0.0047). n.s.: not significant (<0.05). Taken from (11).
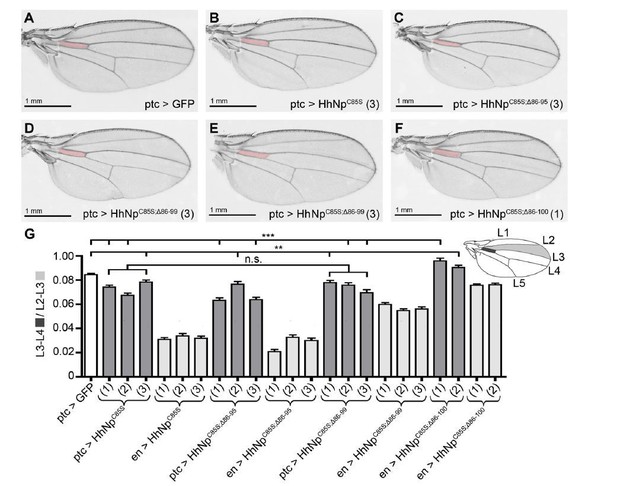
Compared phenotypes resulting from the overexpression of HhC85S and N- terminally truncated variants in the Hh-producing and Hh-receiving compartments of the wing disc.
(A-F) Adult wing structures as a result of ptc-Gal4-mediated overexpression of non- palmitoylated Hh in wild type background (at 25 °C). Note that proximal L3-L4 intervein spaces get slightly reduced (B-D) and, in addition, varying numbers of wings lacked the ACV (E and Table 1 below). Wing patterning is fully restored upon deletion of the most N-terminal 15 amino acids.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Drosophila melanogaster) | Hedgehog; Hh | PMID 8252628 | NCBI Reference sequence: NM_001038976.1 | |
| Cell line (D. melanogaster) | Schneider 2 | Invitrogen | RRID: CVCL_Z232 | |
| Cell line (Homo sapiens) | Bosc23 | PMID: 11395778 | RRID: CVCL_4401 | |
| Transfected construct (Hedgehog) | Hh | PMID 8252628 | NCBI Reference sequence: NM_001038976 | |
| Transfected construct (Sonic hedgehog) | Shh | PMID: 7916661 | NCBI Reference sequence: NM_009170 | |
| Antibody | anti-Hh | Santa Cruz | d300 catalog # sc-25759 | 2000-fold at 4°C over night |
| Antibody | anti-Shh | R and D Systems | Catalog # AF464 | 1000-fold at 4°C over night |
| Antibody | anti-en | DSHB | DSHB # 4D9 | 50-fold at 4°C over night |
| Antibody | anti-LacZ | Cappel, MP Biomedicals | Catalog # 08559761 | 50-fold at 4°C over night |
| Antibody | anti-HA | Sigma | catalog # H9658 | 5000-fold at 4°C over night |
| Strain, strain background (D. melanogaster) | Ptc > Gal4 | Bloomington # 2017 | ||
| Strain, strain background (D. melanogaster) | En > Gal4 | FlyBaseID FBrf0098595 | ||
| Strain, strain background (D. melanogaster) | Hh > Gal4 | Bloomington # 67046 | ||
| Strain, strain background (D. melanogaster) | 34B > Gal4 | Bloomington # 1967 |
Additional files
-
Supplementary file 1
Mutagenesis primers and sequence confirmation for all Hh variants used in this study.
- https://doi.org/10.7554/eLife.33033.023





