NOTCH activity differentially affects alternative cell fate acquisition and maintenance
Figures
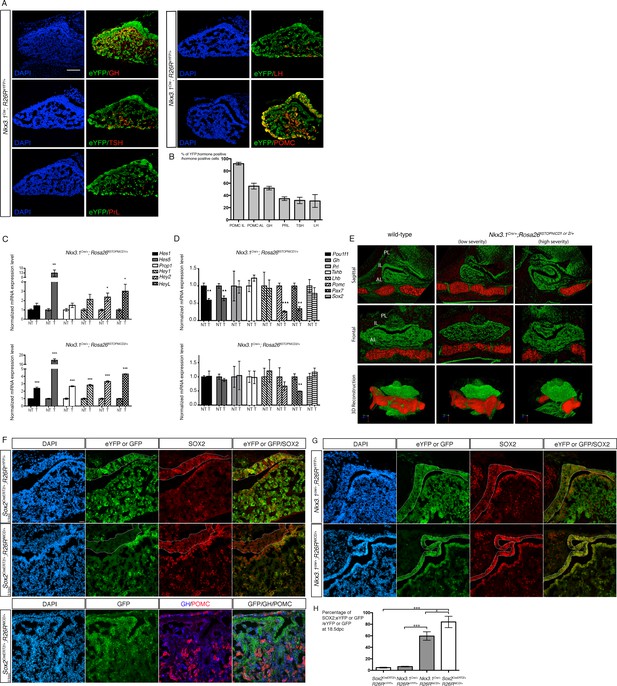
Activation of NOTCH pathway in progenitors efficiently blocks cell fate acquisition.
(A) Immunofluorescence of 18.5dpc Nkx3.1Cre/+; Rosa26ReYFP/+ pituitaries. This lineage tracing analysis shows that NKX3.1Cre is active in precursors for different endocrine cells. (B) Percentage of eYFP positive endocrine cells for each hormone-secreting population in 18.5dpc Nkx3.1Cre/+; Rosa26ReYFP/+ 18.5 dpc pituitaries. Nkx3.1Cre is mostly active in IL precursors with POMC;eYFP double positive representing 92% (±1.8 SD, n = 3) of POMC positive melanotrophs, and 55.4% (±3.6 SD, n = 3) of POMC positive corticotrophs. The other AL cell types are also present in the progeny of NKX3.1 expressing progenitors with GH;eYFP double positive representing 51.9% (±2 SD, n = 2) of somatotrophs, PRL;eYFP double positive representing 34.6% (±2.4 SD, n = 3) of lactotrophs, TSH;eYFP double positive representing 31.7% (±4.2 SD, n = 3) of thyrotrophs and LH;eYFP double positive representing 38.2% (±7.1 SD, n = 3) of gonadotrophs. (C) RT-qPCR analysis of NOTCH target genes in Nkx3.1Cre/+;Rosa26flSTOPNICD1/+ and Nkx3.1Cre/+;Rosa26flSTOPNICD2/+ pituitaries at 18.5dpc (n = 3 to 4 embryos/genotype, Nkx3.1Cre/+;Rosa26flSTOPNIC/+D –T for NICD transgenic - versus Rosa26flSTOPNICD/+ -NT for non-NICD transgenic- littermates, unpaired t test performed). There is a significant induction of NOTCH pathway target gene expression, particularly of Hes5. (D) RT-qPCR analysis of pituitary cell-type markers in Nkx3.1Cre/+;Rosa26flSTOPNICD1/+ and Nkx3.1Cre/+;Rosa26flSTOPNICD2/+ pituitaries at 18.5dpc (n = 3 to 6 embryos/genotype, unpaired t test performed). POMC lineage markers (Pax7 and POMC) are affected by NICD1 and 2 over-expression, while Pit1 and Gh are only affected by NICD1 induction. (E) 3D reconstruction of 15.5dpc Nkx3.1Cre/+;Rosa26flSTOPNICD/+ pituitaries. Soft tissues are false-coloured in green and the basisphenoid bone in red. Severity of the phenotype is variable with most affected embryos showing misfolding of the epithelium lining the Rathke’s pouch lumen, reduction of the lateral pituitary wings and no midline fusion of the basisphenoid bone. (F) Immunofluorescence of 18.5dpc Sox2CreERT2/+;Rosa26ReYFP/+and Sox2CreERT2/+;Rosa26flSTOPNICD1/+pituitaries induced at 9.5dpc. There is a mosaic pattern of eYFP/GFP expression with eYFP expression being more robust than GFP. In contrast with eYFP in control embryos, most NICD1iresGFP positive cells appear SOX2 positive. In addition, GFP positive cells do not express GH or POMC. Finally, there are no GFP positive cells in IL. (G) Immunofluorescence of 18.5dpc Nkx3.1Cre/+;Rosa26ReYFP and Nkx3.1Cre/+;Rosa26flSTOPNICD1/+. IL in Nkx3.1Cre/+;Rosa26flSTOPNICD1/+ is misfolded and hypomorphic. GFP positive cells, both in IL and AL appear to retain high levels of SOX2. Scale bars represent 100 μm for A and 50 μm for F and G. IL is underlined. H.Quantification of the proportion of recombined cells maintaining expression of SOX2 in AL at 18.5dpc. There is a significant increase in the proportion of recombined cells retaining SOX2 expression when NICD is present (n = 3 pituitary/genotype). Moreover this effect is significantly more robust in Sox2CreERT2/+;Rosa26flSTOPNICD1/+ compared to Nkx3.1Cre/+;Rosa26flSTOPNICD1/+. Data are presented as mean ± SD, unpaired t test performed, p=0.0003 when comparing Sox2CreERT2/+;Rosa26ReYFP/+and Sox2CreERT2/+;Rosa26flSTOPNICD1/+, p=0.0001 when comparing Nkx3.1Cre/+;Rosa26ReYFP and Nkx3.1Cre/+;Rosa26flSTOPNICD1/+ and 0.0299 when comparing Sox2CreERT2/+;Rosa26flSTOPNICD1/+ and Nkx3.1Cre/+;Rosa26flSTOPNICD1/. P is calculated after angular transformation of percentages, n = 3 for each genotype.
-
Figure 1—source data 1
Countings for graph H.
- https://doi.org/10.7554/eLife.33318.005
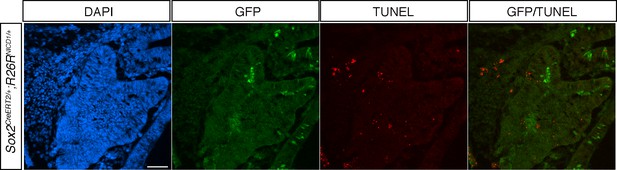
IL recombined cells in 12.5dpc Sox2CreERT2/+;Rosa26flSTOPNICD1/+ Rathke’s pouch are not eliminated by apoptosis.
Immunofluorescence for GFP and TUNEL assay. Recombined GFP positive cells are present in RP and the overlying infundibulum. No TUNEL positive signal is detected in NICD1iresGFP positive cells. Scale bar represents 50 μm.
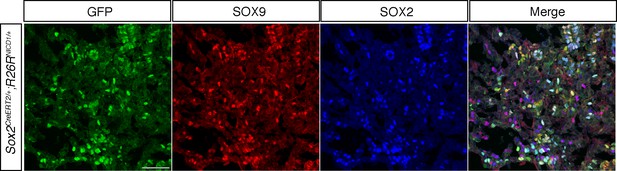
SOX9 is up-regulated in Sox2CreERT2/+;Rosa26flSTOPNICD1/+ progenitors.
Immunofluorescence for SOX2, SOX9 and GFP. Despite sustained activity of NOTCH pathway, most NICD1iresGFP positive cells up-regulate expression of SOX9 in 18.5dpc pituitary progenitors. Scale bar represents 50 μm.
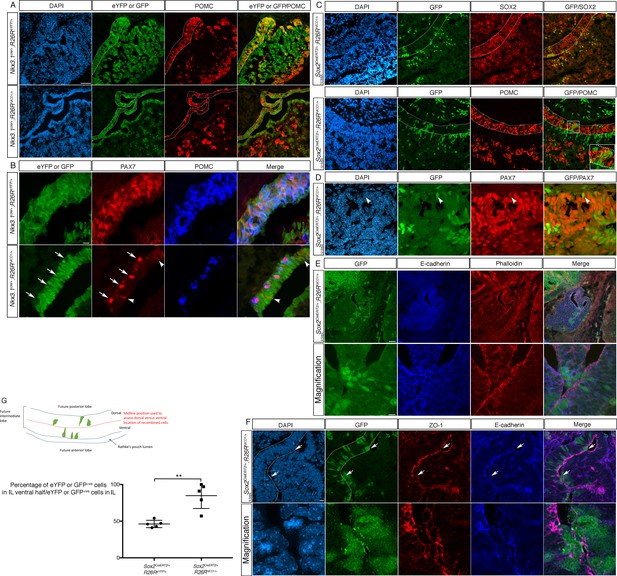
Differential timing of NOTCH activation IL progenitors has contrasting phenotypic consequences.
(A) Immunofluorescence of 18.5dpc Nkx3.1Cre/+;Rosa26ReYFP and Nkx3.1Cre/+;Rosa26flSTOPNICD1/+ pituitaries. NICD1iresGFP positive cells do not express POMC in contrast with eYFP positive cells in control embryos. (B) Immunofluorescence of 18.5dpc Nkx3.1Cre/+;Rosa26ReYFP and Nkx3.1Cre/+;Rosa26flSTOPNICD1/+. In control embryos, IL melanotrophs express PAX7 and POMC while NICD1iresGFP positive cells in mutants are unable to upregulate POMC. Some show low levels of PAX7 expression (arrowhead) suggesting commitment toward melanotroph fate. A few cells upregulate PAX7 and POMC in mutants, but are NICD1iresGFP negative non-recombined cells (arrows). (C, D) Immunofluorescence of 18.5dpc Sox2CreERT2/+;Rosa26flSTOPNICD1/+pituitaries induced at 12.5dpc. NICD1iresGFP positive cells are now observed in IL. These express SOX2, but are POMC negative (high magnification inset). Similarly to what is observed in Nkx3.1Cre/+;Rosa26flSTOPNICD1/+embryos, some NICD1iresGFP cells express PAX7 (D, arrowhead). (E) Immunofluorescence of 12.5dpc Sox2CreERT2/+;Rosa26flSTOPNICD1/+ induced at 9.5dpc. Lower panel represents a magnification of the boxed area. NICD1iresGFP IL positive cells appear clustered ventrally toward AL. E-cadherin staining is lost from the apical membranes of NICD;GFP positive cells. F-actin is stained using phalloidin. (F) Immunofluorescence of 12.5dpc Sox2CreERT2/+;Rosa26flSTOPNICD1/+ induced at 9.5dpc. Lower panel represents a magnification of the boxed area. Expression of ZO-1, along with that of E-cadherin is lost/altered from the apical membranes of the NICD;GFP positive cells that are closest to the cleft. Therefore, both tight and adherent junctions appear abnormal in these cells, suggesting a loss of epithelial polarity. (G) Proportion of recombined cells located in the ventral half of IL in 12.5dpc Sox2CreERT2/+;Rosa26ReYFP and Sox2CreERT2/+;Rosa26flSTOPNICD1/+pituitaries induced at 9.5dpc. In control Sox2CreERT2/+;Rosa26ReYFP pituitaries, eYFP positive cells are homogeneously distributed in IL (n = 5 embryos, 58 (±8.5 SD) cells were counted/embryo). In contrast, 85% of NICD1iresGFP positive cells in mutant embryos are found in the ventral half of IL (n = 5 embryos, 29 (±23.6 SD) cells were counted/embryo). Unpaired t test performed, p=0.0045 after angular transformation of percentages. An annotated picture illustrates how cells were counted in dorsal and ventral IL. Scale bars represent 50 μm for A, C and D (upper panel), 20 μm for B and 10 μm for E (magnification) and F. IL is underlined.
-
Figure 2—source data 1
Countings for graph G.
- https://doi.org/10.7554/eLife.33318.008
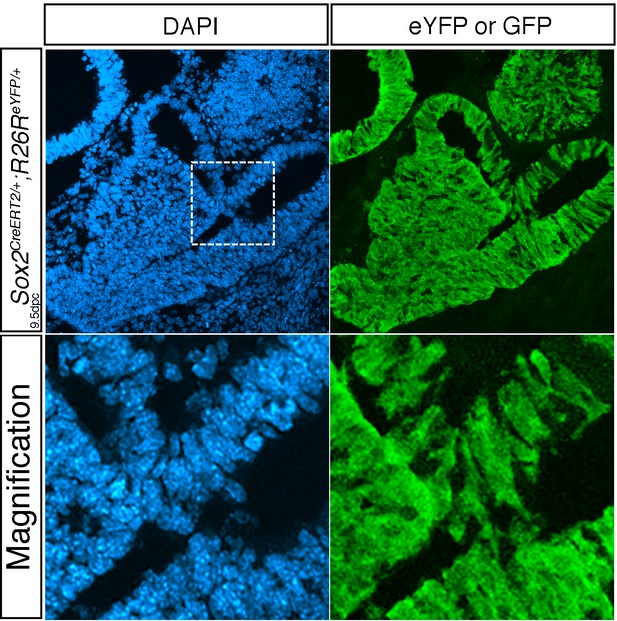
Contact between prospective IL and AL in control embryos.
Immunofluorescence for GFP in 12.5dpc Sox2CreERT2/+;Rosa2ReYF1/+embryos. A zone of apparent contact between the prospective IL and AL is observed.
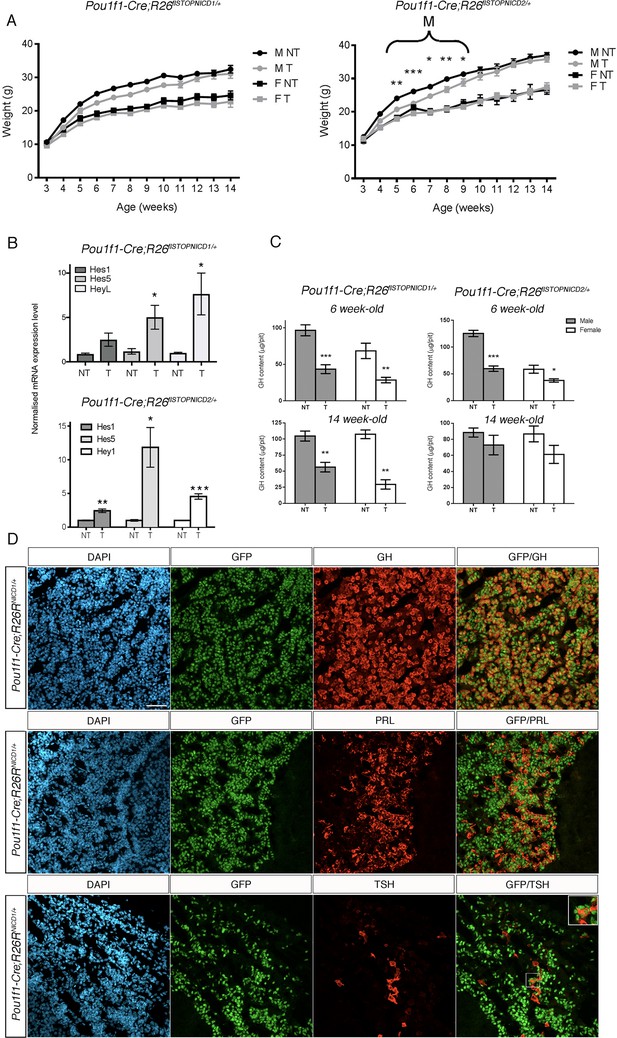
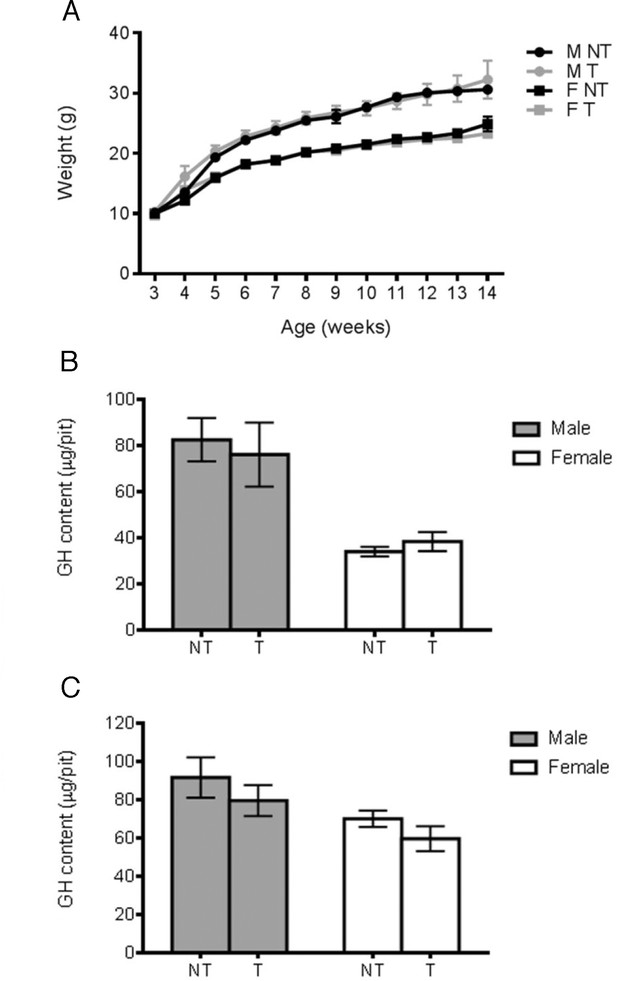
Activation of NOTCH pathway in POU1F1 lineage alters somatotrophs post-natally.
(A) Growth curves of Pou1f1-Cre; Rosa26flSTOPNICD1/+ and Pou1f1-Cre; Rosa26flSTOPNICD2/+ animals. Male (M) and female (F) mutant (T) and wild-type (NT) littermates were monitored from weaning at 3 weeks of age until 14 weeks of age. There is a transient significant weight reduction around puberty in Pou1f1-1Cre;R26flSTOPNICD2/+ animals. A similar tendency is observed in Pou1f1-1Cre;R26flSTOPNICD1/+ animals but this does not reach significance. (n = 3 to 14 mice/sex and genotype, mixed-effects model of weight versus age was used, using genotype as fixed factors and subject (mice) as random factors, with analysis of variance (ANOVA) to test the overall effect of genotype on growth, followed by Tukey post-hoc tests). (B) RT-qPCR analysis of NOTCH target genes in Pou1f1-1Cre;R26flSTOPNICD1 and 2/+ adult pituitaries. There is a significant increase in expression levels of three NOTCH targets, Hes1, Hes5 and Heyl in Pou1f1-1Cre;R26flSTOPNICD1/+ animals and Hes1, Hes5 and Hey1 in Pou1f1-1Cre;R26flSTOPNICD2/+ animals. This demonstrates that the pathway is overactivated (n = 3 to 6 pituitaries/genotype in Pou1f1-Cre; Rosa26flSTOPNICD1/+ panel –NT- are Rosa26flSTOPNICD1/+ and –T- Pou1f1-Cre; Rosa26flSTOPNICD1/+ unpaired t test performed). (C) Growth hormone (GH) pituitary contents were assayed by sandwich ELISA. GH contents of Pou1f1-1Cre;R26flSTOPNICD1/+ male (p=0.0003 at 6 weeks, p=0.0032 at 14 weeks) and female (p=0.0048 at 6 weeks and p=0.0013 at 14 weeks) mice are significantly reduced at both 6 and 14 weeks of age compared to wild-type littermates. In Pou1f1-1Cre;R26flSTOPNICD2/+there is only a transient deficit at 6week-old (p<0.0001 for males and p=0.0219 for females). This shows that somatotrophs function is affected by NICD expression (n = 3 to 6 mice/sex and genotype, unpaired t test performed). (D) Immunofluorescence of 6 week-old Pou1f1-1Cre;R26flSTOPNICD1/+pituitaries. NICD1iresGFP positive cells show expression of GH, PRL and TSH (with high magnification inset), suggesting that somatotroph, lactotroph and thyrotroph cell fate acquisition has not been impaired by NOTCH activation. Scale bar represent 50 μm.
-
Figure 3—source data 1
Weights for Pou1f1Cre;R26 flSTOPNICD1/+ growth curves.
- https://doi.org/10.7554/eLife.33318.010
-
Figure 3—source data 2
Weights for Pou1f1Cre;R26 flSTOPNICD2/+ growth curves.
- https://doi.org/10.7554/eLife.33318.011
-
Figure 3—source data 3
GH contents for Pou1f1Cre;R26 flSTOPNICD1/+.
- https://doi.org/10.7554/eLife.33318.012
-
Figure 3—source data 4
GH contents for Pou1f1Cre;R26 flSTOPNICD2/+.
- https://doi.org/10.7554/eLife.33318.013
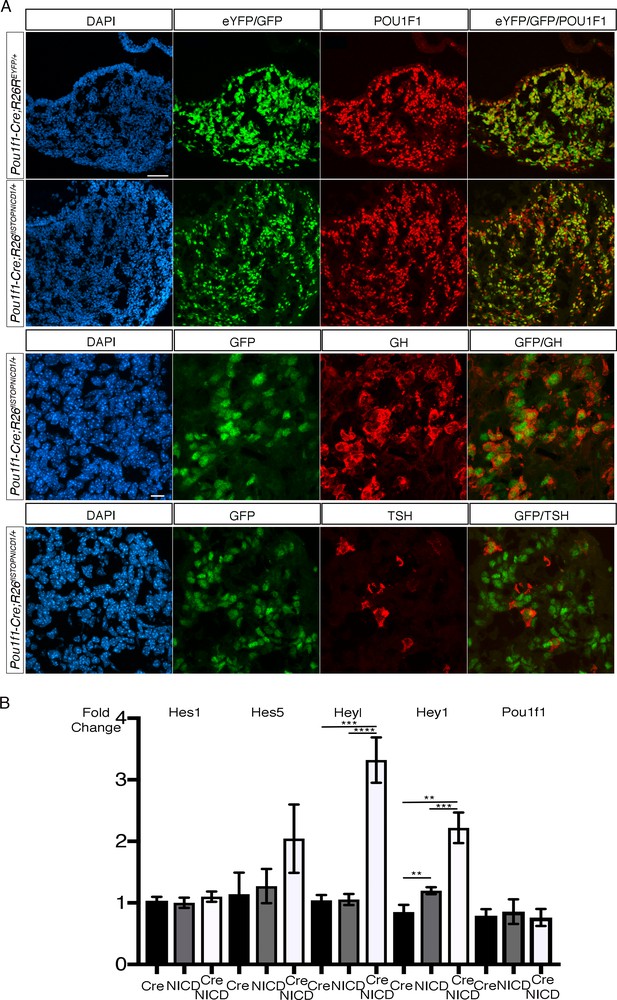
Expression of NICD1 in POU1F1 lineage does not alter cell fate acquisition in the developing pituitary and this correlates with inefficient activation of NOTCH pathway.
(A) Immunofluorescence of 18.5dpc Pou1f1-Cre;Rosa26ReYFP and Pou1f1-Cre;Rosa26flSTOPNICD1/+pituitaries. In both control and mutant embryos, the pattern of recombination appears similar. EYFP, in controls, and NICD1iresGFP positive cells in mutants co-express PIT1, GH or TSH, suggesting that cell fate acquisition is not altered. Scale bars represent 50 μm (upper panels, low magnification) and 10 μm (lower panels, higher magnification). (B) RT-qPCR analysis of NOTCH target genes in Pou1f1-1Cre;R26flSTOPNICD1/+pituitaries at 18.5dpc. We only observe a significant, but mild upregulation of Hey1 and Heyl in Pou1f1-1Cre;R26flSTOPNICD1/+pituitaries compared to both Pou1f1-1Cre and R26flSTOPNICD1/+controls (n = 5 to 10 pituitaries/genotype, unpaired t test performed). Consequently, Pou1f1 expression levels are not affected in mutants. This suggests that the pathway cannot be efficiently activated in the POU1F1 lineage.
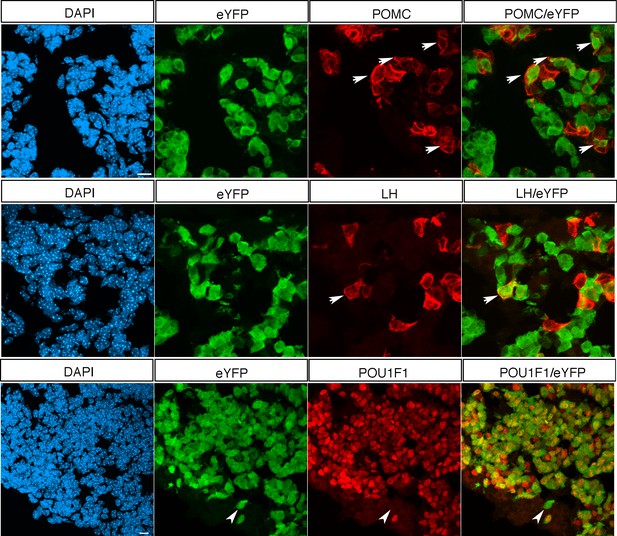
Characterization of POU1F1-Cre by lineage tracing analysis at 18.5dpc.
Immunofluorescence for eYFP, POMC, LH and POU1F1 in Pou1f1-Cre;Rosa26ReYFP/+ 18.5 dpc pituitaries. There is some ectopic activity as a subset of recombined cells (arrows) are positive for POMC (corticotroph) or LH (gonadotroph), as previously reported (Gaston-Massuet et al., 2011). We estimate that 4.9% (±2.5 SD, n = 3) of recombined cells are not POU1F1 positive (arrowhead), reflecting this ectopic activity. However, the vast majority of POU1F1 positive cells −78.2%-(±11.16 SD, n = 3) are, as expected, eYFP positive. The 21.8% of POU1F1 positive;eYFP negative cells are noticeably present mostly near the progenitor layer. This suggests that cells that have recently committed, and upregulated expression of POU1F1, have not undergone recombination yet. Scale bars represent 10 μm.
Deletion of NOTCH pathway transcriptional mediator Rbpj in POU1F1 lineage has no effect on GH levels.
(A) Growth curves of Pou1f1-Cre;Rbpjfl/fl animals. Male (M) and female (F) mutant (T) and wild-type (NT) littermates were monitored from weaning at 3 weeks of age until 14 weeks of age. There is no significant difference between wild-type littermate and mutants (n = 3 to 9 mice/sex and genotype, mixed-effects model of weight versus age was used, using genotype as fixed factors and subject (mice) as random factors, with analysis of variance (ANOVA) to test the overall effect of genotype on growth, followed by Tukey post-hoc tests). (B, C) GH pituitary contents were assayed by sandwitch ELISA in 6 week-old (B, n = 3 to 6 pituitaries/sex and genotype) and 14 week-old (C, n = 3 to 7 pituitaries/sex and genotype) animals. There is no significant difference between wild-type and mutant animals (unpaired t test performed).
-
Figure 5—source data 1
Weights for Pou1f1Cre;RBPJfl/fl growth curves.
- https://doi.org/10.7554/eLife.33318.017
-
Figure 5—source data 2
GH contents for Pou1f1Cre;RBPJfl/fl 42 days-old.
- https://doi.org/10.7554/eLife.33318.018
-
Figure 5—source data 3
GH contents for Pou1f1Cre;RBPJfl/fl 100 days-old.
- https://doi.org/10.7554/eLife.33318.019
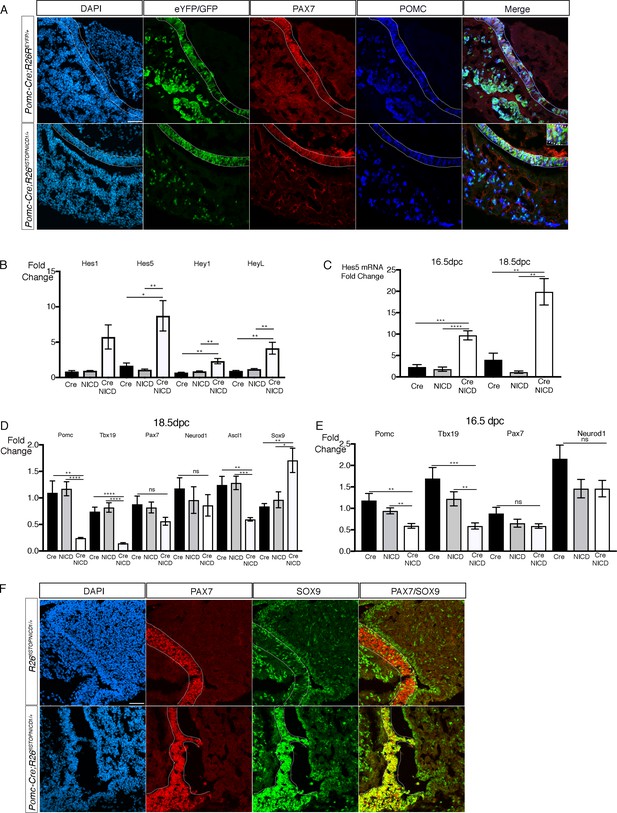
Activation of NOTCH pathway in the POMC lineage results in a gradual loss of differentiation markers in the embryo.
(A) Immunofluorescence of 18.5dpc Pomc-Cre;Rosa26ReYFP and Pomc-Cre;Rosa26flSTOPNICD1/+ pituitaries. In both control Pomc-Cre;Rosa26ReYFP and mutant Pomc-Cre;Rosa26flSTOPNICD1/+ embryos, the pattern of recombination appears similar. EYFP, in controls, and NICD1iresGFP positive cells in mutants co-express PAX7 and POMC (with high magnification inset), suggesting that cell fate acquisition is not altered. Fluorescence detection thresholds are identical between control and mutant. (B) RT-qPCR analysis of NOTCH pathway target genes in 18.5dpc pituitaries. There is a significant induction of Hes5, Heyl, and Hey1 expression following NICD expression (n = 3 to 4 pituitaries/genotype unpaired t test performed). (C) RT-qPCR analysis of Hes5 in 16.5 and 18.5dpc Pomc-Cre;Rosa26flSTOPNICD1/+ pituitaries. Levels of Hes5 are doubling between 16.5 and 18.5dpc (n = 4 to 7 pituitaries/genotype, unpaired t test performed). (D) RT-qPCR analysis of POMC lineage cell-type markers in 18.5dpc Pomc-Cre;Rosa26flSTOPNICD1/+ pituitaries. There is a significant reduction of POMC, Tbx19 and Ascl1 expression while Sox9 is up-regulated. In contrast expression of Pax7 and Neurod1 is not significantly affected (n = 4 to 6 pituitaries/genotype, unpaired t test performed). (E) RT-qPCR analysis of POMC lineage cell-type markers in 16.5dpc Pomc-Cre;Rosa26flSTOPNICD1/+ pituitaries. The same tendency is observed, but downregulation is milder, suggesting that gene expression is further altered as Hes5 induction becomes more robust (n = 5 to 7 pituitaries/genotype, unpaired t test performed). (F) Immunofluorescence of 18.5dpc Rosa26flSTOPNICD1/+ and Pomc-Cre;Rosa26flSTOPNICD1/+ pituitaries. In the control Rosa26flSTOPNICD1/+ section, PAX7 is present in melanotrophs, while SOX9 is restricted to stem cells lining the cleft in IL and AL, and glial cells in PL. PAX7 and SOX9 are hardly expressed by the same cells. In contrast, in Pomc-Cre;Rosa26flSTOPNICD1/+ there is a clear co-localisation of SOX9 and PAX7 in IL. Scale bar represents 50 μm. IL is underlined.
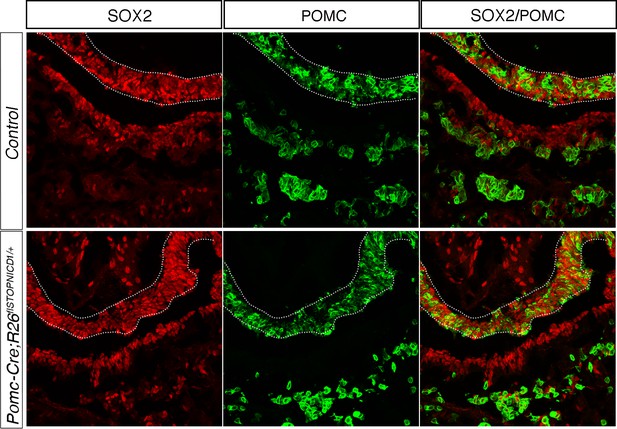
In Pomc-Cre;Rosa26flSTOPNICD1/+ SOX2 expression levels appear similar in progenitors and melanotrophs.
Immunofluorescence for SOX2 and POMC. In control samples, SOX2 expression is more intense in the progenitors lining the cleft than in POMC positive melanotrophs at 18.5dpc (Goldsmith et al., 2016). In contrast, in Pomc-Cre;Rosa26flSTOPNICD1/+ pituitaries, expression levels appear homogeneous across both cell types.
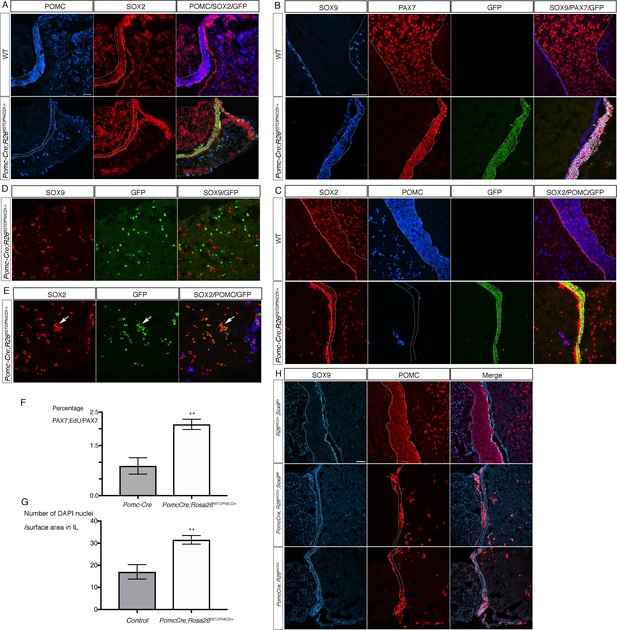
Activation of NOTCH pathway in the POMC lineage results in regression of melanotrophs and corticotrophs toward a progenitor-like fate post-natally.
(A) Immunofluorescence of P7 wild-type and Pomc-Cre;Rosa26flSTOPNICD1/+ pituitaries. Expression of POMC is dramatically down-regulated in IL and NICD1iresGFP positive corticotrophs in AL. SOX2 staining appears of similar intensity in both IL and SCs in mutants, while in control its expression is clearly lower in IL melanotrophs compared to SCs. (B, C) Immunofluorescence of 4 month-old wild-type and Pomc-Cre;Rosa26flSTOPNICD1/+ IL. There is a clear co-localisation of PAX7 and SOX9 in mutant IL that is never observed in wild-type samples (B). POMC expression is almost absent in the SOX2 positive IL cells in mutants (C). In addition, IL is thinner in mutants, while cell density appears increased. (D, E) Immunofluorescence of 4 month-old wild-type and Pomc-Cre;Rosa26flSTOPNICD1/+ AL. In contrast with what is observed in IL, SOX9 is not expressed in AL NICD1iresGFP positive cells (D), but SOX2 is ectopically induced in the mutant cells (arrow, E). (F) Analysis of cell proliferation in PAX7 positive cells at P2. Pups were injected with EdU and pituitaries harvested one hour later. There is a significant increase in cell proliferation in Pomc-Cre;Rosa26flSTOPNICD1/+ PAX7 positive cells (n = 3 to 4 pups/genotype, unpaired t test performed, p=0.0013 is calculated after angular transformation of percentages). (G) Analysis of cell density in 5 month-old IL (n = 3 to 4 pituitaries/genotype, unpaired t test performed, p=0.0011). (H) Immunofluorescence for SOX9 and POMC in 3 week-old pituitaries. Despite loss of ectopic SOX9 expression in Pomc-Cre;Rosa26flSTOPNICD1/+;Sox9 fl/fl POMC expression is still down-regulated in the hypoplastic IL. Scale bar represent 50 μm in A, H and in B for C-E. IL is underlined.
-
Figure 7—source data 1
Countings for graph F.
- https://doi.org/10.7554/eLife.33318.025
-
Figure 7—source data 2
Countings for graph G.
- https://doi.org/10.7554/eLife.33318.026
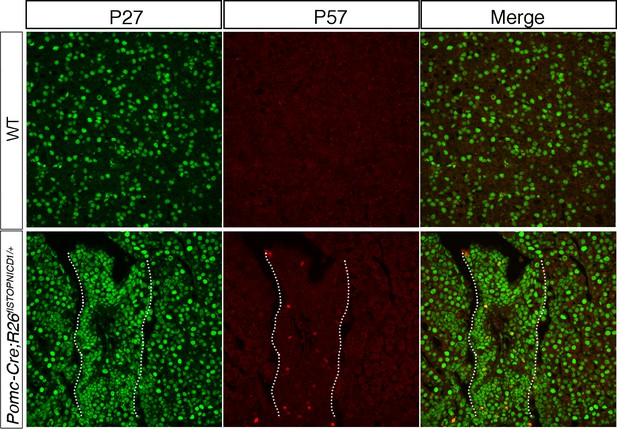
P57 is up-regulated in some cells in Pomc-Cre;Rosa26flSTOPNICD1/+ IL.
Immunofluorescence for P27 and P57. Expression of P27 is ubiquitous in wild-type IL and mutant adult samples. P57 is not normally expressed anymore in adult WT (Bilodeau et al., 2009), while a few cells reactivate its expression in Pomc-Cre;Rosa26flSTOPNICD1/+ pituitaries. IL is underlined in Pomc-Cre;Rosa26flSTOPNICD1/+.
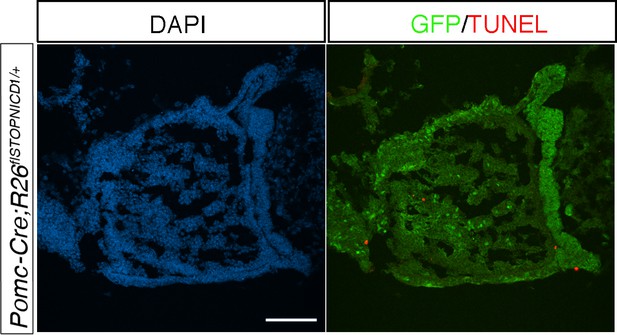
Reduction of intermediate lobe size in Pomc-Cre;Rosa26flSTOPNICD1/+ pituitaries is not due to cell apoptosis.
Immunofluorescence for GFP and TUNEL assay. In P2 pituitaries NICD1iresGFP positive cells are not TUNEL positive. Scale bar represents 50 μm.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| strain, strain background (mus musculus) | Nkx3.1Cre | Lin, Y., Liu, G., Zhang, Y., Hu, Y.P., Yu, K., Lin, C., McKeehan, K., Xuan, J.W., Ornitz, D.M., Shen, M.M., et al. (2007). Fibroblast growth factor receptor 2 tyrosine kinase is required for prostatic morphogenesis and the acquisition of strict androgen dependency for adult tissue homeostasis. Development 134, 723–734. | Nkx3-1tm3(cre)Mms | |
| strain, strain background (mus musculus) | Sox2CreERT2 | Arnold, K., Sarkar, A., Yram, M.A., Polo, J.M., Bronson, R., Sengupta, S., Seandel, M., Geijsen, N., and Hochedlinger, K. (2011). Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 9, 317–329. | Sox2tm1(cre/ERT2)Hoch | |
| strain, strain background (mus musculus) | Pou1f1-Cre | this paper | (nogene)Tg(pou1f1-cre)1Rsd | |
| strain, strain background (mus musculus) | Rbpjfl | Han, H., Tanigaki, K., Yamamoto, N., Kuroda, K., Yoshimoto, M., Nakahata, T., Ikuta, K., and Honjo, T. (2002). Inducible gene knockout of transcription factor recombination signal binding protein-J reveals its essential role in T versus B lineage decision. Int Immunol 14, 637–645. | RBPJtm1Hon | |
| strain, strain background (mus musculus) | Rosa26ReYFP | Srinivas, S., Watanabe, T., Lin, C.S., William, C.M., Tanabe, Y., Jessell, T.M., and Costantini, F. (2001). Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1, 4. | Gt(Rosa26)Sortm1(EYFP)Cos | |
| strain, strain background (mus musculus) | Rosa26floxSTOP-Nicd1 | Murtaugh, L.C., Stanger, B.Z., Kwan, K.M., and Melton, D.A. (2003). Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A 100, 14920–14925. | Gt(Rosa26)Sortm1(Notch1)Dam | |
| strain, strain background (mus musculus) | Rosa26floxSTOP-Nicd2 | Fujimura, S., Jiang, Q., Kobayashi, C., and Nishinakamura, R. (2010). Notch2 activation in the embryonic kidney depletes nephron progenitors. J Am Soc Nephrol 21, 803–810. | Gt(Rosa)26Sortm1(Notch2)Nis | |
| strain, strain background (mus musculus) | Pomc-Cre | Langlais, D., Couture, C., Kmita, M., and Drouin, J. (2013). Adult pituitary cell maintenance: lineage-specific contribution of self-duplication. Mol Endocrinol 27, 1103–1112. | ||
| Antibody | goat anti-Sox2 | ISS | GT15098 | 1/300 |
| Antibody | rat anti-GFP | Fine Chemical products | 04404–84 | 1/1000 |
| Antibodies | anti pituitary hormones | NHPP | ||
| Antibody | mouse anti-Pax7 | DSHB | P3U1 | 1/100 |
Additional files
-
Supplementary file 1
RT-qPCR primer sequences.
- https://doi.org/10.7554/eLife.33318.027
-
Transparent reporting form
- https://doi.org/10.7554/eLife.33318.028





