A new mode of pancreatic islet innervation revealed by live imaging in zebrafish
Figures
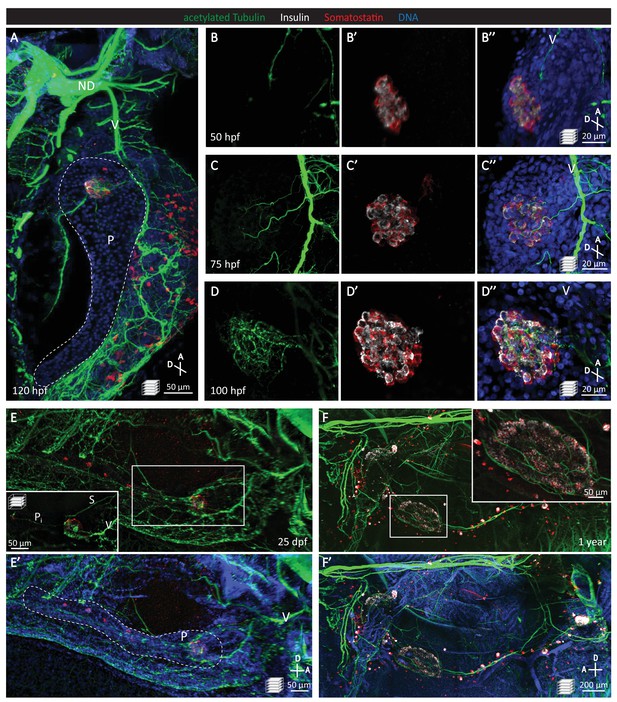
Pancreatic islet innervation in zebrafish is established early in development and maintained in juvenile and adult stages.
(A-D) Whole mount immunostaining of wild-type zebrafish at 50, 75, 100, 120 hr post fertilization (hpf) for acetylated Tubulin (nerves), Insulin (beta cells), Somatostatin (delta cells), and DAPI (DNA). (E–F) Whole mount immunostaining of 25 days post fertilization (dpf) zebrafish and 1 year zebrafish pancreas and intestine following tissue clearing with the CLARITY protocol. Maximum intensity projections are presented; A, anterior; D, dorsal; V, vagus nerve; ND, nodose ganglion; P, pancreas; S, sympathetic innervation; Pi, intra-pancreatic innervation.
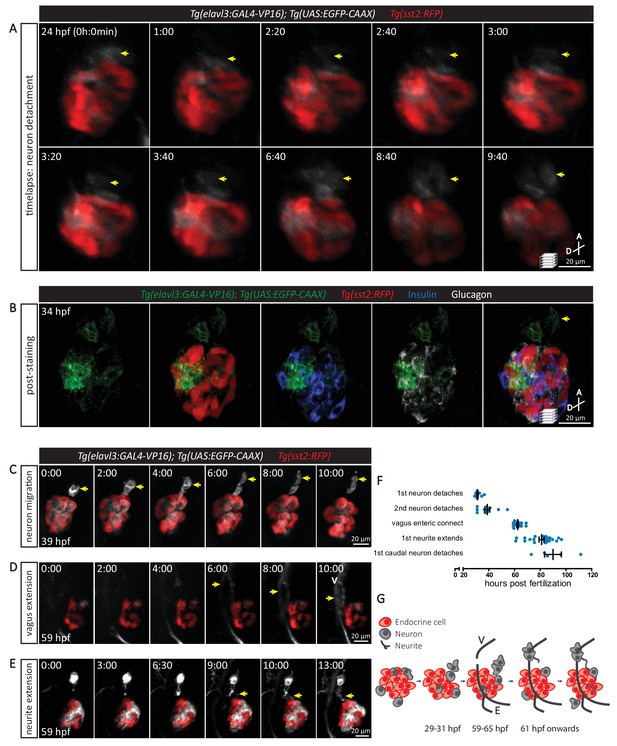
The sequence of cellular events preceding pancreatic islet parasympathetic innervation is revealed with single-cell resolution time-lapse confocal imaging.
(A) Tg(elavl3:Gal4-VP16); Tg(UAS:EGFP-CAAX); Tg(sst2:RFP) zebrafish mounted in 0.5% agarose containing 0.017% tricaine were imaged with laser scanning confocal microscopy at 20 min time intervals. Maximum intensity projections of selected timeframes are presented (A, anterior; D, dorsal). Yellow arrows point to the detachment of neurons from the pancreatic islet. (B) Whole mount immunostaining at 34 hpf for GFP (neurons), RFP (delta cells), Insulin (beta cells), and Glucagon (alpha cells) after 10 hr time-lapse imaging. The detached neurons are not positive for endocrine cell markers. (C-E) Confocal imaging of Tg(elavl3:Gal4-VP16); Tg(UAS:EGFP-CAAX); Tg(sst2:RFP) zebrafish at 20–30 min time intervals. Maximum intensity projections of selected timeframes are presented. Yellow arrows point to the cellular events of interest. (F) Quantification of the time when the indicated cellular events were observed for individual fish (mean ± SEM). (G) Schematic of the sequence of cellular events preceding parasympathetic innervation of the pancreatic islet. V, vagus nerve; E, enteric nerve.

A subset of pancreatic nerve extensions derives from neurons that were once in close contact with endocrine cells.
Whole mount immunostaining at 80 hpf for GFP (elavl3-positive cells), acetylated Tubulin (nerves), Insulin (beta cells), and RFP (delta cells). Yellow arrow points to elavl3 expressing cell projecting neural extensions toward the pancreatic islet.
Time-lapse imaging shows detachment of neurons from the pancreatic islet.
24 hpf Tg(elavl3:Gal4-VP16); Tg(UAS:EGFP-CAAX); Tg(sst2:RFP) zebrafish mounted in 0.5% agarose with tricaine was imaged with laser scanning confocal microscopy at 20 min time intervals. Movie corresponds to Figure 2A.
Time-lapse imaging shows detachment of neurons from the pancreatic islet.
24 hpf Tg(elavl3:Gal4-VP16); Tg(UAS:EGFP-CAAX); Tg(sst2:RFP) zebrafish mounted in 0.5% agarose with tricaine was imaged with laser scanning confocal microscopy at 20 min time intervals.
Time-lapse imaging shows neurons migrating away from the pancreatic islet.
39 hpf Tg(elavl3:Gal4-VP16); Tg(UAS:EGFP-CAAX); Tg(sst2:RFP) zebrafish mounted in 0.5% agarose with tricaine was imaged with laser scanning confocal microscopy at 20 min time intervals. Movie corresponds to Figure 2C.
Time-lapse imaging shows vagus nerve extending past the pancreatic islet.
59 hpf Tg(elavl3:Gal4-VP16); Tg(UAS:EGFP-CAAX); Tg(sst2:RFP) zebrafish mounted in 0.5% agarose with tricaine was imaged with laser scanning confocal microscopy at 30 min time intervals. Movie corresponds to Figure 2D.
Time-lapse imaging shows neurite extending toward the pancreatic islet.
59 hpf Tg(elavl3:Gal4-VP16); Tg(UAS:EGFP-CAAX); Tg(sst2:RFP) zebrafish mounted in 0.5% agarose with tricaine was imaged with laser scanning confocal microscopy at 30 min time intervals. Movie corresponds to Figure 2E.
Time-lapse imaging shows interaction of detached neurons with the vagus nerve.
59 hpf Tg(elavl3:Gal4-VP16); Tg(UAS:EGFP-CAAX); Tg(sst2:RFP) zebrafish mounted in 0.5% agarose with tricaine was imaged with laser scanning confocal microscopy at 30 min time intervals.
Time-lapse imaging shows detachment and caudal migration of neurons from the pancreatic islet.
83 hpf Tg(elavl3:Gal4-VP16); Tg(UAS:EGFP-CAAX); Tg(sst2:RFP) zebrafish mounted in 0.5% agarose with tricaine was imaged with laser scanning confocal microscopy at 45 min time intervals.
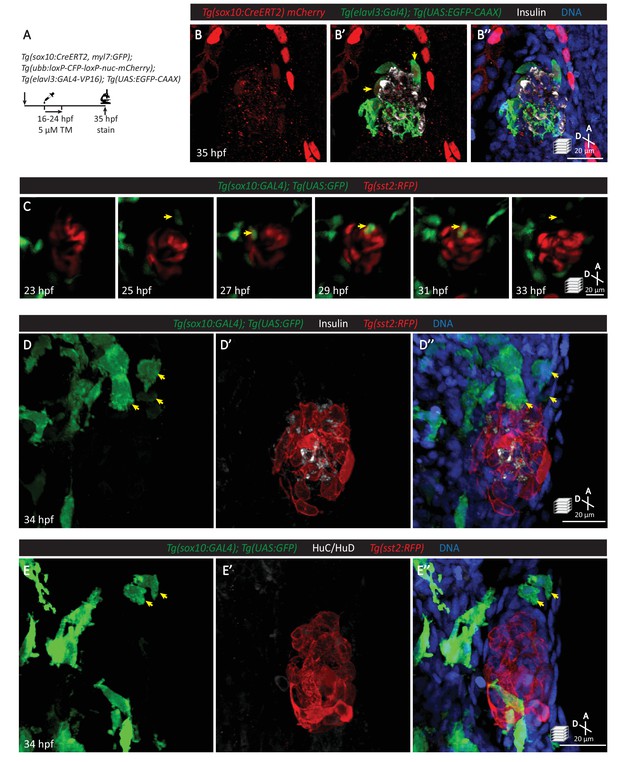
Neural crest cells are in close contact with pancreatic endocrine cells early in development.
(A) Lineage tracing of neural crest cells in Tg(sox10:CreERT2, myl7:GFP); Tg(ubb:loxP-CFP-loxP-nuc-mCherry); Tg(elavl3:GAL4-VP16); Tg(UAS:EGFP-CAAX) zebrafish following 5 µM tamoxifen treatment from 16 to 24 hpf and staining at 35 hpf. (B) Whole mount immunostaining at 35 hpf for mCherry (neural-crest-derived cells), GFP (elavl3-positive cells), Insulin (beta cells), and DAPI (DNA). (C) Confocal imaging of Tg(sox10:GAL4); Tg(UAS:GFP); Tg(sst2:RFP) zebrafish mounted in 0.5% agarose from 23 to 33 hpf. Yellow arrow points to a neural crest cell in close proximity to endocrine pancreatic cells and briefly contacting islet cells before migrating away. (D) Whole mount immunostaining at 34 hpf for GFP (sox10-positive cells), Insulin (beta cells), RFP (delta cells), and DAPI (DNA). Yellow arrows point to neural-crest-derived cells that were once in contact with the pancreatic islet. (E) Whole mount immunostaining at 34 hpf for GFP (sox10-positive cells), HuC/HuD (mature neurons), RFP (delta cells), and DAPI (DNA). Yellow arrows point to neural-crest-derived cells that were once in contact with the pancreatic islet.
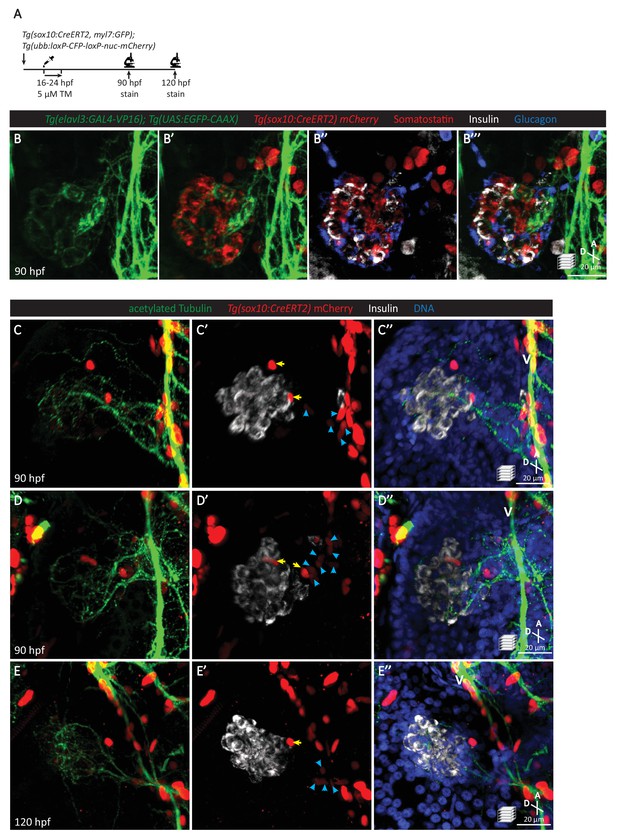
Pancreatic islet parasympathetic innervation is derived from the neural crest.
(A) Lineage tracing of neural crest cells in Tg(sox10:CreERT2, myl7:GFP); Tg(ubb:loxP-CFP-loxP-nuc-mCherry) zebrafish following 5 µM tamoxifen treatment from 16 to 24 hpf and staining at 90 and 120 hpf. (B) Whole mount immunostaining at 90 hpf for GFP (elavl3-positive cells), mCherry (neural-crest-derived cells), Somatostatin (delta cells), Insulin (beta cells), and Glucagon (alpha cells). (C–E) Whole mount immunostaining at 90 (C, D) and 120 (E) hpf for acetylated Tubulin (nerves), Insulin (beta cells), mCherry (neural-crest-derived cells), and DAPI (DNA). Yellow arrows point to neural-crest-derived cells on the periphery of the pancreatic islet; blue arrowheads point to neural-crest-derived cells projecting neural extensions toward the pancreatic islet, and some of these cells are adjacent to the vagus nerve (V).

Neural crest cells are essential for the establishment of islet parasympathetic innervation.
(A) Immunostaining analysis of innervation density in wild-type and sox10 mutants at 80 hpf. (B) Wild-type and sox10-/- zebrafish at 80 hpf. (C) Body length measurements at 80 hpf, mean ± SEM, n = 26–27 animals, p-values from t tests are presented. (D) Whole larva free glucose level measurements at 80 hpf, mean ± SEM, n = 21–22 batches of five larvae per replicate. (E–F) Whole mount immunostaining at 80 hpf for acetylated Tubulin (nerves), Insulin (beta cells), Somatostatin (delta cells), and DAPI (DNA) of wild-type and sox10 mutants. sox10 mutants display a severe decline in enteric nervous density and a complete loss of pancreatic innervation, n = 8 animals.
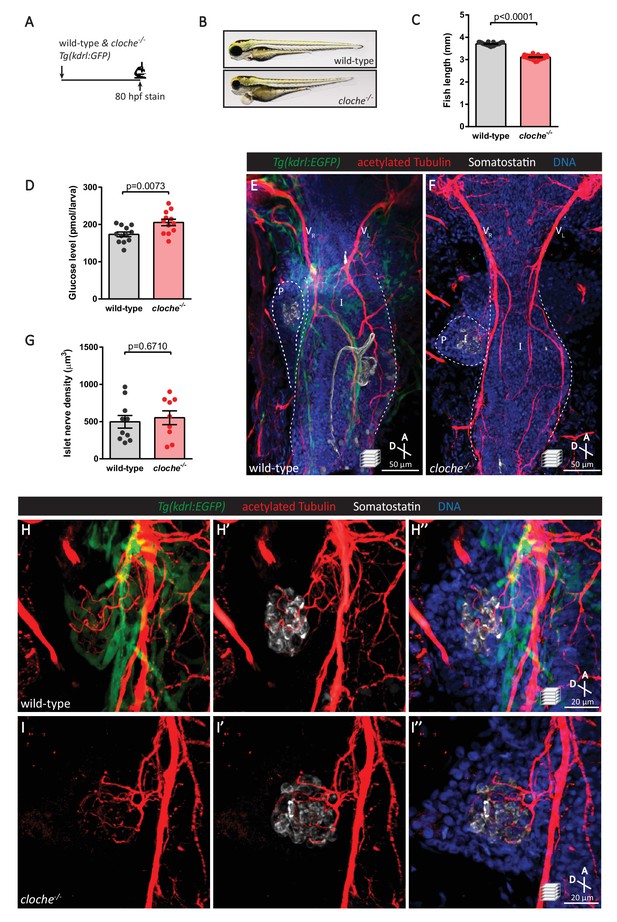
The vasculature is not essential for the initial establishment of pancreatic islet parasympathetic innervation.
(A) Immunostaining analysis of innervation density in Tg(kdrl:GFP) wild-type and cloche mutants at 80 hpf. (B) Wild-type and cloche-/- larvae were imaged at 80 hpf. (C) Body length measurements at 80 hpf, mean ± SEM, n = 21–27 animals. (D) Whole larva free glucose level measurements at 80 hpf, mean ± SEM, n = 12 batches of five larvae per replicate. (E–I) Whole mount immunostaining at 80 hpf for GFP (blood vessels), acetylated Tubulin (nerves), Somatostatin (delta cells), and DAPI (DNA). Maximum intensity projections are presented; A, anterior; D, dorsal. Quantification of islet nerve density (G), mean ± SEM, n = 9–10 animals, p-values from t tests are presented. No significant difference in vagus nerve extension and islet innervation was observed between wild-type and cloche-/- larvae. VR, right vagus nerve; VL, left vagus nerve; P, pancreas; I, intestine.

The vasculature is not essential for the initial establishment of islet parasympathetic innervation.
(A-B) Whole mount immunostaining of 4 dpf Tg(kdrl:EGFP) wild-type and cloche mutants, which lack most endothelial cells, for GFP (blood vessels), acetylated Tubulin (nerves), Insulin (beta cells), and DAPI (DNA). No significant differences are observed in islet innervation between wild-type and cloche mutant larvae.
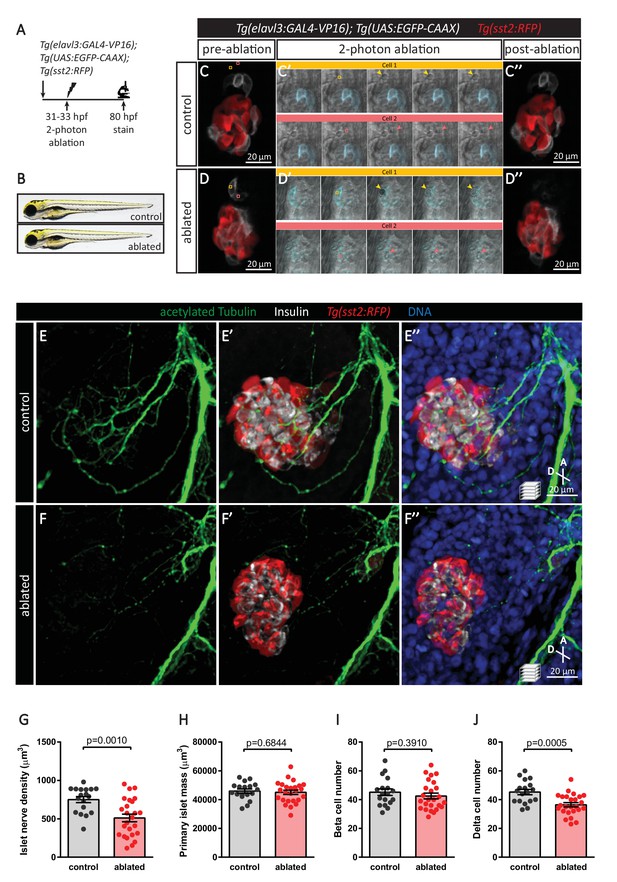
Targeted ablation studies reveal the crucial role of peri-islet neurons for the initiation of pancreatic islet parasympathetic innervation.
(A) Schematic of the two-photon ablation experiment. (B) Gross morphology at 80 hpf was comparable between control and ablated fish. (C–D) Tg(elavl3:Gal4-VP16); Tg(UAS:EGFP-CAAX); Tg(sst2:RFP) zebrafish mounted in 0.5% agarose with tricaine were subjected to two-photon laser ablation. The detached neuron clusters were ablated between 31 and 33 hpf. The control was mock ablation of cells adjacent to the leading edge of the migrating neurons using the same laser intensity. Pre-ablation, short time-lapse immediately following ablation, and post-ablation images are displayed. Orange and pink boxes outline the regions of ablation; arrowheads point to ablated cells. (E–J) Whole mount immunostaining at 80 hpf for acetylated Tubulin (nerves), Insulin (beta cells), RFP (delta cells), and DAPI (DNA). Quantification of islet nerve density (G), primary islet mass (H), beta cell number (I), and delta cell number (J), mean ± SEM, n = 18–25 animals, p-values from t tests are presented.
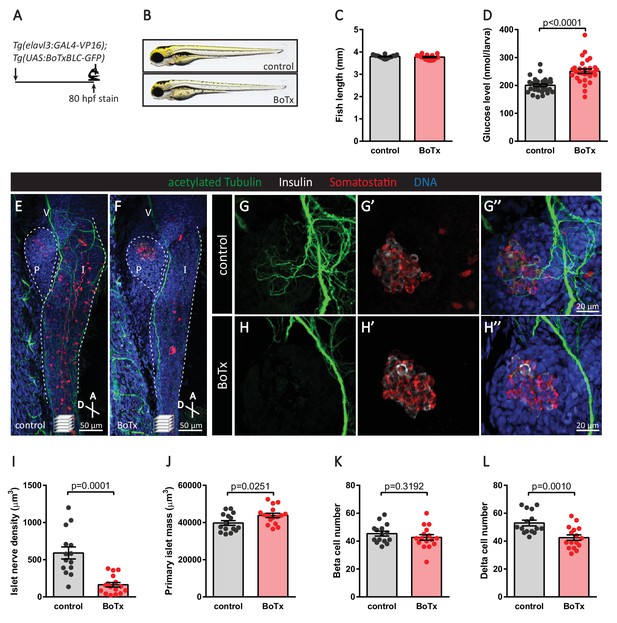
Inhibition of neural synaptic transmission diminishes islet parasympathetic innervation.
(A) Botulinum toxin (BoTx) was expressed in post-mitotic neurons to inhibit neurotransmitter release in Tg(elavl3:Gal4-VP16); Tg(UAS:BoTxBLC-GFP) zebrafish. (B) Control and BoTx-positive fish were imaged at 80 hpf. (C) Body length measurements at 80 hpf, mean ± SEM, n = 17–20 animals. (D) Whole larva free glucose level measurements at 80 hpf, mean ± SEM, n = 30 batches of five larvae per replicate. (E–L) Whole mount immunostaining at 80 hpf for acetylated Tubulin (nerves), Insulin (beta cells), Somatostatin (delta cells), and DAPI (DNA). Maximum intensity projections are presented; A, anterior; D, dorsal; V, vagus nerve; P, pancreas; I, intestine. Quantification of islet nerve density (I), primary islet mass (J), beta cell number (K), and delta cell number (L), mean ± SEM, n = 14–16 animals, p-values from t tests are presented.
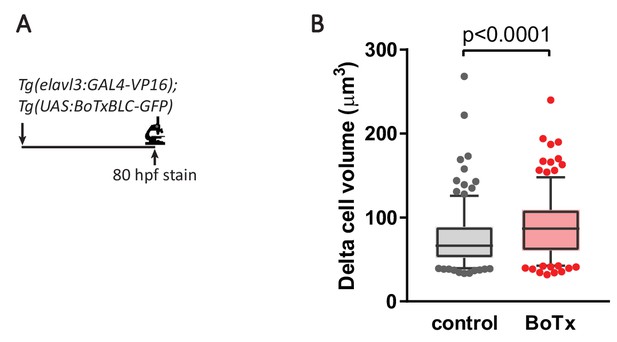
Inhibition of synaptic transmission results in delta cell hypertrophy.
(A) Botulinum toxin (BoTx) was expressed in post-mitotic neurons to inhibit neurotransmitter release in Tg(elavl3:Gal4-VP16); Tg(UAS:BoTxBLC-GFP); Tg(sst2:RFP) zebrafish. (B) Quantification of delta cell volume at 80 hpf, 5–95 percentile box plot, n = 10 animals/230 cells for control, n = 11 animals/236 cells for BoTx.
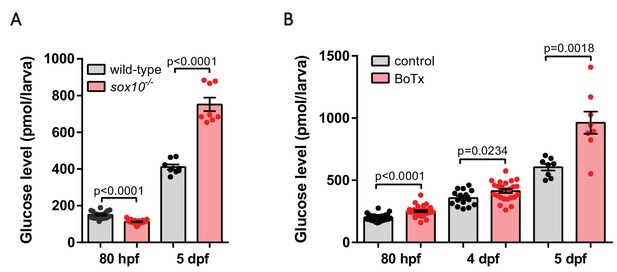
Diminished nerve density and impaired neural output result in hyperglycemia.
(A) Whole larva free glucose level measurements of wild-type and sox10-/- zebrafish at 80 hpf and 5 dpf, mean ± SEM, n = 8–22 batches of five larvae per replicate. Elevated free glucose levels were observed in sox10 mutants at 5 dpf. (B) Whole larva free glucose level measurements of control and Tg(elavl3:Gal4-VP16); Tg(UAS:BoTxBLC-GFP) zebrafish at 80 hpf, 4 dpf and 5 dpf, mean ± SEM, n = 8–30 batches of 5 larvae per replicate, p-values from t tests are presented. Elevated free glucose levels were observed in botulinum-toxin-positive fish from 80 hpf onwards.
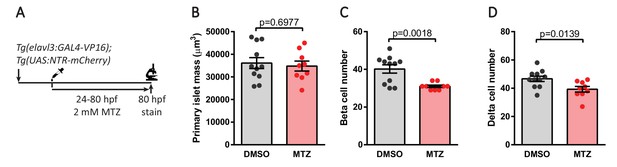
Genetic ablation of neurons decreases pancreatic endocrine cell numbers.
(A) Genetic ablation in Tg(elavl3:Gal4-VP16); Tg(UAS:NTR-mCherry) zebrafish expressing nitroreductase (NTR) in post-mitotic neurons. Treatment with 2 mM metronidazole (MTZ) from 24 to 80 hpf triggers cell death in NTR expressing cells. Whole mount immunostaining at 80 hpf for Insulin (beta cells), Somatostatin (delta cells), and DAPI (DNA). (B–D) Quantification of primary islet mass (B), beta cell number (C), and delta cell number (D), mean ± SEM, n = 9–11 animals, p-values from t tests are presented.
Tables
List of zebrafish transgenic and mutant lines.
https://doi.org/10.7554/eLife.34519.022| Name | Specificity/Purpose/Phenotype | Reference |
|---|---|---|
| Tissue specific promoter lines | ||
| Tg(elavl3:GAL4-VP16)zf357 | Post-mitotic neurons | (Stevenson et al., 2012) |
| Tg(sst2:RFP)gz19 | Pancreatic delta cells | (Li et al., 2009) |
| Tg(sox10:GAL4)sq9 | Neural crest cells | (Lee et al., 2013) |
| Tg(sox10:CreERT2, myl7:GFP)t007 | Neural crest cells, heart marker | (Mongera et al., 2013) |
| Tg(ubb:loxP-CFP-loxP-nuc-mCherry)jh63 | Ubiquitous | (Wang et al., 2015) |
| Tg(kdrl:EGFP)s843 | Endothelial cells | (Jin et al., 2005) |
| UAS lines | ||
| Tg(UAS:EGFP-CAAX)m1230 | Visualize neurons | (Fernandes et al., 2012) |
| Tg(UAS:BoTxBLC-GFP)icm21 | Inhibit neurotransmitter release | (Sternberg et al., 2016) |
| Tg(UAS:NTR-mCherry)c264 | Ablate neurons | (Davison et al., 2007) |
| Tg(UAS:GFP)zf82 | Visualize neural crest cells | (Asakawa et al., 2008) |
| Mutant lines | ||
| cloches5, clochem39 | Lacking endothelial cells | (Stainier et al., 1995; Liao et al., 1997) |
| sox10tw2 | Lacking neural crest cells | (Kelsh et al., 1996) |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.34519.023





