HIF-2α is essential for carotid body development and function
Figures
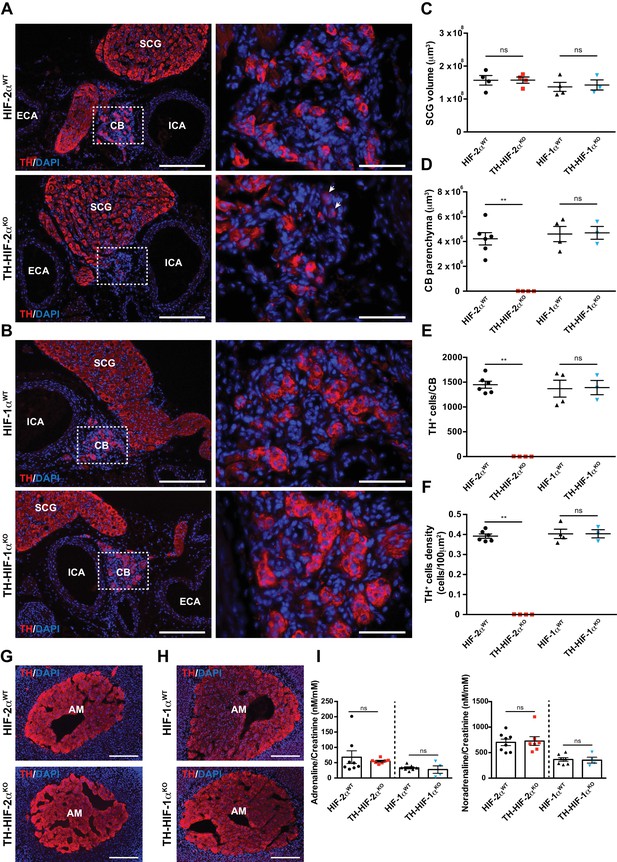
Selective loss of carotid body glomus cells in sympathoadrenal-specific Hif-2α, but not Hif-1α, deficient mice.
(A and B) Tyrosine hydroxylase (TH) immunostaining on carotid bifurcation sections from Epas1WT(A, top panels), Th-Epas1KO (A, bottom panels), Hif1aWT (B, top panels) and Th-HIF1aKO (B, bottom panels) mice (8–12 weeks old). Micrographs showed in A (bottom) were selected to illustrate the rare presence of TH+ glomus cells in Th-Epas1KO mice (white arrowheads). Dashed rectangles (left panels) are shown at higher magnification on the right panels. SCG, superior cervical ganglion; CB, carotid body; ICA, internal carotid artery; ECA, external carotid artery. Scale bars: 200 µm (left panels), 50 µm (right panels). (C–F) Quantification of total SCG volume (C), total CB volume (D), TH+ cell number (E) and TH+ cell density (F) on micrograph from Epas1WT(black dots, n = 4 for SCG and n = 6 for CB), Th-Epas1KO (red squares, n = 4), Hif1aWT (black triangles, n = 4) and Th-HIF1aKO (blue triangles, n = 3) mice. Data are expressed as mean ± SEM. Mann-Whitney test, **p<0.001; ns, non-significant. (G and H) TH-immunostained adrenal gland sections from Epas1WT(G, top panel), Th-Epas1KO (G, bottom panel), Hif1aWT (H, top panel) and Th-HIF1aKO (H, bottom panel) littermates. AM, adrenal medulla. Scale bars: 200 µm. (I) Normalized adrenaline (left) and noradrenaline (right) urine content measured by ELISA. Epas1WT(black dots, n = 8), Th-Epas1KO (red squares, n = 7), Hif1aWT (black triangles, n = 7) and Th-HIF1aKO (blue triangles, n = 4). Mann-Whitney test, ns, non-significant.
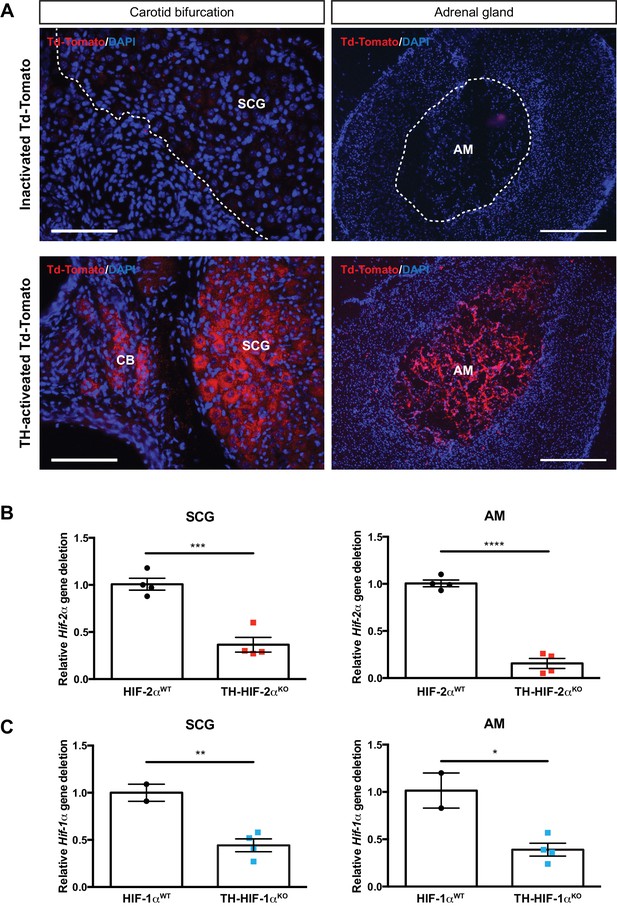
Sympathoadrenal gene deletion by TH-IRES-Cre mouse strain.
(A) TH-Cre-mediated recombination in carotid bifurcation (left panels) and adrenal gland (right panels) sections of TH-activated tdTomato mice (Cre+, lower panels) compared to inactivated tdTomato (Cre-, upper panels) mice. Notice the presence of tdTomato fluorescence into the CB glomus cells, SCG sympathetic neurons and adrenal medulla due to TH-Cre activity (lower panels). Dashed lines delineate the location of SCG and AM within inactivated tdTomato (Cre-) sections. SCG, superior cervical ganglion; CB, carotid body; AM, adrenal medulla. Scale bars: 100 µm for carotid bifurcation and 200 µm for adrenal gland sections. (B–C) Relative Hif-2α (B) and Hif-1α (C) gene deletion of dissected SCG (left graphs) and AM (right graphs) from Th-Epas1KO (red, n = 4) and Th-HIF1aKO (blue, n = 4) compared to their respective littermate controls (HIF-2αWT, n = 4; Hif1aWT, n = 2). Data are expressed as mean ± SEM. Unpaired t-test, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
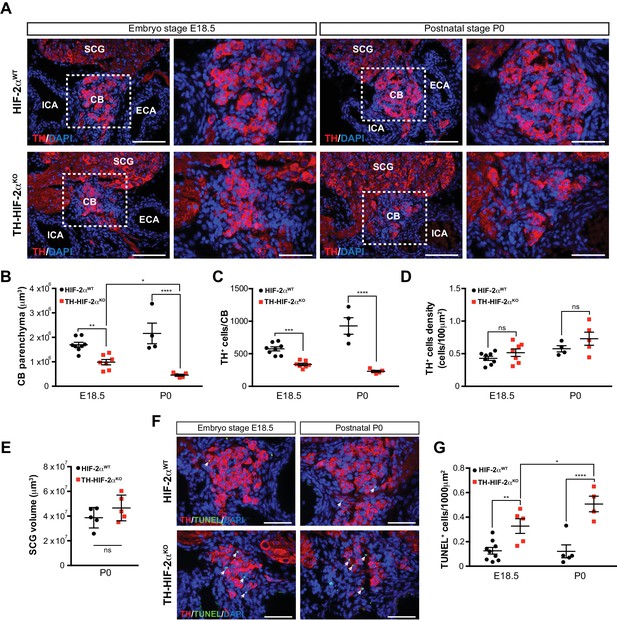
Progressive CB glomus cells death during development of TH-HIF2αKO mouse.
(A) Representative micrographs illustrating CB TH+ glomus cells appearance at embryo stage E18.5 (left) and postnatal stage P0 (right) in Epas1WT(top panels) and Th-Epas1KO (bottom panels) mice. Dashed rectangles (left panels) are shown at higher magnification on the right panels. SCG, superior cervical ganglion; CB, carotid body; ICA, internal carotid artery; ECA, external carotid artery. Scale bars: 100 µm. (B–E) Quantitative histological analysis of CB volume (B), TH+ cells number (C), TH+ cell density (D) and SCG volume (E) from Th-Epas1KO compared to Epas1WTmice. Epas1WT(E18.5, n = 8; P0, n = 4; SCG, n = 5), Th-Epas1KO (E18.5, n = 7; P0, n = 5; SCG, n = 5). SCG, superior cervical ganglion. Data are expressed as mean ±SEM. Two-way ANOVA, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns, non significant. (F) Representative pictures of double TH+ and TUNEL+ (white arrows) stained carotid bifurcation sections from Epas1WT(top panels) and Th-Epas1KO (bottom panels) mice at E18.5 (left) and P0 (right) stages. (G) Number of TUNEL+ cells within the CB (TH+ area) of Epas1WT(E18.5, n = 9; P0, n = 5) and Th-Epas1KO (E18.5, n = 5; P0, n = 4) mice at E18.5 and P0 stages. Data are expressed as mean ±SEM. Two-way ANOVA, *p<0.05, **p<0.01, ****p<0.0001. Scale bars: 50 µm.
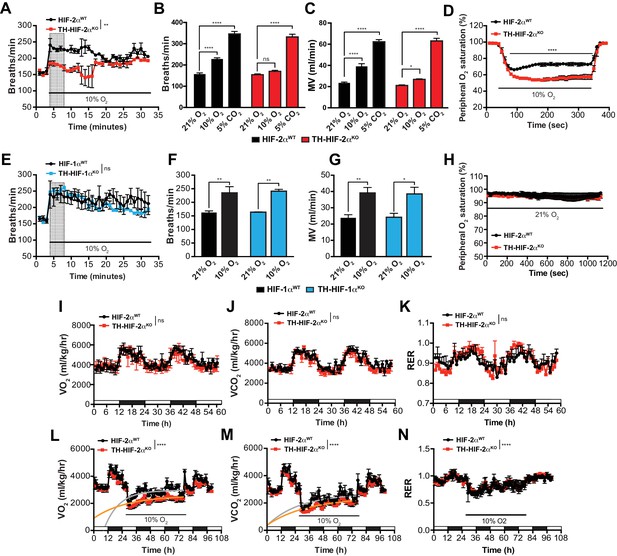
Effects of HIF-2α and HIF-1α sympathoadrenal loss on the HVR and whole-body metabolic activity in response to hypoxia.
(A and E) HVR in Th-Epas1KO (A, red line, n = 4) and Th-HIF1aKO (E, blue line, n = 3) deficient mice compared to control Epas1WT(A, black line, n = 4) and Hif1aWT (E, black line, n = 3) mice. Shift and duration of 10% O2 stimulus (30 min) are indicated. Shaded areas represent the period of time analysed in B and C or F and G, respectively. Data are presented as mean breaths/min every minute ±SEM. Two-way ANOVA, **p<0.01, ns, non-significant. (B and C) Averaged respiratory rate (B) and minute volume (C) of Epas1WT(black, n = 4) and Th-Epas1KO (red, n = 4) mice before and during the first 5 min of 10% O2 (shaded rectangle in A) or 5% CO2 exposure. Data are expressed as mean ±SEM. Two-way ANOVA, *p<0.05, ***p<0.001, ****p<0.0001, ns, non-significant. (D and H) Peripheral O2 saturation before, during and after 10% O2 (D) and 21% O2 (H) in Epas1WT(black, n = 8 in D, n = 4 in H) and Th-Epas1KO (red, n = 9 in D, n = 4 in H) mice. Data are expressed as mean percentage every 10 s ± SEM. Two-way ANOVA, ****p<0.0001. (F and G) Averaged respiratory rate (F) and minute volume (F) of Hif1aWT (black, n = 3) and Th-HIF1aKO (blue, n = 3) mice before and during the first 5 min of 10% O2 exposure (shaded rectangle in E). Data are expressed as mean ±SEM. Two-way ANOVA, *p<0.05, **p<0.01. (I–K) Baseline metabolic activity of Epas1WT(black, n = 3) and Th-Epas1KO (red, n = 3) mice. Data are presented as mean oxygen consumption (VO2, (I), carbon dioxide generation (VCO2, (J) and respiratory exchange ratio (RER, (K) every hour ±SEM. White and black boxes depict diurnal and nocturnal periods, respectively. Area under the curve followed by Mann-Whitney test, ns, non-significant. (L–N) Whole-body metabolic analysis of Epas1WT(black, n = 4) and Th-Epas1KO (red, n = 6) mice in response to 10% O2. Shift and duration of 10% O2 stimulus are indicated. Data are presented as mean oxygen consumption (VO2, (L), carbon dioxide generation (VCO2, (M) and respiratory exchange ratio (RER, (N) every hour ±SEM. White and black boxes depict diurnal and nocturnal periods, respectively. Recovery across the hypoxia period was analysed by one-phase association curve fitting. ****p<0.0001.
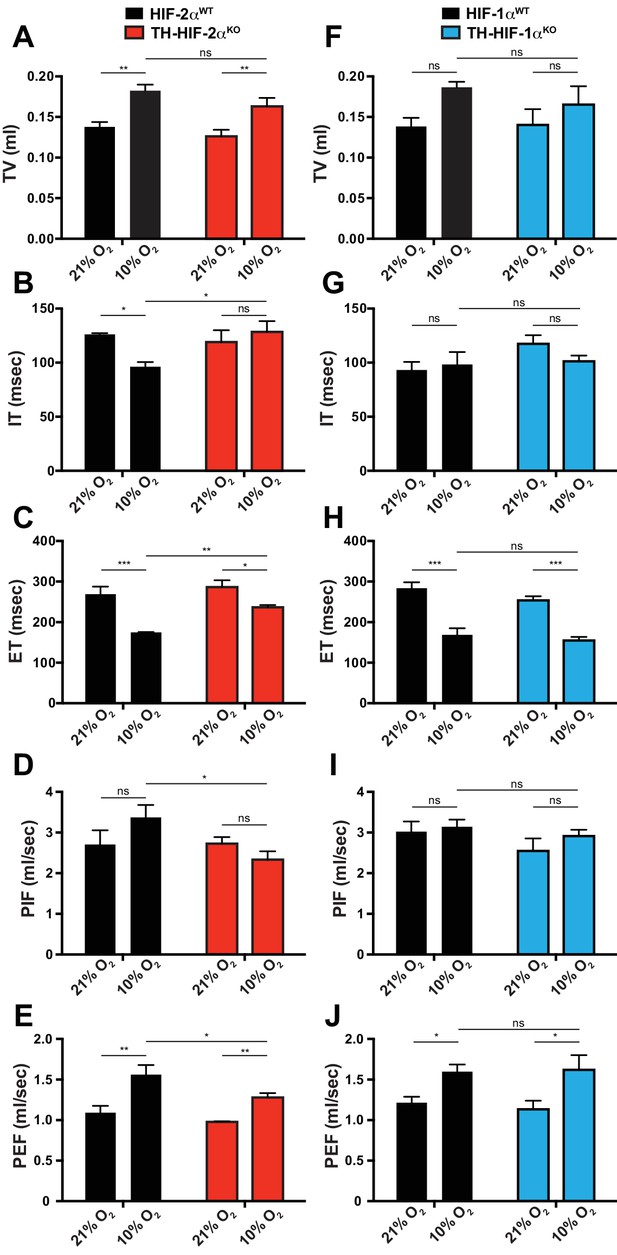
Respiratory parameters of Th-Epas1KO and Th-HIF1aKO mice exposed to acute hypoxia.
(A–J) Averaged tidal volume (A and F), inspiration time (B and G), expiration time (C and H), peak inspiratory flow (D and I) and peak expiratory flow (E and J) of Th-Epas1KO (n = 4) and Th-HIF1aKO (n = 3) respect to control mice (HIF-2αWT, n = 4; Hif1aWT, n = 3) before and during the first 5 min breathing 10% O2. Data are expressed as mean ±SEM. Two-way ANOVA, *p<0.05, **p<0.01, ***p<0.001.
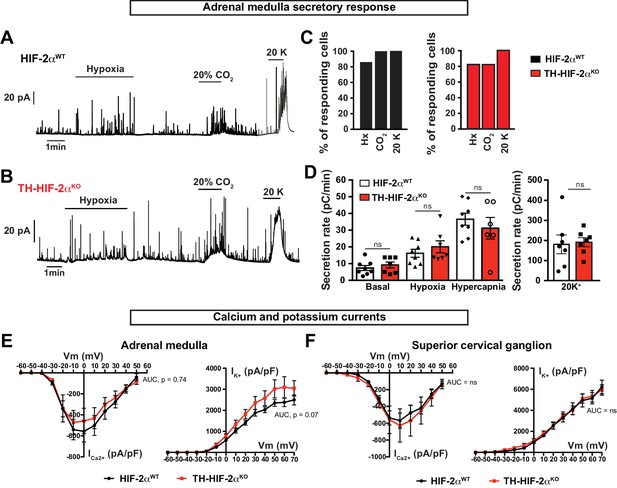
Secretory and electrophysiological properties in AM and SCG from Th-Epas1KO mice.
(A and B) Representative amperometric recordings of catecholamine release by chromaffin cells from Epas1WTand Th-Epas1KO mice (8–12 weeks old) in response to low PO2 (PO2 ≈ 10–15 mmHg), hypercapnia (20% CO2) and 20 mM KCl. (C) Percentage of AM slices with response to hypoxia, 20% CO2 and 20 mM KCl, respectively. Epas1WTnn = 8; TH-HIF-2αKO, n = 7. (D) Averaged secretion rate of chromaffin cells from Epas1WTand Th-Epas1KO mice exposed to hypoxia (PO2 ≈ 10–15 mmHg), hypercapnia (20% CO2) and 20 mM KCl. Each data point represents the mean of 2–3 recordings per mouse. HIF-2αWT, n = 8; TH-HIF-2αKO, n = 7. Data are expressed as mean ±SEM. One-way ANOVA, ns, non-significant. (E and F) Ca2+ and K+ current/voltage (I/V) curves of AM chromaffin cells (E) and SCG sympathetic neurons (F) dissociated from Epas1WT(n = 9 cells from three mice) and Th-Epas1KO (n = 10 cells from three mice) mice. Data are expressed as mean ±SEM. Area under the curve followed by unpaired t-test, ns, non-significant.
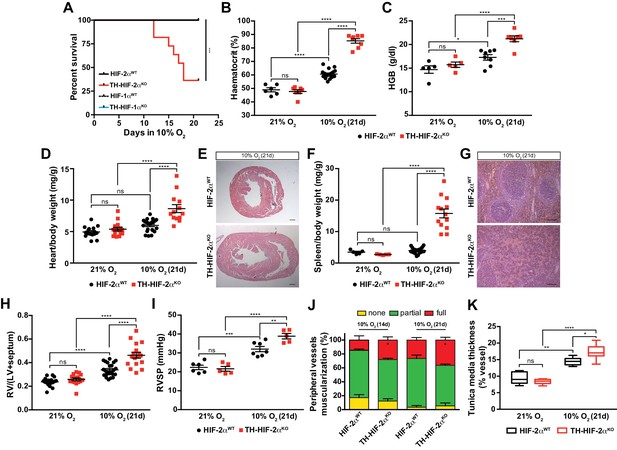
Impaired acclimatisation to chronic hypoxia and severe pulmonary hypertension in mice lacking CBs.
(A) Kaplan-Meier survival curve of the indicated mice housed at 10% O2 up to 21 days. Epas1WT(n = 12), Th-Epas1KO (n = 11), Hif1aWT (n = 8) and Th-HIF1aKO (n = 8). ***p<0.001. (B and C) Haematocrit (B) and haemoglobin (C) levels of Epas1WT(black) and Th-Epas1KO (red) littermates kept in normoxia (21% O2) and after 21 days at 10% O2. In B, Epas1WT(21% O2, n = 6; 10% O2, n = 18), Th-Epas1KO (21% O2, n = 7; 10% O2, n = 8). In C, Epas1WT(21% O2, n = 5; 10% O2, n = 8), Th-Epas1KO (21% O2, n = 5; 10% O2, n = 7). Data are expressed as mean ± SEM. Two-way ANOVA, *p<0.05, ***p<0.001, ****p<0.0001, ns, non-significant. (D and F) Normalized heart (D) and spleen (F) weight of Epas1WT(black) and Th-Epas1KO (red) before and after 21 days in hypoxia (10% O2). In D, Epas1WT(21% O2, n = 16; 10% O2, n = 23), Th-Epas1KO (21% O2, n = 15; 10% O2, n = 13). In F, Epas1WT(21% O2, n = 5; 10% O2, n = 23), Th-Epas1KO (21% O2, n = 5; 10% O2, n = 13). Data are expressed as mean ± SEM. Two-way ANOVA, ****p<0.0001, ns, non-significant. (E and G) Hematoxylin and eosin staining of heart (E) and spleen (G) of Epas1WT(top panel) and Th-Epas1KO (bottom panel) after 21 days in hypoxia (10% O2). Scale bars: 500 μm in E and 100 μm in G. (H) Fulton index on hearts dissected from Epas1WT(black, 21% O2, n = 17; 10% O2, n = 23) and Th-Epas1KO (red, 21% O2, n = 15; 10% O2, n = 17) mice before and after 21 days in hypoxia (10% O2). Data are expressed as mean ± SEM. Two-way ANOVA, ****p<0.0001, ns, non-significant. RV, right ventricle. LV, left ventricle. (I) Right ventricular systolic pressure (RVSP) recorded on Epas1WT(black, 21% O2, n = 6; 10% O2, n = 7) and Th-Epas1KO (red, 21% O2, n = 5; 10% O2, n = 5) mice maintained in normoxia (21% O2) or hypoxia (10% O2) for 21 days. Data are expressed as mean ± SEM. Two-way ANOVA, **p<0.01, ***p<0.001, ****p<0.0001, ns, non-significant. (J and K) Lung peripheral vessels muscularization (J) and arterial medial thickness (K) in Epas1WTand Th-Epas1KO mice exposed to 10% O2 for 14 and/or 21 days. In H, Epas1WT(10% O2 14d, n = 4; 10% O2 21d, n = 3), Th-Epas1KO (10% O2 14d, n = 6; 10% O2 21d, n = 3). In I, Epas1WT(21% O2, n = 5; 10% O2 21d, n = 8), Th-Epas1KO (21% O2, n = 5; 10% O2 21d, n = 8). Data are expressed as mean ± SEM. Two-way ANOVA, *p<0.05, **p<0.01, ****p<0.0001, ns, non-significant.
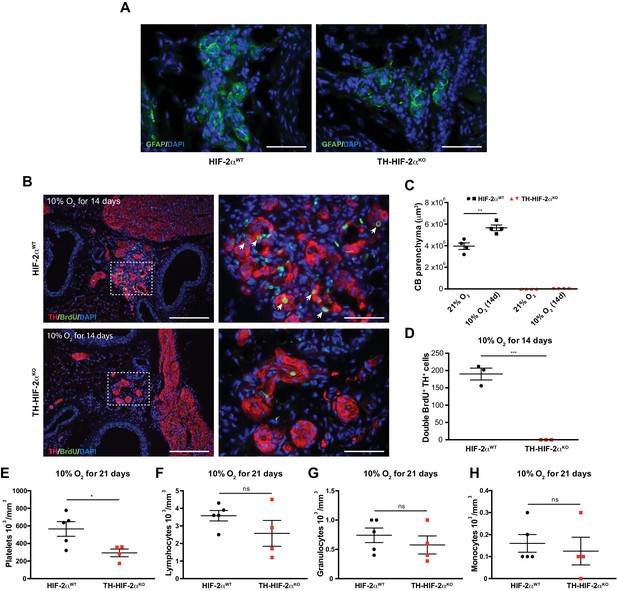
Impaired hypoxia-induced CB proliferation in Th-Epas1KO mice.
(A) Glial fibrillary acidic protein (GFAP) immunostaining to illustrate the presence of CB stem cells in carotid bifurcation of both Epas1WTand Th-Epas1KO mice. Scale bars: 50 µm. (B) Double TH+ BrdU+ immunofluorescence performed on carotid bifurcation sections from Epas1WT(top) and Th-Epas1KO (bottom) mice exposed to 10% O2 for 14 days. Dashed rectangles are shown at higher magnification on the right panels. Newly formed TH+ cells are indicated with white arrows. Scale bars: 200 µm (left pictures), 50 µm (right pictures). (C and D) Quantification of total CB volume (C, n = 4 per genotype and condition) and double BrdU+ TH+ cells number (D, n = 3 per genotype and condition) in Epas1WT(black) and Th-Epas1KO (red) mice maintained for 14 days in hypoxia (10% O2). Data are presented as mean ±SEM. Unpaired t-test, **p<0.01, ***p<0.001. (E–H) Platelets (E), lymphocytes (F), granulocytes (G) and monocytes (H) blood counts in Epas1WT(n = 5, black) and Th-Epas1KO (n = 4, red) mice maintained for 21 days in hypoxia (10% O2). Unpaired t-test, *p<0.05, ns, non-significant.
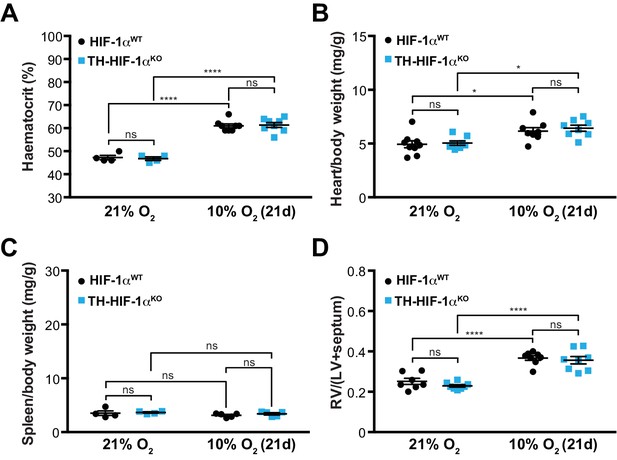
Adaptation to hypoxia in Th-HIF1aKO animals.
(A) Haematocrit levels of Hif1aWT (black, 21% O2, n = 4; 10% O2, n = 8) and Th-HIF1aKO (blue, 21% O2, n = 4; 10% O2, n = 8) mice housed at 21% O2 or after 21 days at 10% O2. Data are expressed as mean ±SEM. Two-way ANOVA, ****p<0.001, ns, non-significant. (B and C) Normalized heart (B) and spleen (C) weight of Hif1aWT (black) and Th-HIF1aKO (blue) mice before and after 21 days of hypoxia (10% O2) exposure. In B, Hif1aWT, 21% O2, n = 9; 10% O2, n = 8; TH-HIF-1αKO, n = 9, per condition. In C, 21% O2, n = 4 per genotype; 10% O2, n = 5 per genotype. Data are expressed as mean ± SEM. Two-way ANOVA, *p<0.05, ns, non-significant. (D) Fulton index on hearts dissected from Hif1aWT (black) and Th-HIF1aKO (blue) mice before and after 21 days in hypoxia (10% O2). Hif1aWT, 21% O2, n = 7; 10% O2, n = 8; TH-HIF-1αKO, n = 8, per condition. Data are expressed as mean ± SEM. Two-way ANOVA, ****p<0.0001, ns, non-significant. RV, right ventricle. LV, left ventricle.
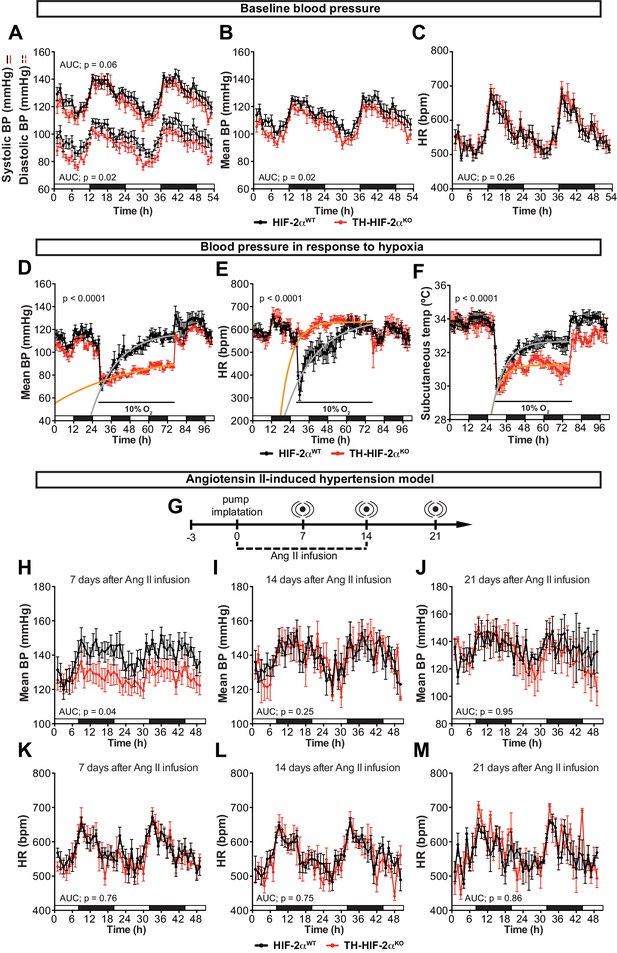
Effects of CB dysfunction on blood pressure regulation.
(A–C) Circadian variations in baseline systolic blood pressure (A, continued line), diastolic blood pressure (A, broken line), mean blood pressure (B) and heart rate (C) of Epas1WT(black, n = 10) and Th-Epas1KO (red, n = 8) littermates recorded by radiotelemetry. White and black boxes depict diurnal and nocturnal periods, respectively. Data are presented as averaged values every hour ±SEM. Area under the curve followed by unpaired t-test are shown. BP, blood pressure. HR, heart rate in beats per minute. (D–F) Mean blood pressure (D), heart rate (E) and subcutaneous temperature (F) alteration in response to hypoxia in radiotelemetry-implanted Th-Epas1KO (red, n = 5) compared to Epas1WT(black, n = 5). Shift and duration of 10% O2 are indicated in the graph. White and black boxes represent diurnal and nocturnal periods, respectively. Data are presented as averaged values every hour ±SEM. Recovery across the hypoxia period was analysed by one-phase association curve fitting. BP, blood pressure. HR, heart rate in beats per minute. (G) Schematic representation of the protocol followed in the Ang II-induced experimental hypertension model. (H–M) Mean blood pressure and heart rate recorded from Epas1WT(black, n = 8) and Th-Epas1KO (red, n = 8) mice after 7 days (H and K), 14 days (I and L) and 21 days (J and M) of Ang II infusion. White and black boxes depict diurnal and nocturnal periods, respectively. Data are presented as averaged values every hour ±SEM. Area under the curve followed by unpaired t-test are shown. BP, blood pressure. HR, heart rate in beats per minute.
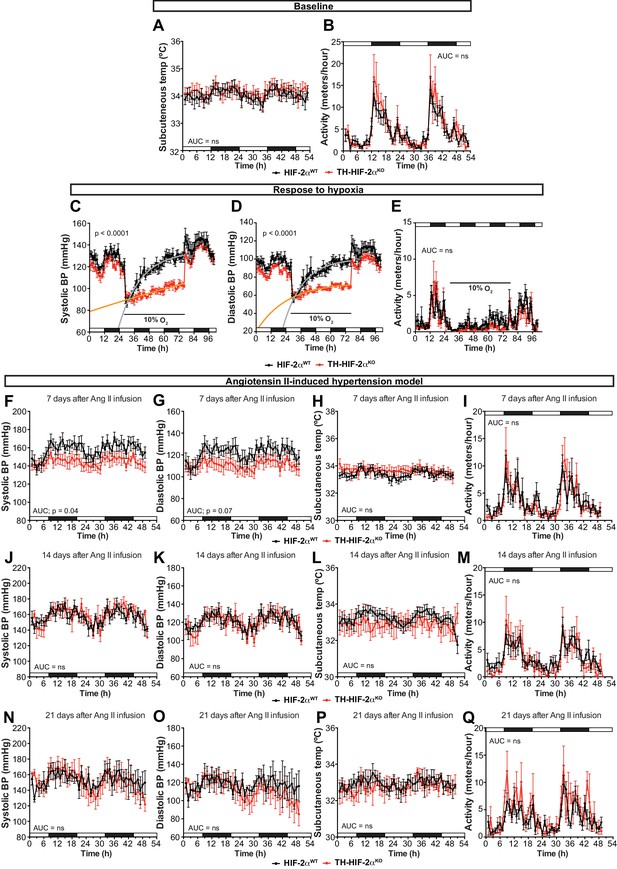
Cardiovascular homeostasis in response to hypoxia and Ang II-induced experimental hypertension.
(A and B) Circadian oscillations in subcutaneous temperature (A) and physical activity (B) of radiotelemetry-implanted Epas1WT(black, n = 10) and Th-Epas1KO (red, n = 8). White and black boxes depict diurnal and nocturnal periods, respectively. Data are presented as averaged values every hour ± SEM. Area under the curve followed by unpaired t-test are shown. (C and D) Systolic blood pressure (C) and diastolic blood pressure (D) alteration in response to hypoxia in Th-Epas1KO (red, n = 5) compared to Epas1WT(black, n = 5) measured by radiotelemetry. Shift and duration of 10% O2 are indicated in the graph. White and black boxes depict diurnal and nocturnal periods, respectively. Data are presented as averaged blood pressure values every hour ± SEM. Recovery across the hypoxia period was analysed by one-phase association curve fitting. (E) Physical activity recorded by radiotelemetry of Epas1WT(black, n = 5) and Th-Epas1KO (red, n = 5) littermates exposed to 10% O2 environment. Shift and duration of 10% O2 are indicated in the graph. White and black boxes depict diurnal and nocturnal periods, respectively. Data are presented as averaged blood pressure values every hour ± SEM. Area under the curve followed by unpaired t-test is shown. (F–Q) Circadian fluctuations in systolic blood pressure, diastolic blood pressure, subcutaneous temperature and physical activity of Epas1WT(black, n = 5) and Th-Epas1KO (red, n = 5) after 7 days (F–I), 14 days (J–M) and 21 days (N–Q) of Ang II infusion recorded by radiotelemetry. White and black boxes represent diurnal and nocturnal periods, respectively. Data are presented as averaged values every hour ± SEM. Area under the curve followed by unpaired t-test are shown. ns, non-significant.
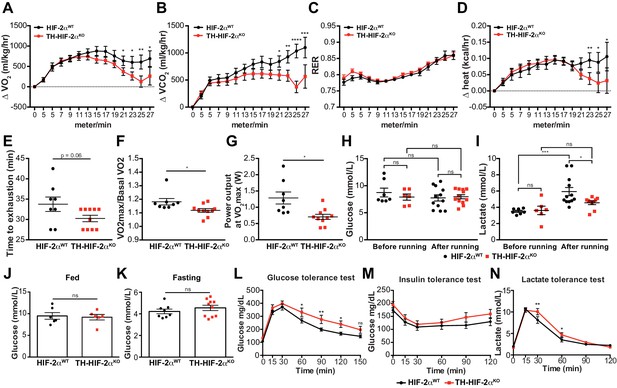
Exercise performance and glucose management in mice lacking CBs.
(A–D). Time course showing the increment in O2 consumption (A), increment in CO2 generation (B), respiratory exchange ratio (C) and increment in heat production (D) from Epas1WT(black, n = 8) and Th-Epas1KO (red, n = 10) mice during exertion on a treadmill. Mice were warmed up for 5 min (5 m/min) and then the speed was accelerated 2 m/min at 2.5 min intervals until exhaustion. Averaged measurements every 2.5 min intervals ± SEM are charted. Two-way ANOVA, *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001. RER, respiratory exchange ratio. (E) Running total time spent by Epas1WT(black, n = 8) and Th-Epas1KO (red, n = 10) mice to become exhausted. Data are expressed as mean ± SEM. Unpaired t-test. (F and G) Aerobic capacity (F) and power output (G) measured in Epas1WT(black, n = 8) mice compared to Th-Epas1KO (red, n = 10) mice when performing at VO2max. Data are expressed as mean ± SEM. Unpaired t-test. *p<0.05. (H and I) Plasma glucose (H) and lactate (I) levels of Epas1WT(black, before, n = 7; after, n = 12) and Th-Epas1KO (black, before, n = 6; after, n = 12) littermates before and after running to exhaustion. Data are expressed as mean ± SEM. Two-way ANOVA, *p<0.05. ***p<0.001. ns, non-significant. (J and K) Plasma glucose levels of Epas1WT(black, n = 6) and Th-Epas1KO (red, n = 6) mice fed (J, n = 6 per genotype) or fasted over night (K, Epas1WT(n = 8) and Th-Epas1KO (n = 10). Data are expressed as mean ± SEM. Unpaired t-test. (L–M) Time courses showing the tolerance of Epas1WTand Th-Epas1KO mice to glucose (L, n = 7 per genotype) insulin (M, n = 11 per genotype) and lactate (N, n = 7 per genotype) injected bolus. Data are presented as mean ± SEM. Two-way ANOVA, *p<0.05, **p<0.01, ns, non-significant.
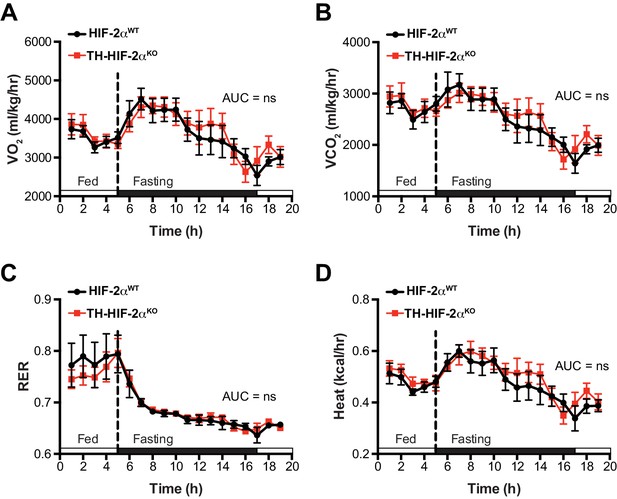
Related to Figure 6.
Whole-body metabolic activity of Th-Epas1KO in response to fasting-induced hypoglycemia. (A–D) Oxygen consumption (A, VO2), carbon dioxide generation (B, VCO2), respiratory exchange rate (C) and heat production (D) of Epas1WT(black, n = 7) and Th-Epas1KO (red, n = 7) littermates under feed and over night fasting conditions. White and black boxes represent diurnal and nocturnal periods, respectively. Data are presented as averaged values every hour ± SEM. Area under the curve followed by unpaired t-test are shown. ns, non-significant.
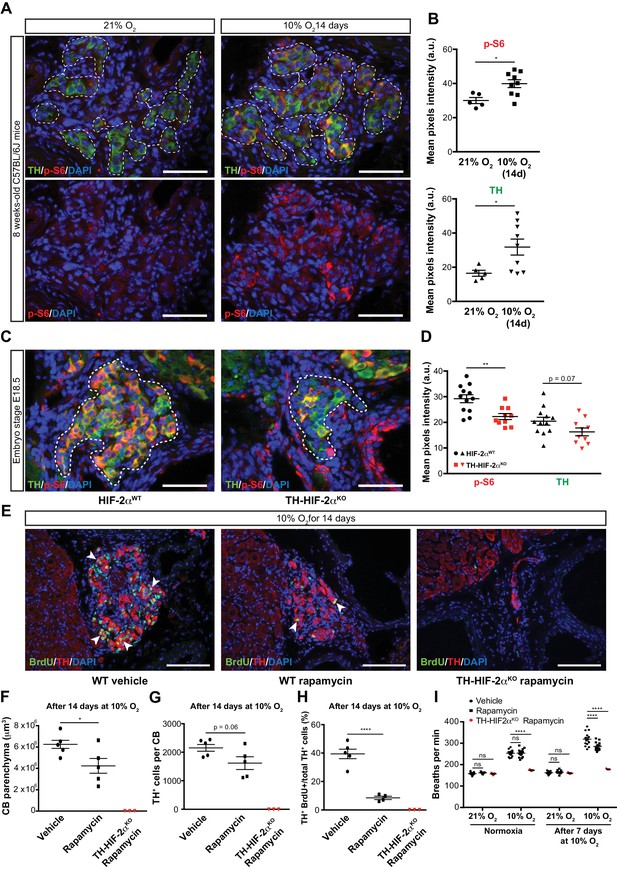
mTORC1 activation in CB development and hypoxia-induced CB neurogenesis.
(A) Double TH and p-S6 (mTORC1 activation marker) immunofluorescence on adult wild type CB slices to illustrate the increased p-S6 expression after 14 days in hypoxia (10% O2). For clarity, bottom panels only show p-S6 staining. TH+ outlined area (top panels) indicates the region used for pixels quantification. Scale bars: 50 µm. (B) Quantification of p-S6 (top) and TH (bottom) mean pixels intensity within the CB TH+ cells of wild type mice breathing 21% O2 or 10% O2 for 14 days. Each data point depicts the average of 3–4 pictures. 21% O2, n = 5; 10% O2 14d, n = 9 per genotype. Data are presented as mean ± SEM. Unpaired t-test, *p<0.05. (C) Representative micrographs of TH and p-S6 double immunostaining of Epas1WTand Th-Epas1KO embryonic (E18.5) carotid bifurcations. TH+ outlined area indicates the region used for pixels quantification. Scale bars: 50 µm. (D) Quantitative analysis performed on Epas1WT(black, n = 12) and Th-Epas1KO (red, n = 10) micrographs previously immuno-stained for p-S6 and TH. Each data point depicts the average of 3–4 pictures. Data are presented as mean ± SEM. Unpaired t-test, **p<0.01. (E) Double BrdU and TH immunofluorescence on carotid bifurcation sections from wild type mice (left and central panels) or Th-Epas1KO mice (right panel) exposed to 10% O2 and injected with rapamycin or vehicle control for 14 days. Notice the striking decrease in double BrdU+ TH+ cells (white arrowheads) in the rapamycin treated wild type mice compared to vehicle control animals. Scale bars: 100 µm. (F–H) Quantification of total CB volume (F), TH+ cells number (G) and percentage of double BrdU+ TH+ over the total TH+ cells (H) in wild type mice (black) or Th-Epas1KO mice (red) breathing 10% O2 and injected with rapamycin or vehicle control for 14 days. Wild type, n = 5 mice per treatment. TH-HIF-2αKO, n = 3. (I) Averaged breaths per minute during the first 2 min of acute hypoxia (10% O2) exposure from vehicle or rapamycin injected wild type mice (black) or Th-Epas1KO (red) mice before and after 7 days of chronic hypoxia (10% O2) treatment. Wild type, n = 14 mice per condition and treatment. TH-HIF-2αKO, n = 3. Data are presented as mean ± SEM. B, D, F, G and H, unpaired t-test, *p<0.05, ****p<0.0001. I, two-way ANOVA followed by Tukey’s multiple comparison test. ****p<0.0001, ns, non-significant.
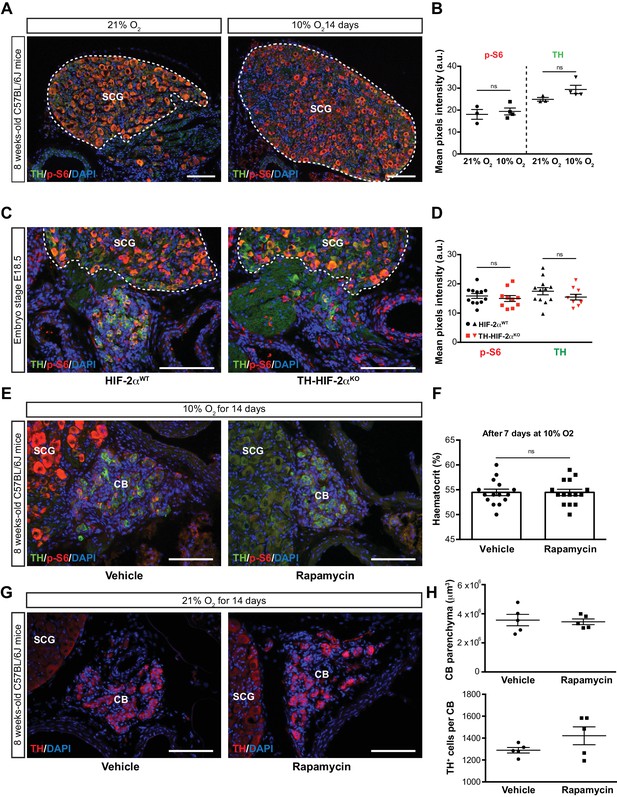
mTORC1 activity in SCG TH+ sympathetic neurons and effect of rapamycin treatment.
(A) Double TH and p-S6 (mTORC1 activation marker) immunofluorescence on adult wild type SCG slices after 14 days in hypoxia (10% O2). TH+ outlined area indicates the region used for pixels quantification. Scale bars: 100 µm. SCG, superior cervical ganglion. (B) Quantification of p-S6 (red) and TH (green) mean pixels intensity within the SCG TH+ area of wild type mice breathing 21% O2 or 10% O2 for 14 days. Each data point depicts the average of 3–4 pictures. 21% O2, n = 3; 10% O2, n = 4. Data are presented as mean ± SEM. Unpaired t-test, ns, non-significant. (C) Representative micrographs of TH and p-S6 double immunostaining of Epas1WTand Th-Epas1KO embryonic (E18.5) carotid bifurcations. SCG TH+ outlined area indicates the region used for pixels quantification. SCG, superior cervical ganglion. Scale bars: 100 µm. (D) Quantitative analysis performed on Epas1WT(black) and Th-Epas1KO (red) micrographs previously immune-stained for TH (green) and p-S6 (red). Each data point depicts the average of 3–4 pictures. 21% O2, n = 12; 10% O2, n = 10. Data are presented as mean ± SEM. Unpaired t-test, ns, non-significant. (E) Double TH and p-S6 immunofluorescence of vehicle control or rapamycin injected adult wild type SCG slices after 14 days breathing in a 10% O2 atmosphere. Notice the absence of p-S6 signal as a consequence of mTORC inhibition by rapamycin. SCG, superior cervical ganglion. CB, carotid body. Scale bars: 100 µm. (F) Haematocrit levels of vehicle control (n = 14) and rapamycin (n = 14) injected mice after 7 days at 10% O2. Un-paired t-test, ns, non-significant. (G) TH immunofluorescence of the carotid bifurcation of wild type mice injected for 14 days with vehicle control or rapamycin breathing room air. SCG, superior cervical ganglion. CB, carotid body. Scale bars: 100 µm. (H) Quantitative analysis of the CB parenchyma volume (top) and TH+ cell number (bottom) of wild type mice injected for 14 days with vehicle control or rapamycin and maintained in normoxic (21% O2) conditions.
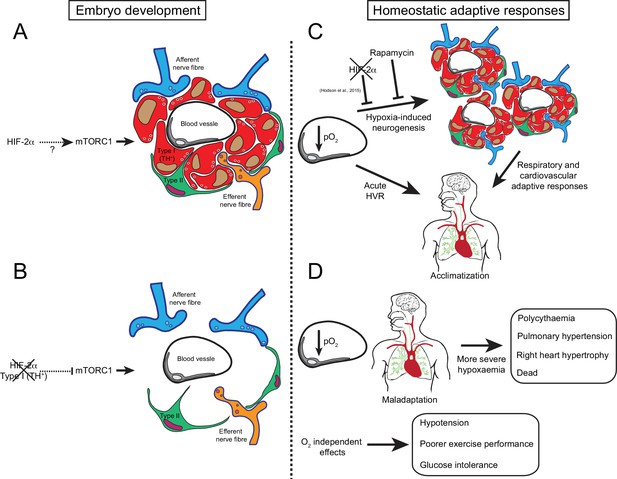
Graphic abstract of the role of HIF-2α in CB development and function.
(A) Schematic representation of a CB glomerulus. In CB development mTORC1 activity is high, which likely contributes to normal CB proliferation and growth. (B) Embryonic Hif-2α ablation in TH+ cells (type I cells) leads to repressed mTORC1 activity and progressive O2-sensitive cell loss via a direct or indirect mechanism. (C) Upon low arterial pO2, CB O2-sensitive glomus cells trigger an acute hypoxic ventilatory response (HVR) increasing O2 uptake by the lungs. Sustained hypoxia induces CB growth, a process that is HIF-2α-dependent (Hodson et al., 2016) and blocked by rapamycin (mTORC1 inhibitor). Hypoxia-induced CB neurogenesis further increases ventilation and regulates cardiovascular function allowing adaptation. (D) The absence of CB responses to low pO2 leads to a more severe hypoxaemia, resulting in pathological polycythaemia, pulmonary hypertension, and right ventricular failure. Other adaptive responses, such as arterial pressure, exercise performance and glucose homeostasis, are also impaired by CB loss. Taken together, we show that HIF-2α is essential for CB development and that its absence results in the disruption of key homeostatic adaptive responses.
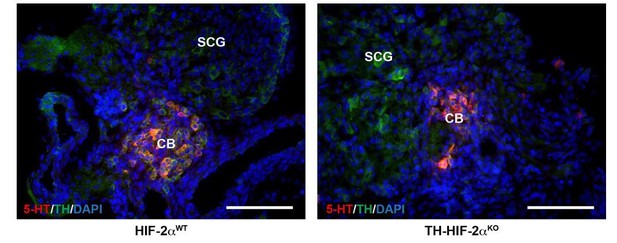
CB glomus cells identification by serotonin expression.
Double serotonin (5-HT, red) and TH (green) immunofluorescence on carotid bifurcations from HIF-2αWT and TH-HIF-2αKO mice at E18.5. Scale bars: 100 µm.
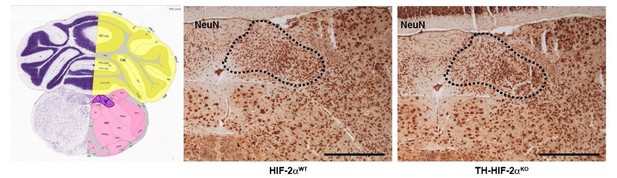
Histological analysis of the nucleus of the solitary tract.
NeuN immunohistochemistry of brain slices from HIF-2αWT andTH-HIF-2αKO mice. Left picture was obtained from Allen brain atlas (http://mouse.brain-map.org/static/atlas). Nucleus of the solitary tract is highlighted in purple. Dash line depicts the area corresponding with the nucleus of the solitary tract. Scale bars: 500 µm.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Mus musculus) | Hif-1α | NA | MGI:106918 | |
| Gene (M. musculus) | Hif-2α | NA | MGI:109169 | Official symbol Epas1 |
| Gene (M. musculus) | Th | NA | MGI:98735 | |
| Strain, strain background (M. musculus) | C57BL/6J | The Jackson laboratory | Jax:000664 | |
| Genetic reagent (M. musculus) | HIF-1aWT | PMID:10945599 | Jax: 007561 | |
| Genetic reagent (M. musculus) | HIF-2aWT | PMID:17284606 | Jax:008407 | |
| Genetic reagent (M. musculus) | TH-Cre | PMID:15452869 | MGI:3056580 | |
| Genetic reagent (M. musculus) | Td-Tomato | PMID:20023653 | Jax:007909 | |
| Antibody | anti-TH (rabbit polyclonal) | Novus Biologicals | Novus Biologicals:NB300-109 | (1:1000) |
| Antibody | anti-TH (chicken polyclonal) | abcam | abcam:ab76442 | (1:1000) |
| Antibody | anti-GFAP (rabbit polyclonal) | Dako | Dako:Z0334 | (1:500) |
| Antibody | anti-BrdU (rat polyclonal) | abcam | abcam:ab6326 | (1:100) |
| Antibody | anti-phospho S6 (rabbit polyclonal) | Cell Signaling Technology | Cell signaling:2211 | (1:200) |
| Antibody | anti-SMAα(mouse monoclonal) | Sigma-Aldrich | Sigma-Aldrich:A-2547 | (1:200) |
| Antibody | Alexa 488- or 568- secondaries | ThermoFisher | (1:500) | |
| Sequence-based reagent | Hif1_probe | IDT | 5’-/56-FAM/CCTGTTGGTTGCGCAGCAAGCATT/3BHQ_1/–3’ | |
| Sequence-based reagent | Hif1_F | IDT | 5’-GGTGCTGGTGTCCAAAATGTAG-3’ | |
| Sequence-based reagent | Hif1_R | IDT | 5’-ATGGGTCTAGAGAGATAGCTCCACA-3’ | |
| Sequence-based reagent | Hif2_probe | IDT | 5’-/56-FAM/CCACCTGGA/ZEN/CAAAGCCTCCATCAT/3IABkFQ/−3’ | |
| Sequence-based reagent | Hif2_F | IDT | 5’-TCTATGAGTTGGCTCATGAGTTG-3’ | |
| Sequence-based reagent | Hif2_R | IDT | 5’-GTCCGAAGGAAGCTGATGG-3’ | |
| Peptide, recombinant protein | AngII | Sigma-Aldrich | Sigma-Aldrich:A2900 | |
| Peptide, recombinant protein | Insulin (human) | Sigma-Aldrich | Sigma-Aldrich:11 376 497 001 | |
| Peptide, recombinant protein | Collagenase type IA | Sigma-Aldrich | Sigma-Aldrich:C9891 | |
| Peptide, recombinant protein | Trypsin | Sigma-Aldrich | Sigma-Aldrich:93610 | |
| Commercial assay or kit | LSAB+, Dako REAL Detection Systems | Dako | Dako:K5001 | |
| Commercial assay or kit | Epinephrine/Norepinephrine ELISA Kit | Abnova | Abnova:KA1877 | |
| Commercial assay or kit | DNeasy Blood and Tissue Kit | Qiagen | Qiagen:69506 | |
| Commercial assay or kit | TaqMan Universal PCR Master Mix | Applied Biosystems | Applied biosystems:4304437 | |
| Commercial assay or kit | Click-iT TUNEL Alexa Fluor 488 Imaging Assay | ThermoFisher | ThermoFisher:C10245 | |
| Chemical compound, drug | BrdU | Sigma-Aldrich | Sigma-Aldrich:B5002 | |
| Chemical compound, drug | Rapamycin | LC Laboratories | LC Laboratories:R-5000 | |
| Chemical compound, drug | D-Glucose | Sigma-Aldrich | Sigma-Aldrich:G8270 | |
| Chemical compound, drug | Sodium L-Lactate | Sigma-Aldrich | Sigma-Aldrich:71718 | |
| Software, algorithm | Fiji | http://imagej.net/Fiji | ||
| Other | EVG stain | Merck | Merck:115974 | |
| Other | Hematoxylin Solution, Mayer’s | Sigma-Aldrich | Sigma-Aldrich:MHS16 | |
| Other | Eosin Y solution, aqueous | Sigma-Aldrich | Sigma-Aldrich:HT110216 | |
| Other | DAPI stain | Molecular probes | Molecular probes:D1306 | (1:5000) |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.34681.018





