Somatic clones heterozygous for recessive disease alleles of BMPR1A exhibit unexpected phenotypes in Drosophila
Figures
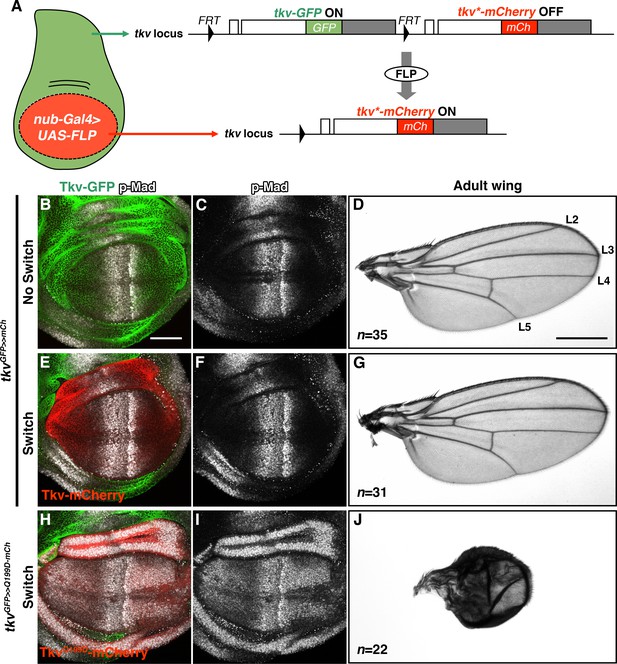
Inducible tkv allele switch system.
(A) Schematic illustration of a FLP/FRT-mediated tkv allele switch. mCherry-tagged Tkv expression was specifically induced in the wing pouch via FLP/FRT-mediated recombination that eliminates tkv-GFP from the locus. White and grey boxes represent tkv coding and 3’UTR sequences, respectively. (B–G) tkvGFP>>mCherry heterozygous animal before and after allele switch. (B, C, E, F) GFP (green) and mCherry (red) represent endogenous Tkv expression in the developing wing disc. BMP/DPP activity was detected by anti-p-Mad staining (grey). (D, G) Both Tkv-GFP and Tkv-mCherry expressing animals developed normal adult wings. Longitudinal veins L2-L5 are indicated. (H–J) Wing pouch-specific allele switch of the constitutively active form of Tkv, TkvQ199D. TkvQ199D-mCherry (red) expression led to ectopic p-Mad activation (grey, H, I), resulting in wing malformation (J). Scale bars: 50 μm for (B), and 0.5 mm for (D). Anterior is oriented to the left in all wing disc figures and to the top in adult wing images.
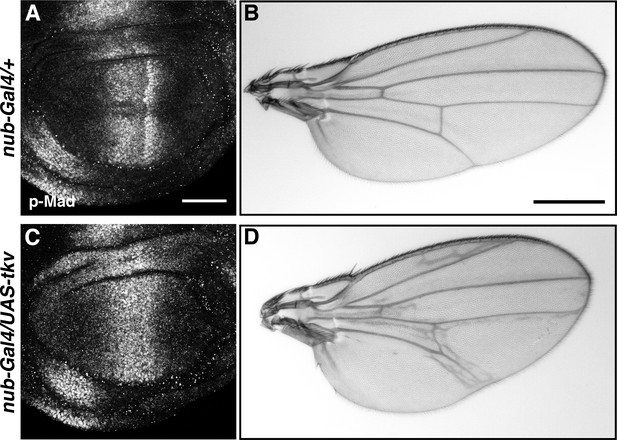
Wing patterning defects caused by tkv overexpression.
(A, B) p-Mad staining of nub-Gal4/+ wing disc (A) and nub-Gal4/+ adult wing (B). (C, D) tkv overexpression in the wing pouch by nub-Gal4 (nub-Gal4/UAS-tkv) led to abnormal p-Mad expression (C), resulting in wing patterning defects (D). Scale bars: 50 μm for (A), 0.5 mm for (B). Anterior is oriented to the left in all wing disc figures and to the top in adult wing images.
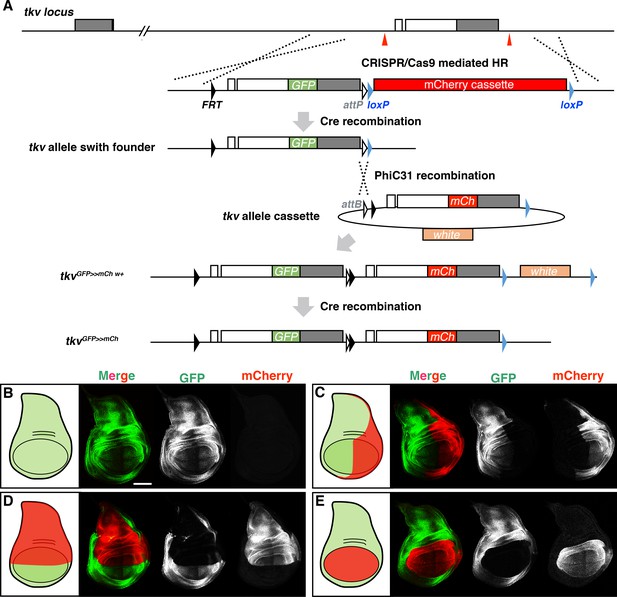
Spatial regulation of tkv allele switch.
(A) Flowchart illustrating the establishment of tkv allele switch transgenic lines. (B–E) Region-specific induction of Tkv-mCherry using the Gal4/UAS system. Control (tkvGFP>>mCherry/+, B), the posterior (tkvGFP>>mCherry/+; hh-Gal4, UAS-FLP/+, C), the dorsal (tkvGFP>>mCherry/ap-Gal4; UAS-FLP/+, D), and the wing pouch switch (tkvGFP>>mCherry/nub-Gal4; UAS-FLP/+, E). Scale bars: 100 μm. Anterior is to the left.
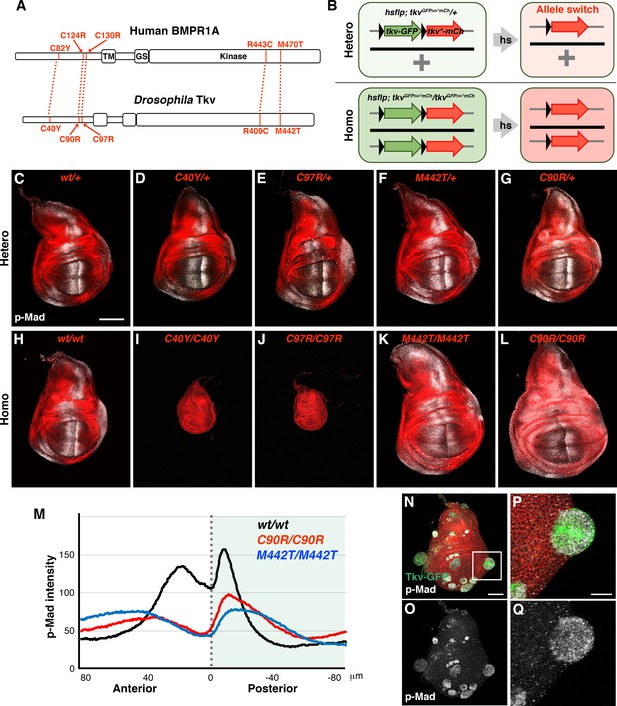
Distinct wing disc phenotypes caused by JPS-associated tkv mutations.
(A) Human BMPR1A and Drosophila Tkv protein structures. Conserved JPS-associated mutations are indicated. (B) Scheme for the induction of tkv hetero- and homozygous mutant cells by heat shock. (C–L) wild-type and mutant forms of Tkv-mCherry (red) together with p-Mad (grey) expression in either hetero- (C–G) or homozygous (H–L) wing discs. Scale bars: 100 μm. (M) Averaged p-Mad intensity plot profiles for wild-type tkvmCherry (n = 16), tkvC90R-mCherry (n = 15) and tkvM442T-mCherry (n = 16) homozygous wing discs. 0 indicates the compartment boundary position (posterior is shaded). (N–Q) High levels of BMP activity observed in residual wild-type clones within predominantly tkvC97R-mCherry homozygous wing discs. Ectopic p-Mad expression (grey) is detected in rounded clusters of wild-type cells (green in N, P). (P and Q) show magnified images of the boxed region in (N). Scale bars: 50 μm for (N), 20 μm for (P). Anterior is oriented to the left side of all images.
-
Figure 2—source data 1
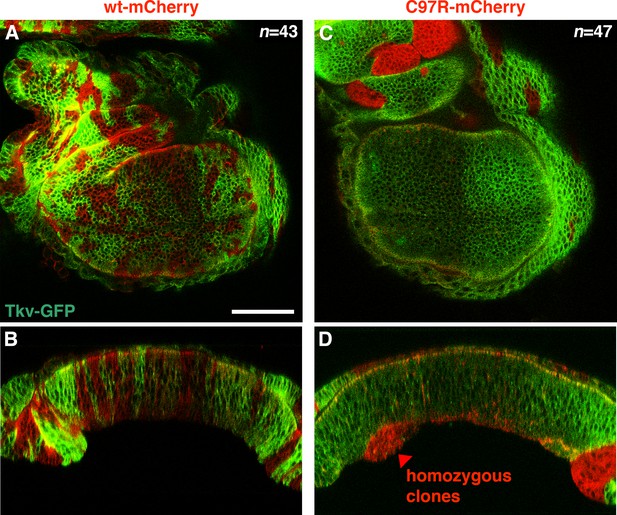
BMP/DPP activation in wild-type clones within the tkvC97R homozygous background.
Wild-type clones (green) showed higher BMP/DPP activity (grey) compared to their tkvC97R homozygous neighbors (red) and frequently exhibited an outgrowth phenotype.
- https://doi.org/10.7554/eLife.35258.008
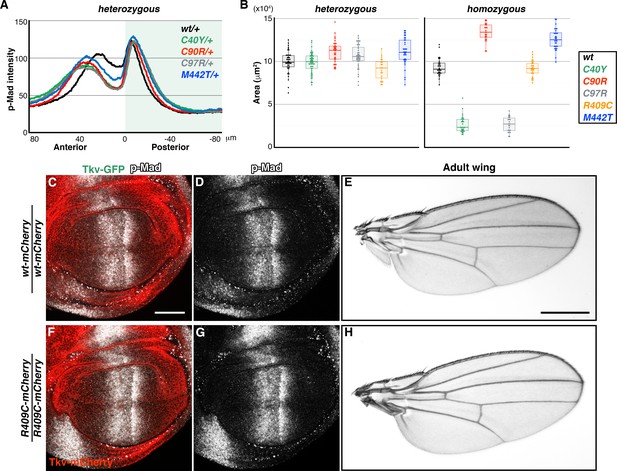
Phenotypes of tkv mutations.
(A) Averaged p-Mad intensity profiles of wild-type tkvmCherry (n = 20), tkvC40Y-mCherry/+ (n = 24), tkvC90R-mCherry/+ (n = 24), tkvC97-mCherry/+ (n = 24), and tkvM442T-mCherry/+ (n = 16) wing discs. (B) Wing disc size comparisons. wild-type tkvmCherry (nhet = 44; nhomo = 31), tkvC40Y-mCherry (nhet = 52; nhomo = 32), tkvC90R-mCherry (nhet = 35; nhomo = 32), tkvC97R-mCherry (nhet = 42; nhomo = 23), tkvR409C-mCherry (nhet = 21; nhomo = 31), and tkvM442T-mCherry (nhet = 37; nhomo = 33). (C–H) tkvR409C homozygous animals developed normal wings. Wing discs and adult wings from wild-type tkvmCherry (C–E) and tkvR409C-mCherry (F–H) homozygous animals. Scale bars: 50 μm for (C), 0.5 mm for (E). Anterior is oriented to the left in (C), (D), (F and G), and to the top in (E and H).
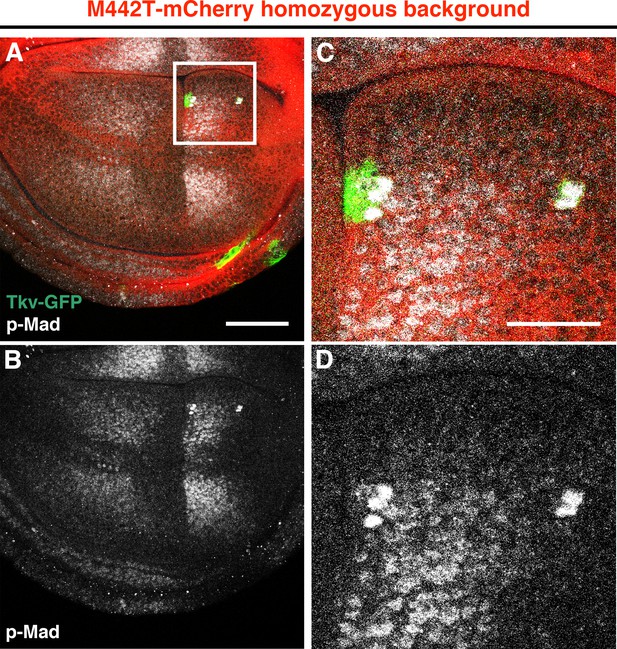
Strong BMP activation in cells expressing wild-type Tkv within the tkvM442T background.
(A–D) Small residual wild-type Tkv-GFP clones in tkvM442T-mCherry homozygous wing discs. Tkv-GFP (green) cells in the wing pouch showed a higher p-Mad (grey) expression than tkvM442T mutant cells. Intriguingly, wild-type clones did not appear to proliferate normally in this background. Magnified images of the boxed area in (A) (C, D). Scale bars: 50 μm for (A), 20 μm for (C). Anterior is oriented to the left side of all images.
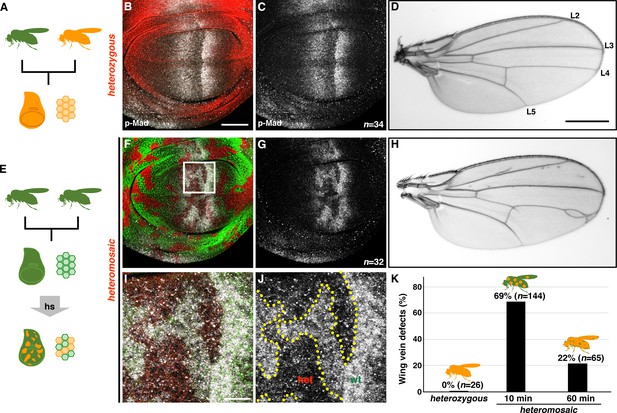
tkv heterozygous mosaicism disrupts wing pattern formation.
(A–D) Germline inheritance of the recessive tkvC97R-mCherry mutation (A). TkvC97R-mCherry (red) and p-Mad (grey) expression in a heterozygous wing disc (B, C). tkvC97R heterozygotes developed normal wings (D). Positions of longitudinal vein L2-L5 are shown. (E–J) Generating somatic clones of tkvC97R heterozygous cells by heat shock (E). wild-type and tkvC97 heterozygous cells are shown by green and red, respectively (F, G, I, J). tkvC97R-mCherry heteromosaicism disrupted the p-Mad activity gradient (grey) and caused wing vein patterning defects (H). (I, J) Magnified images of the boxed area in (F). A similar effect on p-Mad activity was observed in male wing discs, although adult males exhibited milder wing phenotypes. Scale bars: 50 μm for (B), 0.5 mm for (D), and 10 μm for (I). Anterior is oriented to the left side of wing disc images and to the top side of adult wing pictures. (K) Quantification of adult wing phenotypes in tkvC97R-mCherry heterozygous and heteromosaic animals. Longer heat shock generated more uniformly heterozygous cell populations and reduced mosaicism, rescuing wing vein phenotypes.
Basal extrusion of tkvC97R homozygous mutant clones from the wing epithelia.
(A–D) tkvC97R homozygous mutant clones were basally eliminated from wing epithelia. (A, B) To generate mCherry-labelled wild-type control clones, hs-flp; tkvGFP>>mCherry/tkvmCherry animals were heat shocked for 10 min at 72 hr AEL. (C, D) Homozygous mCherry-labeled tkvC97R clones were generated with an identical heat shock regimen in larvae of the genotype: hs-flp; tkvGFP>>C97R-mCherry/tkvCC97R-mCherry. The upper and lower panels show standard XY and optical cross section XZ images of each wing disc, respectively. Arrowhead (red) indicates eliminated tkvC97R homozygous mutant cells. Scale bars: 50 μm. Anterior is left.
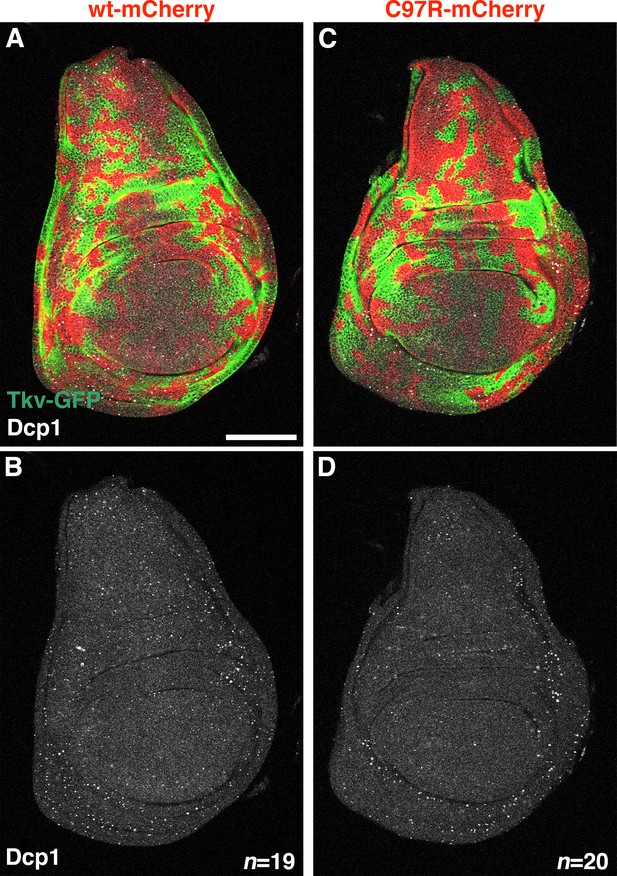
tkv heteromosaicism does not induce cell death.
(A–D) Cell death was not observed in wing discs possessing tkvC97R heteromosaic clones. Wing discs from hs-flp; tkvGFP>>mCherry/+ (control, A, B), and hs-flp; tkvGFP>>C97R-mCherry/+ (C, D) animals heat shocked at 72 hr AEL for 10 min were stained with anti-cleaved Drosophila Dcp-1. Scale bars: 100 μm. Anterior is left.
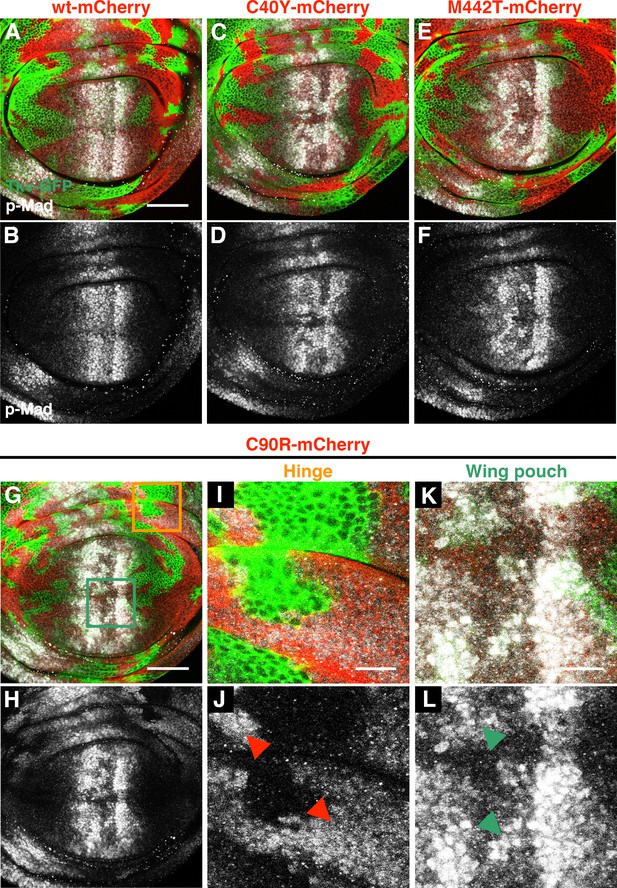
Additional JPS-associated tkv mutations exhibit deleterious heteromosaicism.
(A–F) Deleterious mosaic effects of tkvC40Y and tkvM442T. Wing discs from wild-type control (A, B), tkvGFP>>C40Y-mCherry (C, D), and tkv GFP>>M442T-mCherry (E, F) animals. Tkv-GFP and Tkv*-mCherry are depicted by green and red, respectively. tkvC40Y and tkvM442T heteromosaic resulted in aberrant p-Mad expression (grey, C–F). (G–L) Context dependent TkvC90R activity. The hinge cells expressing TkvC90R-mCherry induced ectopic p-Mad expression (grey, orange box in G), (I, J), while the same mutation showed a weaker signaling capability in the wing pouch (green box in G), (K, L). Red and green arrowheads indicate locations of tkvC90R heterozygous and wild-type cells, respectively. Scale bars: 50 μm in (A) and (G), 10 μm in (I) and (K). Anterior is left.
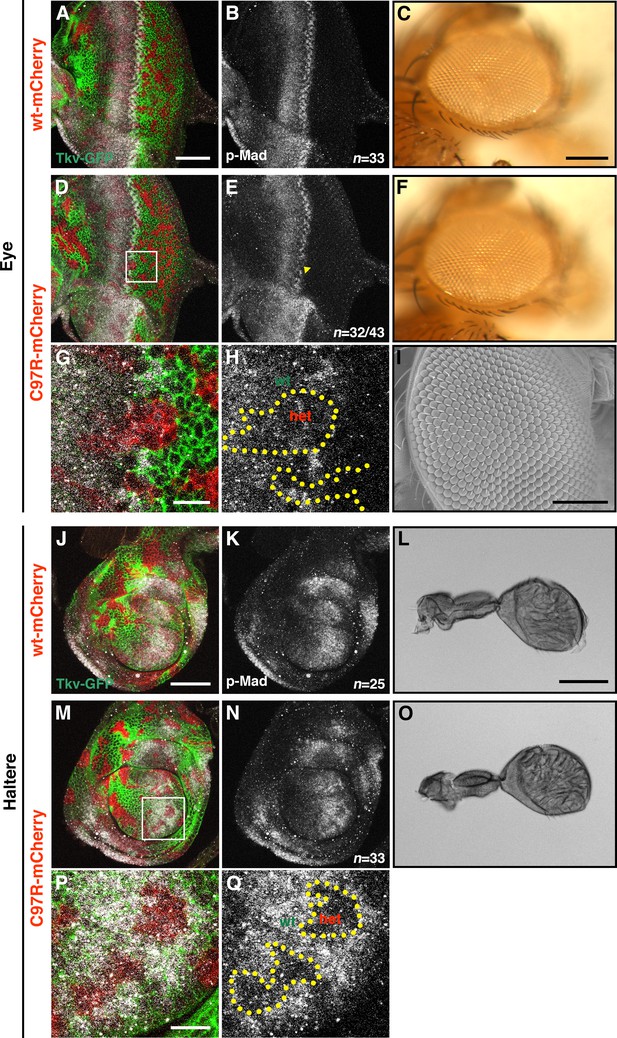
Effects of tkvC97R heterozygous mutant clones in the developing eye and haltere discs.
(A–Q) tkvC97R heterozygous clones in the developing eye (A–I), and haltere discs (J–Q). For these experiments, hs-flp; tkvGFP>>mCherry/+ (control, (A, B, J, K), and hs-flp; tkvGFP>>C97R-mCherry/ + (experimental, (D, E, M, N) animals were heat shocked at 72 hr AEL for 10 min and discs were stained with anti-p-Mad antibody. The boxed areas in (D and M) are shown at higher magnification in (G) and (H), and (P) and (Q), respectively. While p-Mad levels in both discs were mildly disrupted, animals carrying tkvC97R heterozygous clones developed normal eyes and halteres. Scale bars: 50 μm (A, J), 0.2 mm (C), 10 μm (G, P), 100 μm (I, L).
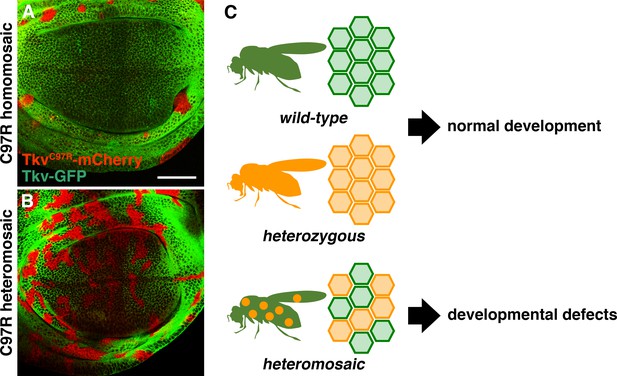
Deleterious heteromosaicism.
(A, B) tkvC97R-mCherry homozygous clones were eliminated from wing disc epithelia (A), while heterozygous cells were retained (B). Scale bars: 50 μm. Anterior is to the left. (C) While wild-type animals and heterozygous carriers both develop normally, animals carrying heterozygous somatic clones exhibit developmental defects.
Additional files
-
Supplementary file 1
Primers used in this study.
EcoRI and AscI sites are underlined in 17 and 22, respectively. Lowercase characters indicate point mutations.
- https://doi.org/10.7554/eLife.35258.015
-
Transparent reporting form
- https://doi.org/10.7554/eLife.35258.016





