Extinction recall of fear memories formed before stress is not affected despite higher theta activity in the amygdala
Figures
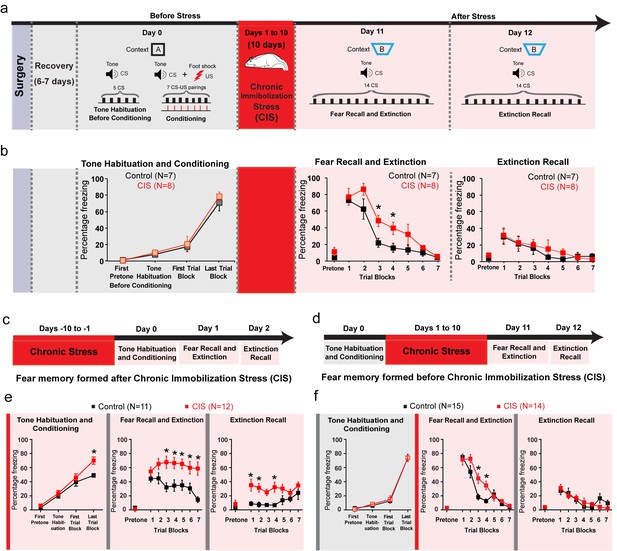
Normal expression of fear memories formed before stress.
(a) Experimental design. Rats were subjected to surgery for implanting recording electrodes, and then allowed to recover for 6–7 days. Next the rats were subjected to tone habituation followed by fear conditioning on Day 0. 24 hr later some of these rats were subjected to chronic immobilization stress (CIS; 2 hr/d, 10d) while others were controls. Both groups underwent fear extinction 10 days later (Day 11), and extinction recall on Day 12. (b) Freezing at different time points. Significant increase in freezing relative to tone habituation after fear conditioning in both control and CIS groups together (F(3,42)=91.71, p<0.01). No difference in fear recall at the start of the session between CIS (N = 8) and control (N = 7) rats (Day 11). CIS rats exhibited higher CS-induced freezing in the third and fourth trial blocks (p<0.05) indicating delay in acquisition of fear extinction. However, both groups eventually decreased freezing to the same level at the end of the 7 trial blocks (Factor: CIS F(1,13)=4.85, p=0.05; Factor: Learning F(6,78)=45.44, p<0.01; Factor: Interaction F(6,78)=1.60, p=0.16). There was no difference in freezing between CIS and control animals during extinction recall (Factor: CIS F(1,13)=0.14, p=0.72; Factor: Learning F(6,78)=9.72, p<0.01; Factor: Interaction F(6,78)=0.57, p=0.75). (c–d) Two different experimental designs to compare the effects of stress on fear recall and extinction by administering the same 10 day CIS either before (c) or after (d) the same fear conditioning protocol. (c) Animals were subjected to CIS from Day −10 to −1 followed by tone habituation and fear conditioning on Day 0. The animals were then subjected to fear recall and extinction on Day 1 followed by extinction recall on Day 2. (d) Animals were subjected to tone habituation and fear conditioning on Day 0 followed by CIS from Day 1 to 10. Next the animals were subjected to fear recall and extinction on Day 11 followed by extinction recall on Day 12. (e) When fear memory was formed after stress, the CIS animals (N = 12) show higher freezing compared to control animals (N = 11) only at the end of the conditioning session (Factor: CIS F(1,21)=6.23, p=0.02; Factor: Learning F(3,63)=75.15, p<0.01; Factor: Interaction F(3,63)=2.48, p=0.07). Interestingly, both CIS and control groups show similar levels of freezing during fear recall at the start of the fear extinction session. However, the animals subjected to CIS fail to undergo extinction down to the same levels of freezing as seen in control animals (Factor: CIS F(1,21)=15.23, p<0.01; Factor: Learning F(6,126)=2.04, p=0.07; Factor: Interaction F(6,126)=1.78, p=0.11). Notably, the CIS animals also exhibit significantly higher freezing during extinction recall relative to control rats (Factor: CIS F(1,21)=15.64, p<0.01; Factor: Learning F(6,126)=1.39, p=0.22; Factor: Interaction F(6,126)=1.04, p=0.40). (f) Both control and CIS groups show learning induced enhancement in freezing (F(3,84)=262.7, p<0.01). There was no difference in fear recall between CIS (N = 14) and control (N = 15) rats (Day 11). However, CIS rats exhibited higher CS-induced freezing in the third and fourth trial blocks (p<0.05) indicating delay in acquisition of fear extinction (Factor: CIS F(1,27)=4.19, p=0.05; Factor: Learning F(6,162)=71.05, p<0.01; Factor: Interaction F(6,162)=4.13, p<0.01). But similar to the rats implanted with electrodes (b), both groups eventually reduced freezing to the same level at the end of the 7 trial blocks. There was no difference in freezing behavior during extinction recall (Factor: CIS F(1,27)=0.03, p=0.86; Factor: Learning F(6,162)=14.67, p<0.01; Factor: Interaction F(6,162)=2.28, p=0.04). Data are mean ±s.e.m. in blocks of two trials except pretone. *p<0.05.
-
Figure 1—source data 1
Data for animals across groups representing freezing response to CS and pretone during the different phases of behaviour (Figure 1b,e and f).
- https://doi.org/10.7554/eLife.35450.004
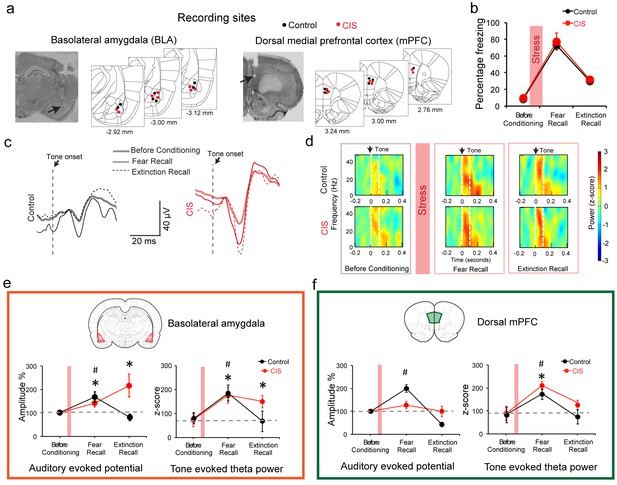
Stress-triggered persistent hyperactivity in the BLA but not the dmPFC.
(a) Representative micrographs and diagrams of recording electrode placements (red: CIS, black: Control) in the BLA (left) and dmPFC (right). (b) Summary of changes in tone-induced freezing behavior before conditioning, and during fear and extinction recall. Stress (red bar) did not affect fear retrieval or extinction (Factor: CIS F(1,13)=0.26, p=0.62; Factor: Learning F(2,26)=51.95, p<0.01; Factor: Interaction F(2,26)=0.02, p=0.98). (c) Representative raw LFP traces depicting changes in AEPs recorded in the BLA in response to CS presented during habituation before conditioning, fear and extinction recall in animals subject to CIS (red) and unstressed control (black) animals. (d) Representative spectrograms of BLA LFPs before conditioning (left), fear recall (center) and extinction recall (right) recorded from control (top) and CIS (bottom) animals. Dotted white lines on the spectrogram indicate onset (arrow) and end of CS. (e) Percentage changes (normalized to tone habituation before conditioning) in auditory evoked potential amplitude (AEP, left) and auditory evoked theta power (right) in the BLA. All statistical comparisons are done within groups across the three time points, not between CIS (N = 8) and control (N = 7) rats. In unstressed control animals, AEP amplitudes increased during fear recall compared to tone habituation (F(2,12)=7.39, p<0.01) and was subsequently reversed during extinction recall. In CIS animals, by contrast, BLA AEP amplitudes were enhanced during both fear recall and extinction recall (F(2,14)=5.78, p=0.01). The same pattern of changes were observed in BLA theta power during fear recall in control animals (F(2,12)=5.66, p=0.02), while CIS animals exhibited theta power enhancement during fear recall that persisted even during extinction recall (F(2,14)=13.70, p<0.01). (f) Percentage changes (normalized to tone habituation before conditioning) in AEPs (left) and auditory evoked theta power (right) in the dmPFC. All statistical comparisons are done within groups across the three time points, not between CIS (N = 8) and control (N = 7) rats. AEP amplitudes in control animals increased only during fear recall (F(2,12)=62.01, p<0.01) whereas the animals subjected to CIS exhibited no changes (F(2,14)=1.59, p=0.24). But dmPFC theta power increased in both control and CIS animals during fear recall relative to tone habituation (Control F(2,12)=5.89, p=0.02; CIS F(2,14)=11.15, p<0.01). And these increases were reversed during extinction recall in both groups. Data are mean ±s.e.m. in each block; #p<0.05, Control animals; *p<0.05, CIS animals.
-
Figure 2—source data 1
Data for animals representing freezing response to CS (Figure 2b) and AEP amplitude and theta power in dmPFC and BLA (Figure 2e,f)
- https://doi.org/10.7554/eLife.35450.008
-
Figure 2—source data 2
Data for animals representing freezing response to CS (Figure 2—figure supplement 2b) and AEP amplitude and theta power in dmPFC and BLA (Figure 2—figure supplement 2c,d)
- https://doi.org/10.7554/eLife.35450.009
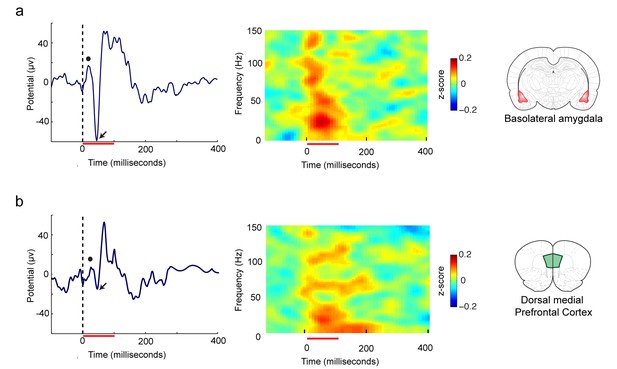
Representative traces and power spectra of tone evoked local field potentials (LFPs).
Auditory evoked potentials (AEPs) and power spectra evoked by the CS recorded from the (a) basolateral amygdala (BLA), and (b) the dorsal medial prefrontal cortex (dmPFC). The red line at the bottom indicates the duration of the CS tone. The black dot represents the first maxima and the arrow represents the first minima.
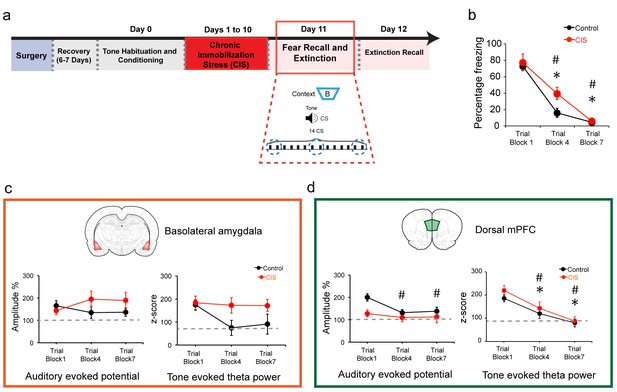
Theta activity in the dmPFC, but not the BLA, mirrors the gradual decrease in freezing levels during the acquisition of fear extinction.
(a) Outline of experimental design depicted in Figure 1. The focus of analysis here is on behavioral and electrophysiological changes during fear recall and extinction learning on Day 11. (b) As described in Figure 1, both control and CIS animals exhibited a gradual reduction in freezing across trial blocks. A significant difference in freezing was found only during trial block 4 such that CIS animals were slower in achieving the same reduction in freezing at the end of the extinction session (Factor: CIS F(1,13)=2.63, p=0.13; Factor: Learning F(2,26)=74.34, p<0.01; Factor: Interaction F(2,26)=2.122, p=0.14). (c) Therefore, we investigated if any of the electrophysiological parameters recorded in these animals matched the behavioral changes in freezing during this session. Accordingly, changes in amplitude of auditory evoked potential (AEP) and theta power evoked by the CS are plotted for trial blocks 1, 4 and 7 (blue dotted circles in (a)). Percentage changes (normalized to tone habituation before conditioning) in AEP (left) and CS-evoked theta power (right) in the BLA. Control and CIS animals did not show any significant changes in AEP amplitudes (left) across trial blocks (Control group: F(2,12)=2.33, p=0.14; CIS group: F(2,14)=0.91, p=0.43). In contrast, a noticeably larger decrease in theta power was seen in the BLA of control animals (right) during trial blocks 4 (Tukey’s Post hoc test; p=0.07) and 7 (Tukey’s Post hoc test; p=0.14), although this did not reach statistical significance (F(2,12)=3.44, p=0.07). In contrast, CIS animals exhibited no such decreasing trends in theta power during trial blocks 4 and 7 (F(2,14)=0.12, p=0.89). (d) Percentage changes (normalized to tone habituation before conditioning) in AEPs (left) and CS-evoked theta power (right) in the dmPFC. In contrast to the BLA, AEP amplitudes in control animals showed significant reductions during trial blocks 4 and 7 (F(2,12)=5.30, p=0.02), but this was not seen in CIS animals (F(2,14)=0.44, p=0.65). Notably, a significant and gradual reduction in dmPFC theta power was seen in both control and CIS animals during trial blocks 4 and 7 relative to trial block 1 (Control group F(2,12)=14.86, p<0.01; CIS group F(2,14)=11.26, p<0.01). However, unlike the difference in freezing levels seen in trial block 4, there was no significant difference in dmPFC theta power between the two groups. All statistical comparisons are done within groups across the three time points, not between CIS (N = 8) and control (N = 7) rats. Data are mean +s.e.m. in each block; #p<0.05, Control animals; *p<0.05, CIS animals.
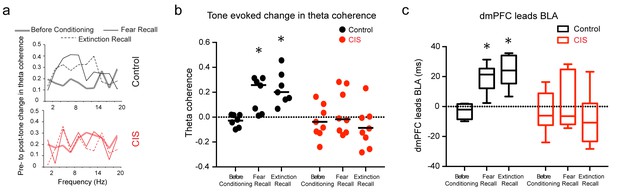
Stress disrupts enhanced dmPFC-BLA theta synchrony and directional coupling during fear expression.
(a) Tone-evoked changes in theta-frequency coherence between the BLA and dmPFC in exemplars of control (top) and CIS (bottom) animals. Control animal exhibited higher synchrony during fear recall and extinction recall than tone habituation. These changes were absent in the rat subjected to CIS. (b) Means and distribution of tone-evoked changes in theta-frequency coherence for both groups (Control N = 7, CIS N = 8). The control animals show enhanced theta coherence during fear recall and extinction recall (F(2,12)=10.81, p<0.01). But animals subjected to CIS did not show any change in theta coherence across sessions (F(2,14)=1.01, p=0.39). Data are presented as scatter plots with medians. (c) Estimation of leads between the dmPFC and BLA using the amplitude cross-correlation. The dmPFC leads more than chance over the BLA during fear and extinction recall in control (N = 7), but not CIS (N = 8) rats. Data are presented as medians ± maxima/minima. *p<0.05 significantly different from chance for each time point in each group.
-
Figure 3—source data 1
Data for animals representing theta coherence between dmPFC and BLA (Figure 3b) and dmPFC-BLA phase difference (Figure 3c)
- https://doi.org/10.7554/eLife.35450.012
-
Figure 3—source data 2
Data for animals representing theta coherence between dmPFC and BLA (Figure 3—figure supplement 1b) and dmPFC-BLA phase difference (Figure 3—figure supplement 1c)
- https://doi.org/10.7554/eLife.35450.013

Stress also disrupts dmPFC-BLA theta synchrony and directional coupling during the acquisition of fear extinction.
(a) Analyses, similar to that shown in Figure 3, of theta coherence and directional coupling between dmPFC and BLA across trial blocks (1, 4, and 7, blue dotted circles) during the fear recall and extinction session on Day 12. (b) Medians and distribution of tone-evoked changes in theta-frequency coherence for both groups (Control N = 7, CIS N = 8). In control animals theta coherence was significantly enhanced during trial blocks 1 and 7. In contrast, no change in theta coherence was seen in CIS animals across sessions. *p<0.05 significantly different from chance for each time point in each group. (c) Estimation of leads between the dmPFC and BLA using the amplitude cross-correlation. The dmPFC leads over the BLA during all the trial blocks in the control (N = 7), but not CIS (N = 8) rats. Data are presented as medians ± maxima/minima. *p<0.05 significantly different from chance for each time point in each group.
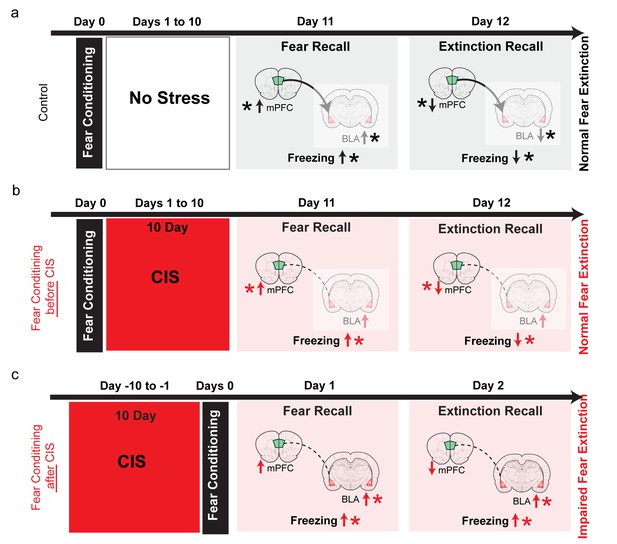
Summary of results and proposed model for how the effects of chronic stress on extinction recall depend on whether the fear memory was formed before or after stress.
(a) In control animals, fear conditioning enhances freezing, which is subsequently reduced by extinction (black vertical arrows). This is accompanied by, first an increase and then a reduction, in theta activity in both the BLA and dmPFC (black vertical arrows). Also, stronger dmPFC-BLA theta synchrony and dmPFC-to-BLA directional influence (thicker curved line with arrow from dmPFC to BLA) is seen during fear and extinction recall. Thus, the direction of changes in freezing are mirrored by in vivo electrophysiological changes in both the BLA and mPFC (indicated by *), although 10 days after conditioning fear expression no longer depends (faded colors) on BLA activity (Do-Monte et al., 2015), Figure 4—figure supplement 1). (b) When fear memories were formed before exposure to chronic immobilization stress (CIS; 2 hr/d for 10d), recall of fear extinction was not affected (red vertical arrows). Although theta activity in the BLA exhibited a persistent increase, its bidirectional regulation was normal in the mPFC (red vertical arrows). CIS also disrupted mPFC-BLA theta-frequency synchrony and directional coupling (dotted lines). However, fear memories formed 10 days ago no longer depend on BLA activity (faded colors), (Do-Monte et al., 2015), Figure 4—figure supplement 1). Consequently, the expression of fear after extinction reflects normal regulation of theta activity in the mPFC (indicated by *), not theta hyperactivity in the BLA. (c) Consistent with earlier behavioral studies, when fear memories were formed after exposure to the same CIS, the behavioural effects on freezing are strikingly different. Stressed animals show enhanced freezing during both acquisition and recall of extinction memory (red vertical arrows). Based on these behavioral findings, and electrophysiological data from our post-conditioning stress experiments (shown in panel b), we hypothesize that CIS will cause a sustained increase in BLA theta activity, but not affect normal bidirectional regulation of dmPFC theta activity (red vertical arrows). Unlike the pre-stress conditioning situation, the CIS animals are now recalling and extinguishing fear memories that were formed only 1 day ago. As a result, CIS-induced BLA hyperactivity will be manifested as stronger fear memories that are resistant to subsequent extinction (indicated by higher freezing during extinction recall in CIS rats). Thus, now it is the sustained increase in BLA theta activity, not normal regulation of dmPFC activity, that better reflects higher freezing in CIS rats during extinction learning as well as extinction recall (indicated by *). Moreover, CIS-induced disruption of mPFC-BLA theta-frequency synchrony and directional coupling (dotted lines) is likely to impair the regulation of BLA activity by the mPFC.
-
Figure 4—source data 1
Data for animals across groups representing freezing response to CS during the different phases of behaviour (Figure 4—figure supplement 1c,d)
- https://doi.org/10.7554/eLife.35450.016
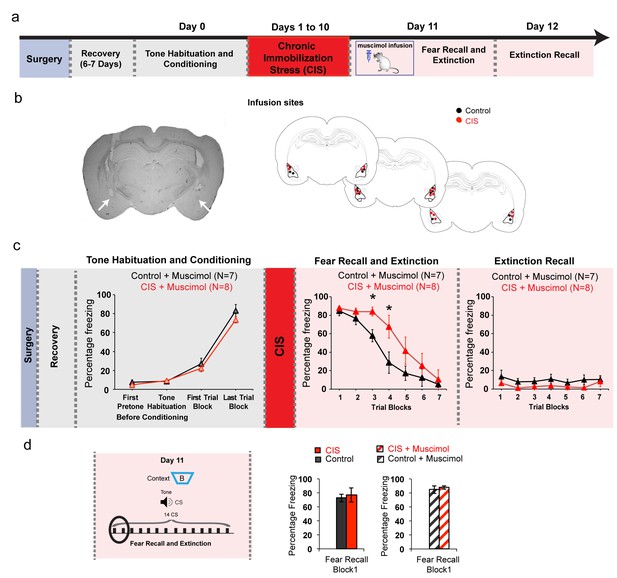
Preliminary results on how pharmacological inactivation of the BLA has no impact on recall of fear memories formed before stress.
(a) Experimental design. Rats were subjected to surgery for implanting cannulae targeting the BLA, and then allowed to recover for 6–7 days. Next the rats were subjected to tone habituation followed by fear conditioning on Day 0. 24 hr later, some of these rats were subjected to chronic immobilization stress (CIS; 2 hr/d, 10d) while others were controls. Both groups underwent muscimol infusion into the BLA followed by fear recall and extinction after 30 min on Day 11. Finally, these animals were subjected to extinction recall on Day 12. (b) Representative micrograph (left) and diagrams (right) showing location of infusion sites in the BLA (red: CIS, black: Control) (c) Freezing levels at different time points. Significant increase in freezing after fear conditioning relative to tone habituation was seen in both control and CIS groups (F(3,42)=179.1, p<0.01). Similar to what was seen in earlier experiments without infusions (Figure 1), BLA inactivation using muscimol led to no difference in fear recall between CIS (N = 8) and control (N = 7) rats (Day 12). CIS rats exhibited higher CS-induced freezing in the third and fourth trial blocks (p<0.05) indicating delay in acquisition of fear extinction, similar to earlier results shown in Figure 1 (Factor: CIS F(1,13)=4.31, p=0.06; Factor: Learning F(6,78)=32.79, p<0.01; Factor: Interaction F(6,78)=1.49, p=0.19). However, both groups eventually decreased freezing to the same level at the end of the 7 trial blocks. Also, there was no difference in freezing during extinction recall between CIS and control groups (Factor: CIS F(1,13)=4.11, p=0.06; Factor: Learning F(6,78)=0.89, p=0.51; Factor: Interaction F(6,78)=0.1937, p=0.98). (d) Comparison of freezing levels during the first block of fear recall with (right) and without (left) muscimol inactivation of the BLA. Importantly, there is no difference in freezing levels between CIS and control rats in these two sets of experiments (i.e. with and without without BLA inactivation) (Factor: CIS F(1,26)=0.31, p=0.58; Factor: BLA inactivation F(1,26)=2.83, p=0.10; Factor: Interaction F(1,26)=0.01, p=0.91). Data are mean ±s.e.m. in blocks of two trials. *p<0.05.
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.35450.017





