Different contributions of preparatory activity in the basal ganglia and cerebellum for self-timing
Figures
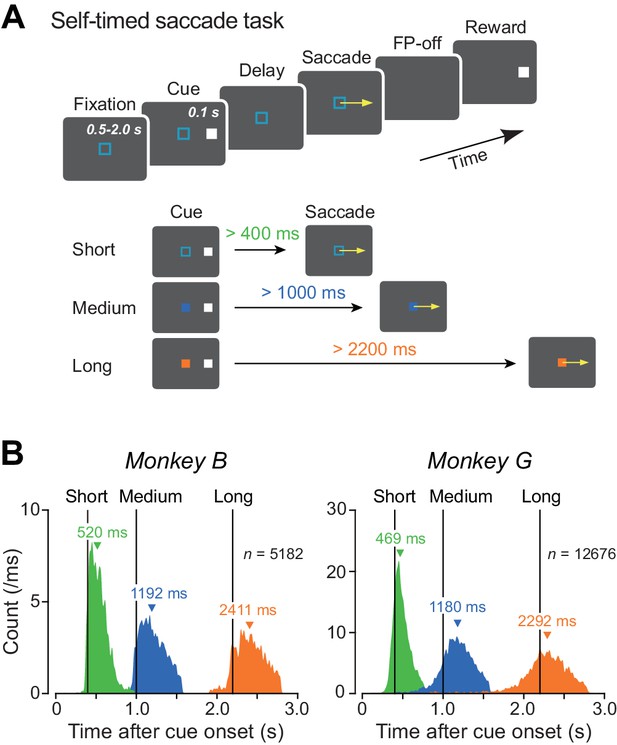
Behavioral task and performance.
(A) Sequence of events in the self-timed saccade task (upper panel). During central fixation, a cue flashed briefly (100 ms) in the peripheral visual field. Monkeys were required to remember the cue location and maintain fixation until expiration of the predetermined mandatory delay interval that was indicated by color of the fixation point (lower panel). Animals received a reward if they correctly made a self-timed memory-guided saccade to the cue location after the mandatory delay period. (B) Distributions of saccade latency during recording sessions in two monkeys. Differently colored histograms represent the data for different mandatory intervals. Vertical lines indicate the end of the mandatory intervals (400, 1000, and 2200 ms). Inverted triangles denote medians.
-
Figure 1—source data 1
Data for Figure 1B.
- https://doi.org/10.7554/eLife.35676.003
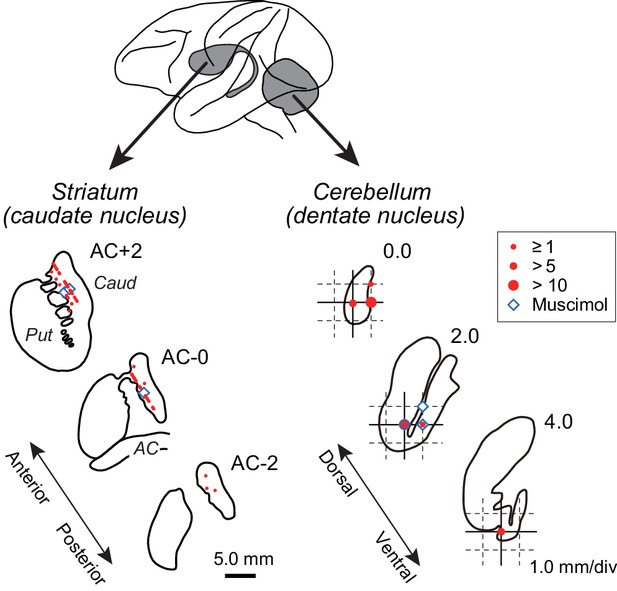
Recording and inactivation sites in monkey G.
Drawings indicate coronal sections of the striatum (left panel) and horizontal sections of the cerebellar dentate nucleus (right panel). Red and blue symbols indicate the sites of recording and muscimol injection, respectively. Data for the intermediate sections are projected anteriorly (striatum) or dorsally (dentate nucleus). Note that the scales for the left and right panels differ by three times. AC, anterior commissure; Caud, caudate nucleus; Put, putamen.
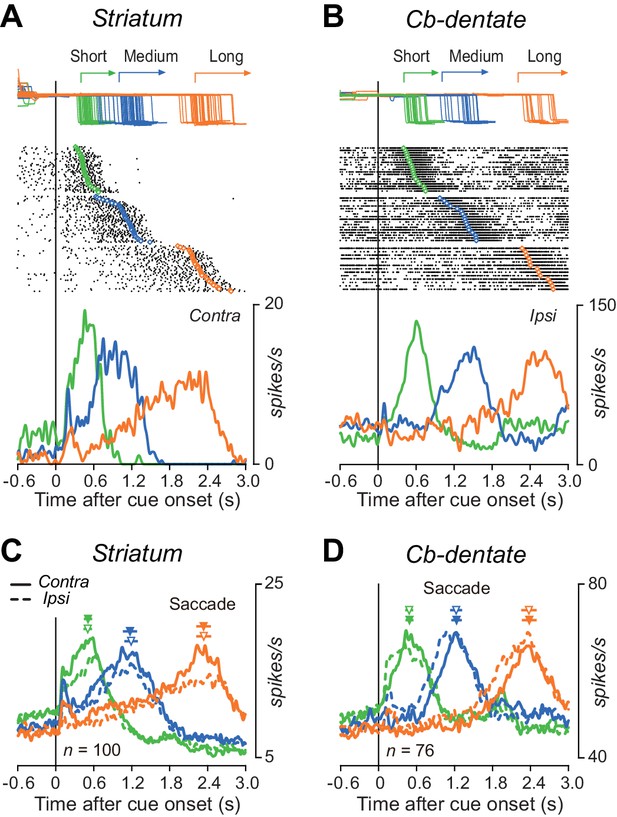
Comparison of single neuronal activity in the striatum and the cerebellar nucleus during the self-timed saccade task.
(A) A representative neuron in the caudate nucleus showing a ramp-up of activity during the delay period. Trials are sorted by saccade latency, and the rasters and corresponding spike density are shown for saccades in contralateral direction. Green, blue, and orange traces indicate data for short, medium, and long mandatory intervals, respectively. (B) A representative neuron in the cerebellar dentate nucleus. Data are shown for saccades in ipsilateral direction. (C) Time courses of the population activity for neurons in the caudate nucleus. Traces are the means of spike densities for individual neurons and are aligned on the cue onset (vertical line). Continuous and dashed traces indicate data for saccades in contralateral and ipsilateral directions, respectively. The filled and open triangles with horizontal bars indicate the mean ± SD of average saccade latency for contralateral and ipsilateral directions in each session, respectively. (D) Time courses of the population activity for neurons in the dentate nucleus. The population data in both structures aligned with saccade initiation are shown in Figure 3—figure supplement 1.
-
Figure 3—source data 1
Data for Figure 3.
- https://doi.org/10.7554/eLife.35676.009
-
Figure 3—source data 2
Data for Figure 3—figure supplement 1.
- https://doi.org/10.7554/eLife.35676.010
-
Figure 3—source data 3
Data for Figure 3—figure supplement 2.
- https://doi.org/10.7554/eLife.35676.011
-
Figure 3—source data 4
Data for Figure 3—figure supplement 3.
- https://doi.org/10.7554/eLife.35676.012
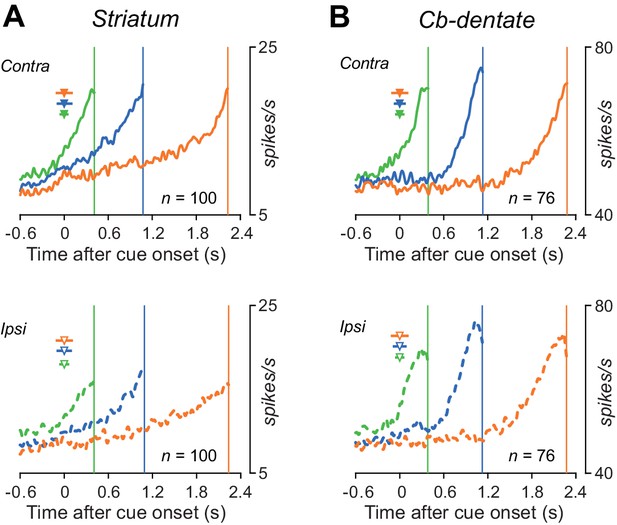
Time courses of the population activity aligned with saccade initiation.
(A) Neurons in the caudate nucleus. Data were aligned with self-timed saccades and were shifted in time to place the end of traces at the mean saccade latencies (vertical lines). The triangles with horizontal bars indicate the mean ± SD of average cue onset time in individual recording sessions. (B) Neurons in the dentate nucleus.
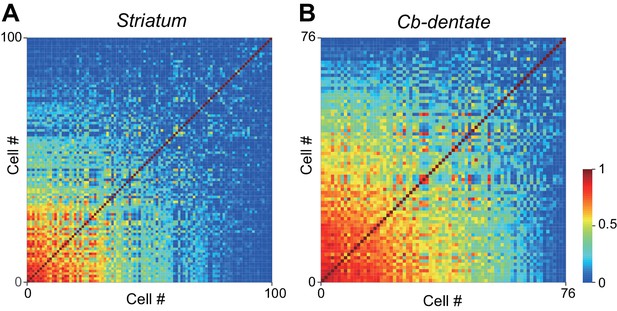
Matrix of inter-neuronal correlation.
(A) Neurons in the caudate nucleus. Correlation of the time course of neuronal activity during the delay period in the self-timing task (medium delay condition, preferred direction) was computed for each pair of striatal neurons. Colors represent r2 values. (B) Neurons in the dentate nucleus. Note that the proportion of neuron pairs showing a strong correlation (r2 >0.7) in the dentate nucleus was greater than that in the caudate nucleus (χ2 test, p=2.1E-50), indicating that cerebellar neurons were more stereotyped than striatal neurons.
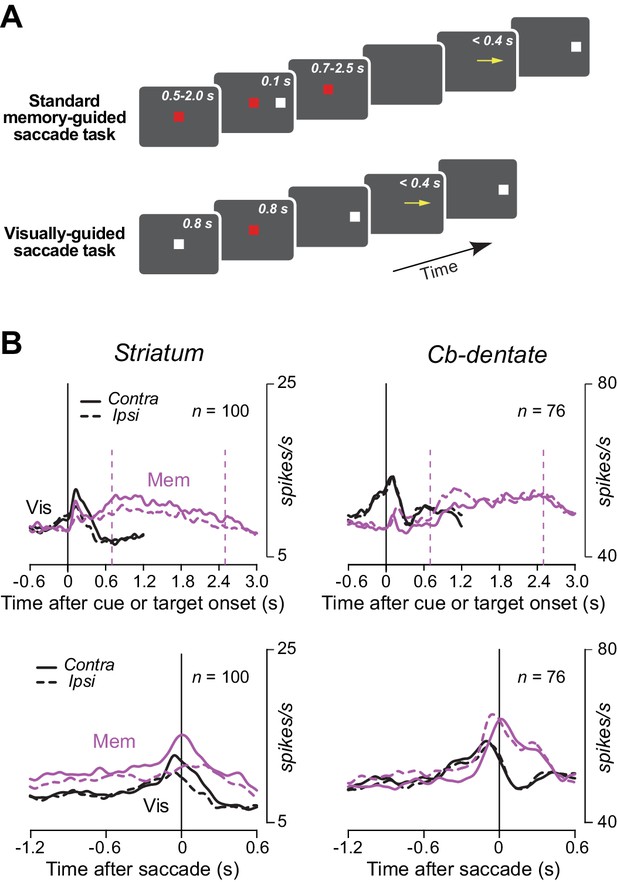
Neuronal activity during the standard memory-guided saccade task and the visually-guided saccade task.
(A) Event sequence in the two tasks. In the standard memory-guided saccade task, monkeys made a saccade to the location of previously presented visual cue in response to the fixation point offset that occurred random 700–2500 ms following the cue onset. In the visually-guided saccade task, the animals made an immediate saccade toward a visible target within 400 ms. (B) Population activity for neurons in the striatum and the cerebellar dentate nucleus during the two saccade tasks. Note that neurons in neither structure showed a ramping activity during the delay period in the standard memory-guided saccade task likely because of the uncertainty about timing of the fixation point offset. In contrast, neurons in both structures often exhibited preparatory activity for visually-guided saccades because the fixation interval was always 800 ms in this task as shown in A. Contra, contralateral; Ipsi, ipsirateral; Mem, standard memory-guided saccade task; Vis, visually-guided saccade task.
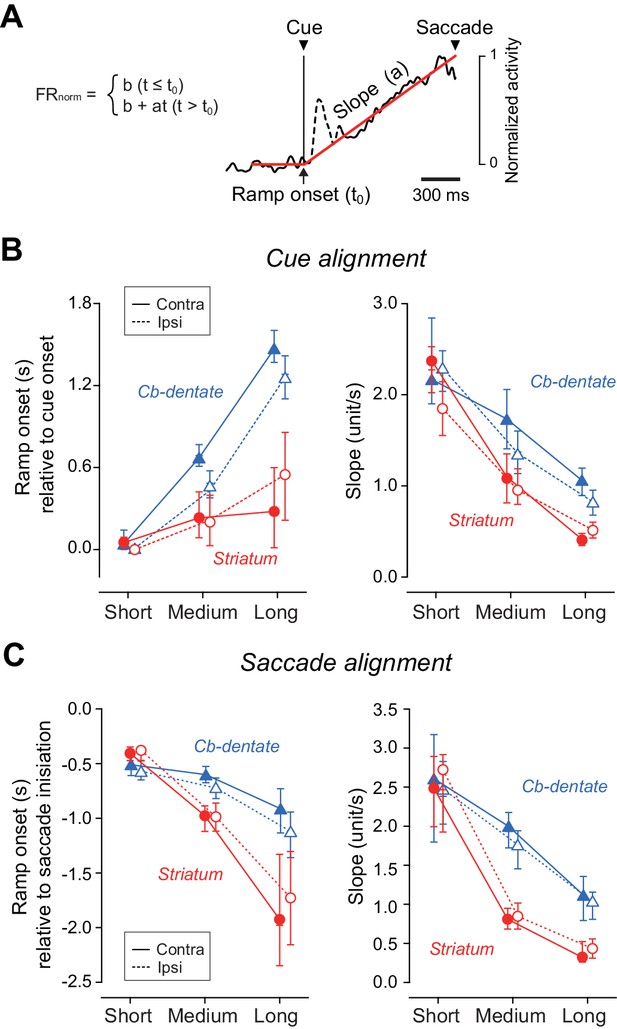
Quantitative analysis for the time course of ramping activity during the delay interval.
(A) An example illustrating how we measured the onset and slope of ramp-up activity. For each condition, the trace of population of normalized activity (starting from 300 ms before cue onset and ending at the mean saccade latency) was fitted with two lines defined by three parameters (least squares, red lines). Data during 200 ms following 50 ms after cue onset were excluded to remove visual transients (dashed line). (B) Summary of ramp onset (left panel) and ramp-up slopes (right panel) for neurons in the striatum (n = 100, red circles) and the cerebellar nucleus (n = 76, blue triangles) based on data aligned with cue onset. Each data point indicates the values computed from the population data. Error bars with tick marks denote 2.5, 50 and 97.5 percentile of the results of the bootstrap analysis. Data points connected with solid and dashed lines indicate the data for ipsiversive and contraversive saccades, respectively. (C) Summary of ramp onsets (left panel) and slopes (right panel) computed for the data aligned with saccade initiation. Note that ramp onsets and slopes for the medium and long delay conditions differed significantly between the striatum and cerebellum.
-
Figure 4—source data 1
Data for Figure 4.
- https://doi.org/10.7554/eLife.35676.014
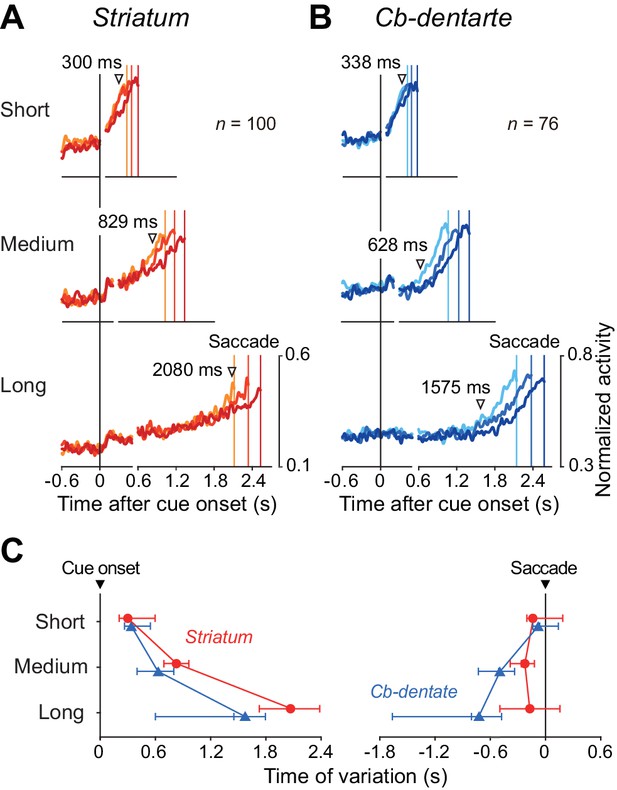
Timing of trial-by-trial variation of ramp-up activity.
(A, B) For each condition, trials were divided into three groups according to saccade latency. Then, the data were normalized for each neuron, aligned with saccade initiation, and were shifted in time so that the times of saccades (colored vertical lines) were placed at the mean saccade latencies relative to the cue onset (right panels). On the left panels, data of the population activity were aligned with the cue onset (vertical black line). Inverted triangles indicate the time when the traces of normalized neuronal firing rate started to diverge as detected by repeated measures ANOVAs (p<0.01 for consecutive 40 ms). The baseline fluctuation (SD) of normalized neuronal activity was comparable between the recording sites (unpaired t-test, p=0.84). (C) Onset of trial-by-trial variation relative to the cue (left panel) or saccade initiation (right). Each point indicates the data derived from the analysis shown in (A) and (B). Error bars with three tick marks denote 2.5, 50 and 97.5 percentile of the results of the bootstrap analysis. Note that the trial-by-trial variation started earlier in the cerebellum than the striatum for medium and long intervals.
-
Figure 5—source data 1
Data for Figure 5.
- https://doi.org/10.7554/eLife.35676.016
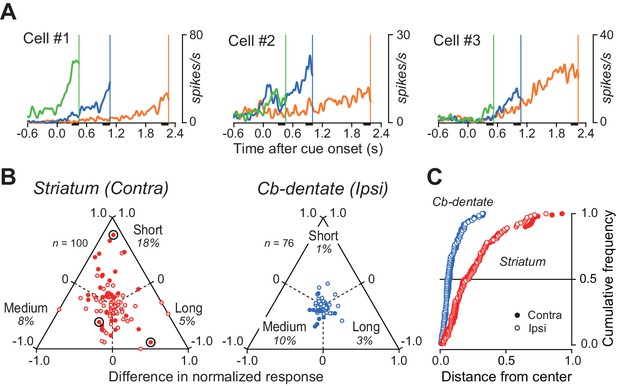
Duration preference of ramping activity.
(A) Three striatal neurons exhibiting a preference for specific mandatory delay interval. For each neuron, data were aligned with self-timed saccades and were shifted in time to place the end of traces at the time of mean saccade latencies (vertical lines). (B) Comparison of the magnitude of firing modulation between the striatum and the cerebellar dentate nucleus. Filled symbols indicate the data showing a significant difference (ANOVA, p<0.01). Bull’s eyes indicate the data for neurons shown in (A). Data for contraversive saccades only are shown for the striatum, while those for ipsiversive saccades only are shown for the cerebellum. Data for the opposite saccade directions are shown in Figure 6—figure supplement 1. Note that the data for the cerebellum clustered around the center, while those for the striatum varied. (C) Cumulative density functions for the distance from the center of the triangles for the data points in (B). Open and filled symbols indicate the data for saccades in ipsilateral and contralateral directions, respectively.
-
Figure 6—source data 1
Data for Figure 6.
- https://doi.org/10.7554/eLife.35676.019
-
Figure 6—source data 2
Data for Figure 6—figure supplement 1.
- https://doi.org/10.7554/eLife.35676.020
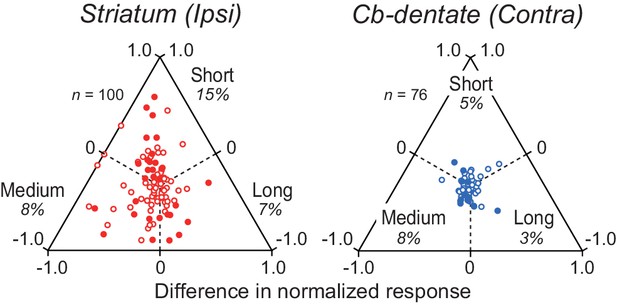
Duration preference of ramping activity.
Comparison of the magnitude of firing modulation across delay intervals for neurons in the striatum and the cerebellar dentate nucleus. Data for ipsiversive saccades only are shown for the striatum (left panel), while those for contraversive saccades only are shown for the cerebellum (right panel). Conventions are the same as Figure 6B.
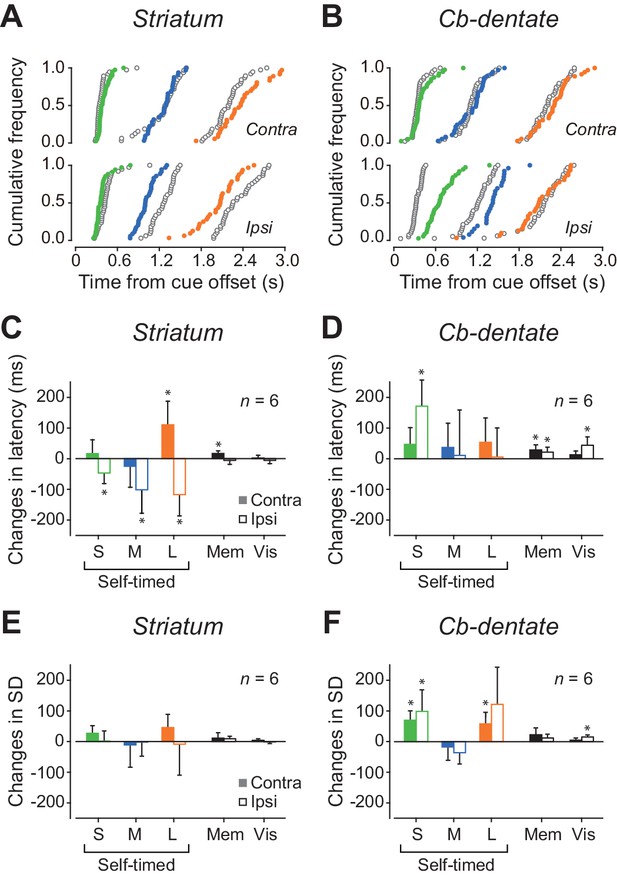
Effects of inactivation.
(A) Data from a representative experiment in the caudate nucleus. Cumulative distributions of saccade latencies are compared between trials before (black open circles) and during (colored circles) inactivation with muscimol. Different colors indicate different mandatory delay intervals. (B) A representative experiment in the cerebellar dentate nucleus. Note that inactivation effects were greatest for ipsiversive trials with short mandatory intervals. (C) Summary of inactivation effects on saccade latency for the caudate nucleus. Bars and whiskers indicate the means and 95% confidence intervals of the changes in median latencies for different conditions. Filled and open bars indicate the data for contraversive and ipsiversive saccades, respectively. Asterisk denotes a significant inactivation effect (paired t-test, p<0.05). (D) Inactivation effects on saccade latency in the cerebellar nucleus. (E, F) Summary of inactivation effects on variation of saccade latency for the caudate nucleus and the cerebellar nucleus, respectively. SD, standard deviation.
-
Figure 7—source data 1
Data for Figure 7.
- https://doi.org/10.7554/eLife.35676.022
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.35676.023





