Mitochondria reorganization upon proliferation arrest predicts individual yeast cell fate
Figures
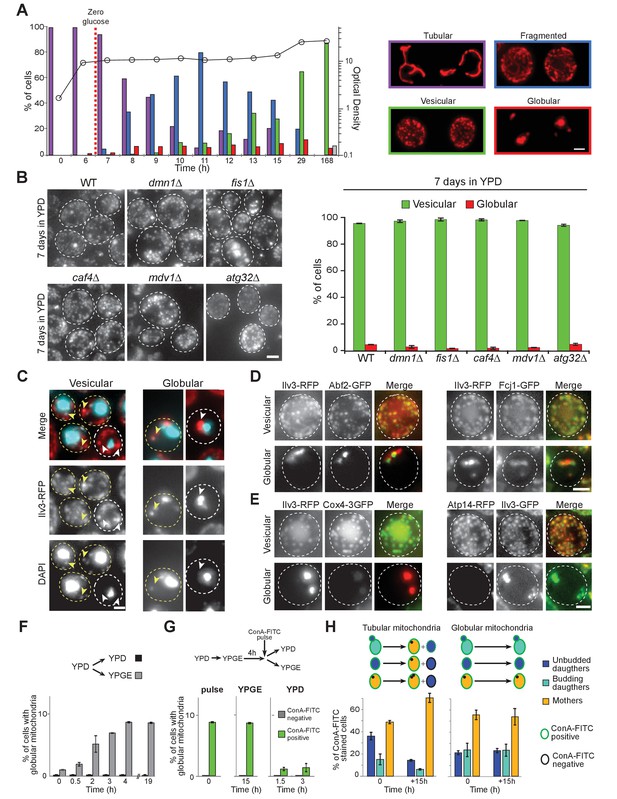
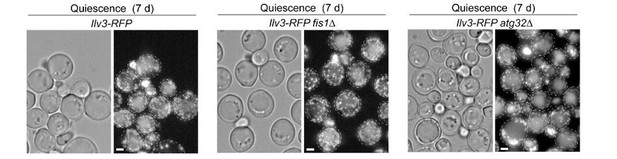
Mitochondrial network reorganization in non-proliferating cells.
(A) Mitochondrial network organization in WT cells expressing Ilv3-RFP as a function of time in YPD (OD600nm: circles). Cells with a tubular (violet), a fragmented (blue), a vesicular (green), a globular (red) mitochondrial network or no Ilv3-RFP signal (grey) were scored. Representative cells of each category are shown; n > 500, N = 2. (B) WT, dnm1Δ, fis1Δ, caf4Δ, mdv1Δ, and atg32Δ cells expressing Ilv3-RFP were grown 7 d in YPD. Histograms display the percentage of Ilv3-RFP positive cells with a vesicular (green) or a globular (red) mitochondrial network; n > 200, N = 2. (C) WT cells expressing Ilv3-RFP were grown 7 d in YPD and stained with DAPI. Yellow and white arrows point to stained or unstained mitochondria respectively. (D) WT cells grown 7 d in YPD co-expressing Ilv3-RFP and the mitochondrial DNA-binding protein Abf2-GFP or the MICOS complex protein Fcj1-GFP. (E) WT cells grown 7 d in YPD co-expressing Ilv3-RFP and the respiratory chain component Cox4-GFP or co-expressing Ilv3-GFP and the ATP synthase subunit Atp14-RFP. (F) WT cells expressing Ilv3-RFP were grown in YPD to OD600nm ~ 1. Cells were then shifted to YPD (black) or YPGE (grey). Cells with globular mitochondria were scored; n > 300, N = 2. (G) WT cells expressing Ilv3-RFP were grown in YPD to OD600nm ~ 1. Cells were then shifted to YPGE. After 4 hr, cells were pulse stained with ConA-FITC and shifted to YPD or YPGE. The percentage of cells with globular mitochondria was scored within both ConA-FITC stained (green) and unstained (grey) cell populations; n > 250, N = 2. (H) Cells from (G) were stained with calcofluor white concomitantly to the ConA-FITC pulse or 15 hr after the transfer to YPGE. The percentage of ConA-FITC positive mother (orange), unbudded daughter (dark blue) and budding daughter (light blue) cells were scored; n > 250, N = 2. Histograms show means, error bars are SD, bar is 2 μm.
-
Figure 1—source data 1
Cell category scoring for each replicated experiment in Figure 1 panel A, B and F to H.
- https://doi.org/10.7554/eLife.35685.005
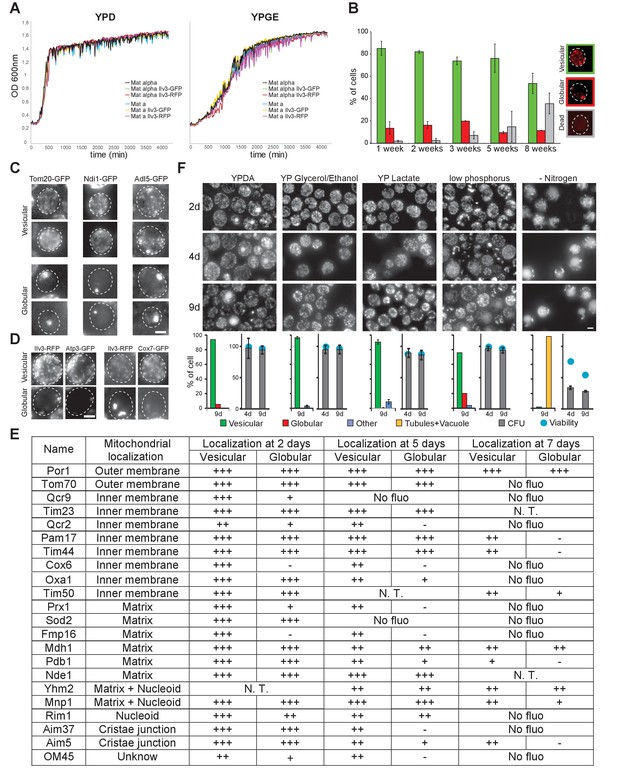
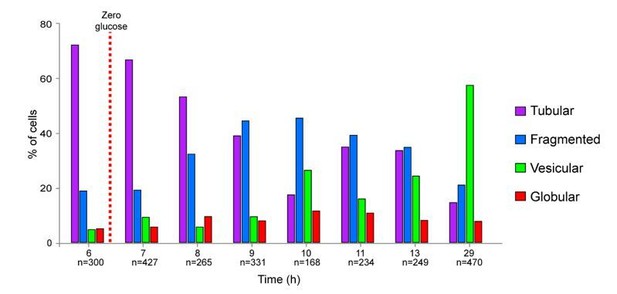
Mitochondrial network organization in WT cells experiencing an extended period of non-proliferation or upon starvation for various nutrients and localization of various mitochondrial proteins in non-proliferating cells.
(A) Proliferation in YPD or YPGE of WT cells of the indicated genotype monitored by measuring OD600nm as a function of time. (B) Mitochondrial network organization of WT cells expressing Ilv3-RFP experiencing an extended period of non-proliferation. Cells with a vesicular (green), a globular (red) or no Ilv3-RFP signal (grey) were scored (n > 300, N = 2). (C) Tom20-GFP, Ndi1-GFP and Adl5-GFP localization in WT cells grown for 4 d in YPD. (D) Sdh2-GFP or Atp4-GFP localization in WT cell expressing Ilv3-RFP grown for 7 d in YPD. (E) Localization of various mitochondrial proteins fused to GFP (endogenous locus) and expressed in WT cells grown for the indicated time in YPD. (+) indicates that the protein is detected within the mitochondria of each cell category, (-) indicates that no fluorescence was detected in a specific cell category, (no fluo) means that no fluorescence was detected whatever the cell category, (N.T.) means not tested. (F) Mitochondrial network organization in WT cells expressing Ilv3-RFP grown in the indicated medium and imaged after 2, 4, 9 days. Graphs present the mitochondria organization at 9 days (left, n > 200, N = 2), cell viability and cell capacity to form a colony (right, viability: n > 200, N = 2; CFU: n = 750, N = 9). Of note, more than 95% of the cells grown 7 days in YPGE were individually able to re-enter proliferation after re-feeding on microscope pad.
-
Figure 1—figure supplement 1—source data 1
Cell category scoring for each replicated experiment in Figure 1—figure supplement 1 panel A, B and F.
- https://doi.org/10.7554/eLife.35685.004
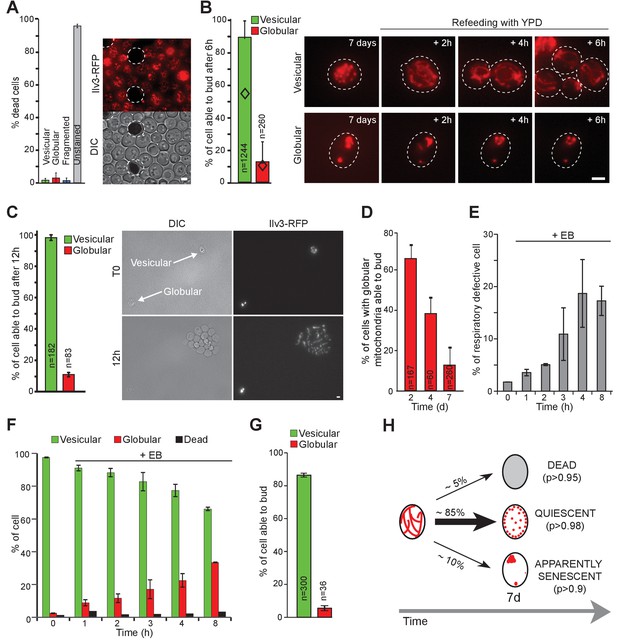
Mitochondrial network reorganization can predict cell fate.
(A) WT cells expressing Ilv3-RFP were grown 7 d in YPD and stained with methylene blue to reveal dead cells. Cells surrounded by a white dashed line are methylene blue positive. The histogram shows the percentage of dead cells, n > 500, N = 2. (B) WT cells expressing Ilv3-RFP were grown 7 d in YPD and re-fed onto a microscope agarose pad containing YPD. Individual cells were imaged every hour. The percentage of cells able to form a new bud within 6 hr is shown (N = 4). Diamonds represent the theoretical independence value. (C) Same as in (B) but cells were imaged for 12 hr. The percentage of cells able to form a bud within 12 hr is shown (N = 3). (D) WT cells expressing Ilv3-RFP were grown in YPD for the indicated time and then refed onto a YPD microscope pad. The percentage of cells with globular mitochondria able to form a bud within 6 hr was scored (N = 2). (E–G) WT cells expressing Ilv3-RFP were grown in YPD to OD600nm~ 0.1. Cells were then treated with 3.5 µM etidium bromide (EB). After incubation for the indicated time, cells were washed and cultured in YPD. After 5 d, cells were plated on YPD, then replicated on YPGE. (E) The percentage of respiratory deficient colonies were scored n > 200, N = 3. (F) After 7 d, the percentage of dead cells (methylene blue positive) or cells with vesicular (green) or globular (red) mitochondria were scored. n > 300, N = 2. (G) After 7 d, cells incubated 8 hr with EB were individually tested for their ability to re-proliferate on YPD microscope pads. The percentage of cells able to form a bud within 6 hr was scored (N = 2). (H) Mitochondrial network organization after 7 days in YPD and individual cell fate; (p) indicates the probability for each cell category 12 hr after refeeding on YPD. Bars are 2 μm. Error bars are SD.
-
Figure 2—source data 1
Cell category scoring for each replicated experiment in Figure 2 panel A, B, C, D and E to G.
- https://doi.org/10.7554/eLife.35685.009
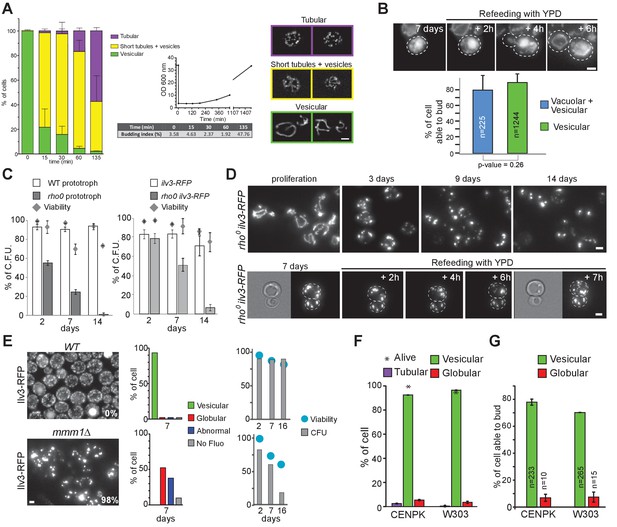
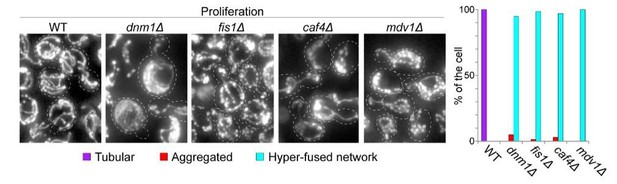
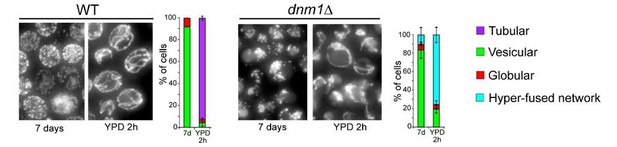
Mitochondrial network organization in rho zero cells, ERMES mutant and WT cells of several genetic backgrounds upon proliferation cessation and quiescence exit.
(A) Upon quiescence exit, the mitochondrial network re-forms tubule before bud emergence. WT cells were grown for 7 days in YPD and allow to exit quiescence in new YPD liquid medium. The morphology of the mitochondrial network is described together with the variation of the budding index and the OD600nm (n > 200, N = 3). (B) WT cells with a vesicular Ilv3-RFP signal or with a Ilv3-RFP signal both in vesicles and in the vacuole have the same ability to emit a bud after refeeding on a YPD microscope pad (N = 4). WT cells expressing Ilv3-RFP were grown for 7 d in YPD and refed on an YPD microscope pad. The ability to form a new bud within 6 hr after refeeding was scored. (C) Cells able to give rise to a colony, that is quiescent cells (CFU, n > 140, N = 2) and dead cells (methylene blue positive, grey diamonds, n > 250, N = 2) were determined in WT and rho zero prototroph (left panel) and auxotroph cells expressing Ilv3-RFP (right panel) grown for the indicated time in YPD. (D) Mitochondrial network organization of rho zero cells expressing Ilv3-RFP upon glucose exhaustion in liquid YPD (upper panel) and upon re-feeding on YPD microscope pad after 7 days of culture. Two cells are shown. (E) WT and mmm1∆ cells expressing Ilv3-RFP were grown for 7 days in YPD. The morphology of the mitochondrial network was analyzed (n > 150, N = 3). After 2, 7 and 16 days in YPD, cell viability (methylene blue staining, n > 200, N = 2, blue dots) and cell’s ability to form colony on YPD plate (micromanipulation of 72 cells, grey) were scored. The percentage of cells unable to proliferate on YPGE is indicated. (F) Morphology of the mitochondrial network of 7 days old WT cells of the CEN-PK or W303 genetic background expressing Ilv3-RFP; n > 200, N = 2. (G) Individual re-proliferation capacity of 7-d-old WT cells of the CEN-PK or W303 genetic background expressing Ilv3-RFP, within 6 hr after re-feeding on a YPD microscope pad (N = 2).
-
Figure 2—figure supplement 1—source data 1
Cell category scoring for each replicated experiment in Figure 2—figure supplement 1 panel A, C, E, F and G.
- https://doi.org/10.7554/eLife.35685.008
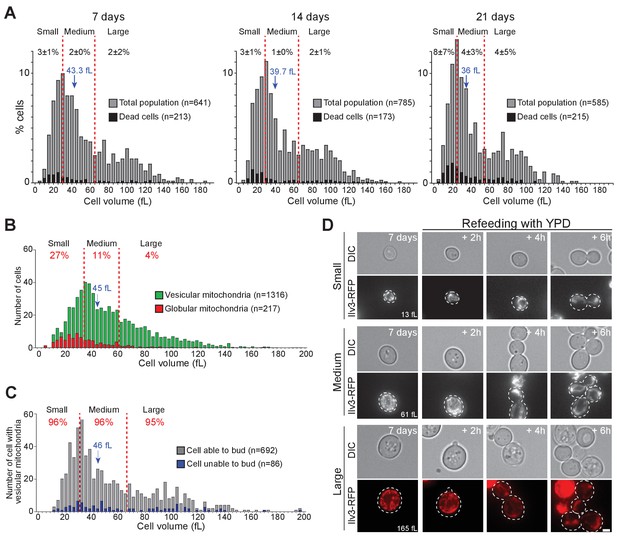
Cell volume and individual cell fate.
(A) Cell volume distribution of WT cells grown for 7, 14, and 21 d in YPD. In A to C, red dashed lines define three sub-populations each representing 1/3rd of the total population. Dead cells (black bars) were identified using methylene blue staining (N = 3). Black numbers indicate the percentage of dead cells within each cell sub-population. (B) Cell volume distribution according to the mitochondrial organization (vesicular: green, globular: red, N = 3) of 7-d-old WT cells expressing Ilv3-RFP. Red numbers indicate the percentage of cells with globular mitochondria within each cell sub-population. (C) Cell volume distribution of 7-d-old WT cells expressing Ilv3-RFP with vesicular mitochondria according to cell re-proliferation capacity. Red numbers indicate the percentage of cells able to bud within 6 hr after refeeding onto a YPD microscope pad within each sub-population (for small cells, n = 235, medium cells n = 226 and large cells n = 231). In A to C, the population median cell volume is indicated in blue. (D) Image series of individual small, regular and large cells with vesicular mitochondria upon refeeding on a YPD microscope pad. Cell volumes before re-feeding are indicated. Bar is 2 μm.
-
Figure 3—source data 1
Cell category scoring for each replicated experiment in Figure 3 panel A, B, and C.
- https://doi.org/10.7554/eLife.35685.013
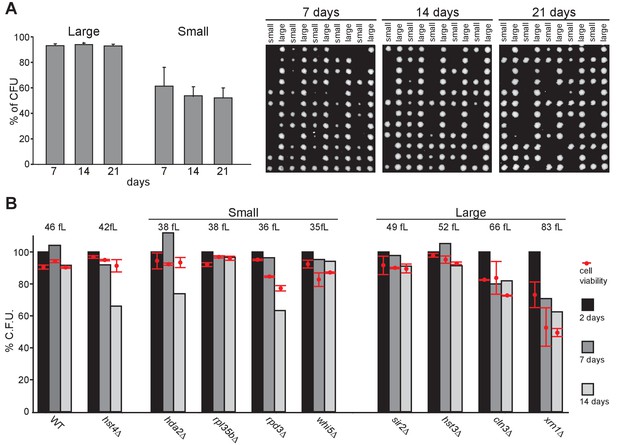
Cell viability and quiescence exit capacity after proliferation cessation in function of cell volume.
(A) WT prototroph cells grown for the indicated time in YPD were individually separated by micro-manipulation onto YPD plates according to their volume (n = 120, N = 2). Representative images of the plates after 3 d at 30°C are shown. (B) Percentage of viable cells (red dot) and colony forming units (CFU) of WT and mutants after 2, 7 and 14 d of growth in YPD. The colony numbers obtained after 2 d were used for normalization. Median cell volume in proliferation and SD are indicated.
-
Figure 3—figure supplement 1—source data 1
Cell category scoring for each replicated experiment in Figure 3—figure supplement 1 panel A and B.
- https://doi.org/10.7554/eLife.35685.012
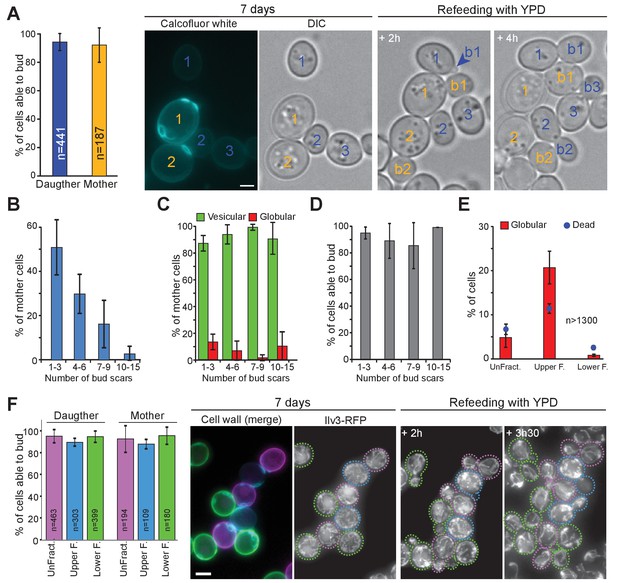
The capacity to enter quiescence does not correlate with a cell’s replicative age or density.
(A–D) WT cells expressing Ilv3-RFP were grown 7 d in YPD, stained with calcofluor white and refed onto a YPD microscope pad. (A) Percentage of mother or daughter cells with vesicular mitochondria able to form a bud within 6 hr after refeeding; n > 270, N = 3. Representative image series are shown. Number in yellow indicate mothers, in blue, daughters. (B) Distribution of the different mother cell categories according to the number of bud scars within the population of mother cells; n > 600, N = 3. (C) Percentage of cells with vesicular (green) or globular (red) mitochondrial network within each mother cell category; n > 400, N = 3. (D) Individual cell’s ability to form a new bud within 6 hr after refeeding onto a YPD microscope pad according to the mother cell replicative age category; n > 400, N = 3. (E) and (F) WT cells expressing Ilv3-RFP were grown 7 d in YPD then centrifuged through a percoll density gradient. (E) The percentage of dead cells and cells with globular mitochondria were scored; n > 500, N = 2. (F) A non-fractionated population (unFract.) was stained with ConA-AlexaFluor647 (pink). Within the fractionated population, the lower fraction (lower F.) was stained with ConA-FITC (green). These two cell subpopulations were re-mixed with the upper fraction subpopulation (upper F.) that was left unstained (blue). Cells were then stained with calcofluor white and allowed to re-proliferate on a YPD microscope pad. The percentage of individual cells with vesicular mitochondria from each fraction that were able to bud within 6 hr after refeeding was scored (N = 3). A representative image series is shown. On the left a field merging the FITC (green), the AlexaFluor647 (pink) and the calcofluor white (blue) channels is shown. Cells are surrounded by dashed line with the color code indicated above. Bar are 2 μm. Error bars are SD.
-
Figure 4—source data 1
Cell category scoring for each replicated experiment in Figure 4 panel A, B to D, E and F.
- https://doi.org/10.7554/eLife.35685.015
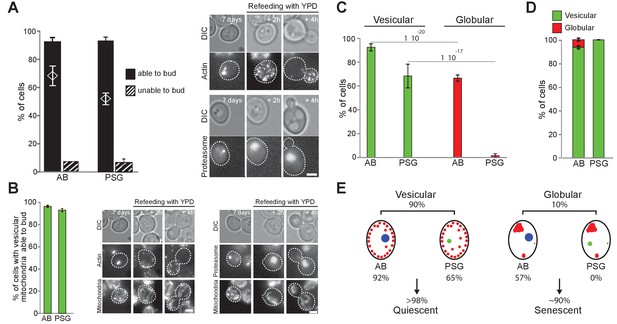
Actin, proteasome and mitochondrial network reorganization.
WT cells expressing Ilv3-RFP and Abp1-3xGFP (actin) or Scl1-GFP (proteasome) were grown 7 d in YPD and refed on a YPD microscope pad. (A) Histograms show the percentage of individual cells displaying AB or PSG able to form a bud within 6 hr after re-feeding. The theoretical ‘independence’ value (diamond) and representative image series are shown; n > 200, N = 2. (B) Percentage of individual cells able to form a bud among cells with both a vesicular mitochondrial network and AB or PSG. Representative image series are shown; n > 300, N = 2. (C) Actin and proteasome organization in cells within vesicular and globular mitochondria cell populations; n > 300 N = 2. (D) Mitochondria organization in cells with AB or PSG; n > 450 and>300 respectively, N = 2. Bars are 2 μm. (E) Cell type and fate distribution among a WT population grown for 7 d. Vesicular mitochondria (small red dots), globular mitochondria (big red dots), AB (big blue dot) and PSG (green dot) are schematized.
-
Figure 5—source data 1
Cell category scoring for each replicated experiment in Figure 5 panel A, B and D.
- https://doi.org/10.7554/eLife.35685.019
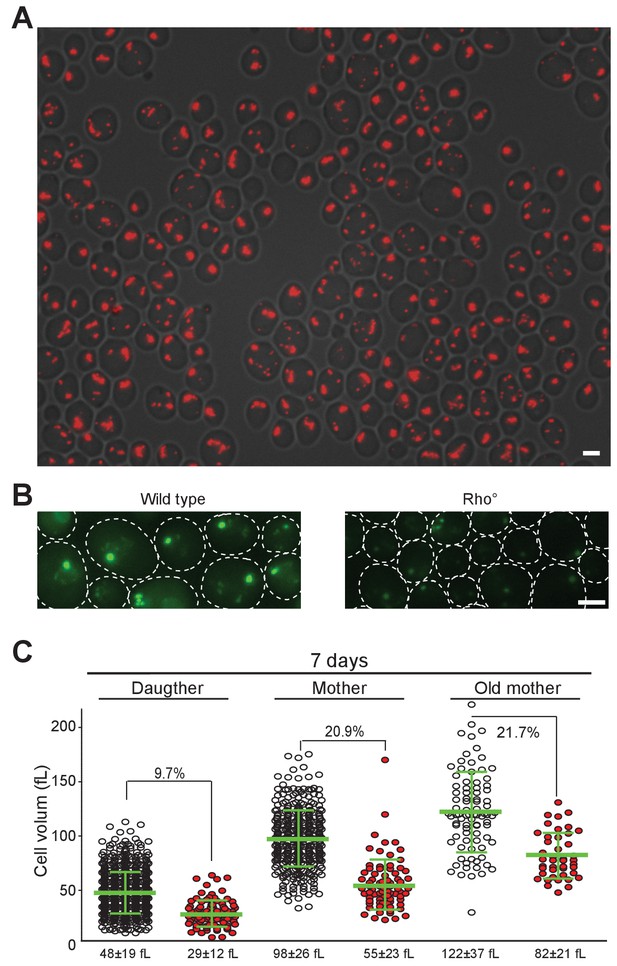
Actin and Proteasome localization in WT and rho zero cells, cell volume variation according to mitochondrial network organization.
(A) Actin phalloidin staining of WT prototroph rho zero cells after 24 hr of culture in YPD. The image is a superposition of the DIC and the RFP channel. (B) Proteasome (Scl1-GFP) localization in WT and WT rho zero cells after 6 d in YPD. Bars are 2 μm. (C) Cell volume of daughter, mother and old mother (>5 bud scars) cells after 7 d in YPD according to their mitochondria organization. The median cell volume and the percentage of cells with globular mitochondria (in red) within each sub-population are indicated.
-
Figure 5—figure supplement 1—source data 1
Cell volume measurements in daughter and mother cells depending on their mitochondrial network organization (panel C).
- https://doi.org/10.7554/eLife.35685.018
Tables
| Resource | Designation | Source or reference | Identifiers |
|---|---|---|---|
| Software | ImageJ | Department of Health and Human Services, NIDA | RRID:SCR_003070 |
| Software | GraphPad Prism 5 | GraphPad Software, Inc. La Jolla, USA | RRID:SCR_015807 |
Additional files
-
Supplementary file 1
Table with the genotype of the strains used in this study.
- https://doi.org/10.7554/eLife.35685.020
-
Transparent reporting form
- https://doi.org/10.7554/eLife.35685.021





