PACAP neurons in the ventral premammillary nucleus regulate reproductive function in the female mouse
Figures
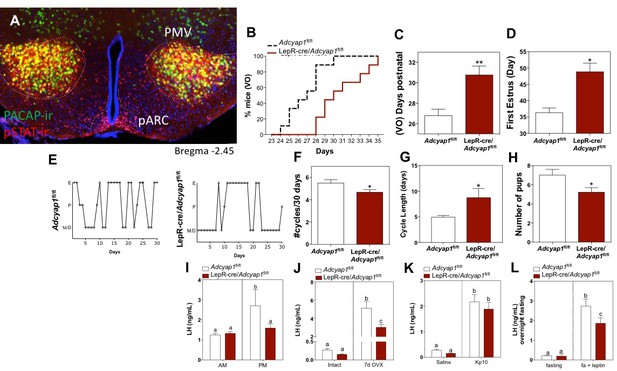
PACAP release from leptin-responsive neurons is essential for normal timing of puberty onset and fertility.
(A) Representative microphotograph of a coronal section depicting PACAP (green) and pSTAT (red) immunoreactivity in the PMV of adult female PACAP-i-cre;L10-GFP mice treated with 5 mg/kg leptin two hours before perfusion. Yellow cells show co-localization. (B–D) Puberty onset is delayed in LepR- Adcyap1fl/fl mice. (B) Accumulated percentage of mice with vaginal opening (VO), an indirect assessment of puberty onset by daily visualization for two weeks post wean (Adcyap1fl/fl n = 9; LepRcre- Adcyap1fl/fl n = 9). (C) Mean postnatal day of VO (Adcyap1fl/fl n = 9; LepRcre- Adcyap1fl/fl n = 9). **p<0.01, Student t-test. (D) Mean postnatal day of first estrous as determined by histology samples after VO taken over the course of one month. (Adcyap1fl/fl n = 4; LepRcre-Adcyap1fl/fl n = 7). *p<0.05, Student t-test. (E–G) Estrous cyclicity is dysregulated in LepR-Adcyap1fl/fl mice. (E) Representative examples of daily (30 days) estrous cycle phases of control Adcyap1fl/fl (n = 9) and LepRcre- Adcyap1fl/fl mice (n = 9). Mouse estrous cycle is 4 days on average, including estrous phase (E), proestrous phase (P) and met/diestrous phase (M/D) which are combined due to similarity by histology. (F) Mean number of estrous cycles in 30 days. *p<0.05 Student t-test (Adcyap1fl/fl n = 9; LepRcre-Adcyap1fl/fl n = 9). (G) Mean cycle length in days. *p<0.05 Student t-test (Adcyap1fl/fl n = 9; LepRcre-Adcyap1fl/fl n = 9). (H) Number of pups per litter for three sets of breeding pairs over five months of ongoing breeding is significantly lower in LepR- Adcyap1fl/fl mice. *p<0.05 Student t-test (Adcyap1fl/fl n = 18; LepRcre- Adcyap1fl/fl n = 18). (I) Preovulatory LH surge is blunted in LepR-Adcyap1fl/fl mice. Circulating levels of LH in the morning (AM 10:00 hr) and afternoon (PM 19:00 hr) under an LH-surge inducing protocol. Adcyap1fl/fl n = 5; LepRcre- Adcyap1fl/fl n = 5). Different letters indicate statistically different values (2 Way ANOVA followed by Fisher’s post hoc test, p<0.05). (J) Circulating LH levels before and one week after OVX. The lack of steroid hormone feedback in control animals leads to a marked increase in LH, which is blunted in LepR-Adcyap1fl/fl mice. Different letters indicate statistically different values (2 Way ANOVA followed by Bonferroni post hoc test, p<0.05), (intact: Adcyap1fl/fl n = 7; LepRcre-Adcyap1fl/fl n = 4. OVX: Adcyap1fl/fl n = 3; LepRcre-Adcyap1fl/fl n = 5). (K) Circulating LH levels 25 min after the injection of vehicle (saline) or 1 nmol kisspeptin 10 (kp10) are not significantly different between the knockout and control animals. Different letters indicate statistically different values (2 Way ANOVA followed by Bonferroni post hoc test, p<0.05), (saline: Adcyap1fl/fl n = 7; LepRcre-Adcyap1fl/fl n = 3. Kp10: Adcyap1 n = 4; LepRcre-Adcyap1fl/fl n = 9). (L) Circulating LH levels after overnight fast and 30 min after central leptin administration are blunted in LepR-Adcyap1fl/fl mice. Different letters indicate statistically different values (2 Way ANOVA followed by Fisher’s post hoc test, p<0.05), (fasting: Adcyap1fl/fl n = 3; LepRcre-Adcyap1fl/fl n = 7. Fasting + leptin: Adcyap1fl/fl n = 3; LepRcre-Adcyap1fl/fl n = 9).
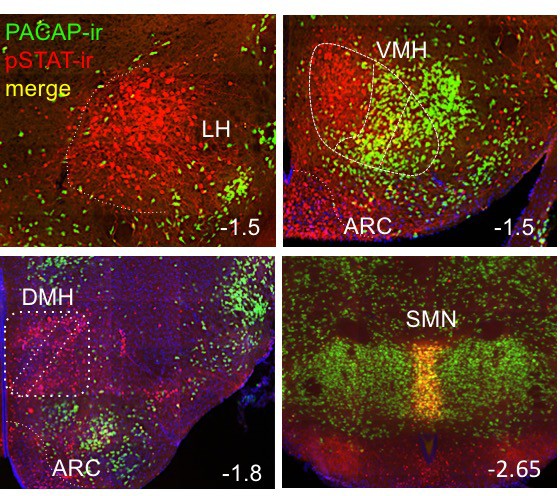
Representatives images of hypothalamic nuclei coronal sections depicting areas of co-labeling (yellow) of PACAP-ir (green) and pSTAT-ir (red) in adult female PACAP-i-cre;L10-GFP mice treated with 5 mg/kg IP leptin two hours before perfusion.
LH: lateral hypothalamus; VMH: ventromedial hypothalamus; ARC: arcuate; DMH; dorsomedial hypothalamus; SMN: supra mammillary nucleus. Numbers at the bottom right of each image detail the distance from bregma in mm for reference.
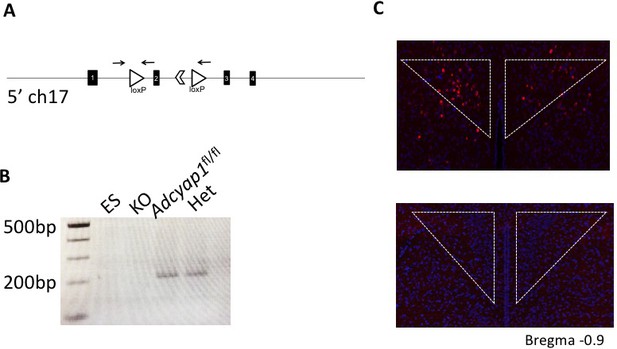
Validation of PACAP conditional allele.
(A) Schematic of recombined allele with loxP sites (open triangles) around exon 2 of PACAP gene on chromosome 17 to create the conditional knock out. Arrows denote primers used for PCR. (B) PCR validation of germline deletion of Adcyap1fl/fl by genetic cross to E2A-cre in punches from mouse hypothalamus. First lane (ES) is ES cell DNA, second lane (KO) is from E2A-cre; Adcyap1fl/fl mouse, third lane from Adcyap1fl/fl, and fourth lane (Het) from E2A-cre; Adcyap1fl/+ mouse. (C) FISH showing Adcyap1 expression in the hypothalamus for validation of the lox allele. Top image is brain of Adcyap1fl/fl and bottom is from E2A-cre; Adcyap1fl/fl mouse.

Additional characterization of the metabolic and reproductive phenotype of LepRCre-Adcyap1fl/fl male and female mice.
(A) RT-qPCR (upper panel) and FISH (lower panel) of PACAP mRNA in the PMV of control (Adcyap1fl/fl) and LepRCre-Adcyap1fl/fl female mice. For qPCR, brain tissue was microdissected from two 1 mm thick sections per animal (n = 3). For FISH, coronal sections of 12 μm thickness were used across the rostro-caudal border, representative image is from the approximate midpoint, *p<0.05 Student t-test. (B) BW of female LepRCre Adcyap1fl/fl and Adcyap1fl/fl female mice during 16 weeks. All animals were switched to a 60% high fat diet in week 8, and there is no significant change in body weight, though LepRCre- Adcyap1fl/fl females are slightly protected from weight gain due to HFD compared to controls. (2 Way ANOVA followed by Bonferroni post hoc test). (C) Number of corporal lutea in the middle section of the ovary from control (n = 5 and LepRCre- Adcyap1fl/fl (n = 6) female mice. Student t-test.
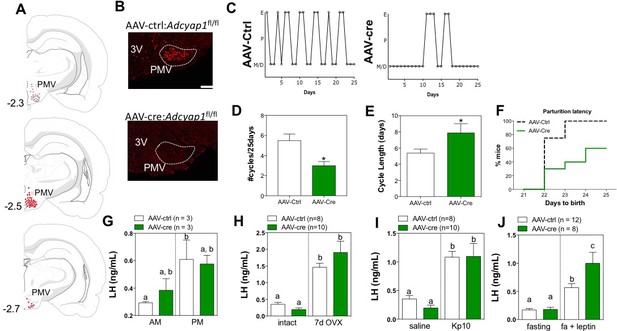
Ablation of PACAP expression from the PMV leads to impairment in the gonadotropic axis.
(A) Schematic representation of the sites of injection of AAV8.CMV.HI.eGFP-Cre showing the extent of viral spread around the PMV for Adcyap1fl/fl study animals. Animals were included in analyses only if they had bilateral injection across >70% of the rostro-caudal diameter of the PMV that did not spread into surrounding areas of PACAP expression (i.e. VMH, dorsal premammillary nucleus). Control virus (AAV8.CMV.PI.eGFP.WPRE.bGH) was injected bilaterally in littermate controls of the same genotype (data not shown). (B) Representative ISH depicting coronal section with Adcyap1 mRNA in the PMV of AAV8.CMV.PI.eGFP.WPRE.bGH control injected (left panel) and AAV8.CMV.HI.eGFP-Cre injected Adcyap1fl/fl female mice. Only animals with bilateral deletion of >70% Adcyap1 mRNA were included in analyses. (C – E) Assessment of estrous cyclicity shows marked dysregulation in the AAV-cre treated Adcyap1fl/fl mice. (C) Representative examples of daily (25 days) estrous cycle phases of control (n = 8) and AAV-cre injected mice (n = 6). (D) Mean number of estrous cycles in 25 days. *p<0.05 Student t-test (AAV-ctrl:Adcyap1fl/fl n = 8; AAV-cre:Adcyap1fl/fl n = 10). (E) Mean cycle length in days. *p<0.05 Student t-test (control n = 6; AAV-cre n = 8). (F) AAV-cre mediated deletion of PACAP from PMV prolongs parturition latency represented as accumulated percentage of animals giving birth per day after mating with C57Bl/6 male for 1 week (AAV-ctrl:Adcyap1fl/fl n = 8; AAV-cre:Adcyap1fl/fl n = 6). (G) Analysis of the magnitude of the preovulatory LH surge. Circulating levels of LH in the morning (AM 10:00 hr) and afternoon (PM 19:00 hr) under an LH-surge inducing protocol. (AAV-ctrl:Adcyap1fl/fl n = 3; AAV-cre:Adcyap1fl/fl n = 3). Different letters indicate statistically different values. Only AAV-Ctrl injected mice showed a significant increase from the AM to the PM. (2 Way ANOVA followed by Bonferroni post hoc test, p<0.05). (H) Circulating LH levels before and one week after OVX are not significantly different between the AAV-cre and AAV-ctrl treated Adcyap1fl/fl mice. Different letters indicate statistically different values (2 Way ANOVA followed by Bonferroni post hoc test, p<0.05), (AAV-ctrl:Adcyap1fl/fl n = 8; AAV-cre:Adcyap1fl/fl n = 10). (I) Circulating LH levels 25 min after the injection of vehicle (saline) or 1 nmol kisspeptin 10 (kp10) are intact. Different letters indicate statistically different values (2 Way ANOVA followed by Bonferroni post hoc test, p<0.05), (AAV-ctrl:Adcyap1 fl/fl n = 8; AAV-cre:Adcyap1fl/fl n = 10). (J) Circulating LH levels after overnight fast and 30 min after central leptin administration indicate PACAP is involved in, but is not necessary for leptin signal transduction through the PMV. Different letters indicate statistically different values (2 Way ANOVA followed by Fisher’s post hoc test, p<0.05), (AAV-ctrl:Adcyap1fl/fl n = 12; AAV-cre:Adcyap1fl/fl n = 8).
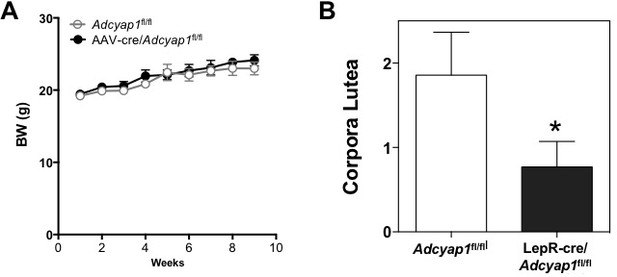
PACAP deletion from PMV neurons in adult females does not affect body weight but decreases the number of corpora lutea.
(A) BW of female Adcyap1fl/fl injected with AAV8.CMV.PI.eGFP.WPRE.bGH (n = 5) or AAV8.CMV.HI.eGFP-Cre (n = 5) during 10 weeks post injection. (2 Way ANOVA followed by Bonferroni post hoc test). (B) Number of corporal lutea in the middle section of the ovary from Adcyap1fl/fl treated with AAV-Ctrl (n = 7) or AAV-cre (n = 6). *p<0.05 Student t-test.
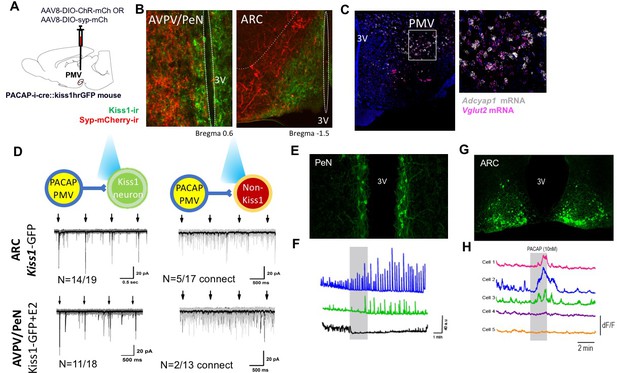
PMVPACAP neurons monosynaptically contact a subset of ARC and AVPV Kisspeptin neurons.
(A) Schematic representation of the site of injection of AAV8.2-eF1a-DIO-syp-mCherry-WPRE (projection tracing, n = 3) or AAV8-hSyn-DIO-ChR2(H134R)-mCherry (circuit mapping, n = 5). (B) Representative microphotographs depicting immunoreactivity of projections from PMVPACAP neurons (red) and Kiss1 neurons (green) in the ARC and AVPV/PeN nuclei after injection of AAV8.2-eF1a-DIO-syp-mCherry-WPRE into the PMV in PACAP-i-cre female mice. (C) Representative double label ISH depicting co-localization of Adcyap1 (white) and Vglut2 (pink) mRNA in the PMV of female C57Bl/6 mice in a coronal section. Right image is an enlarged view of the boxed section in the left image. (D) Channelrhodopsin assisted circuit mapping (CRACM) analysis of projections from PACAP-i-cre mice injected with AAV-DIO-syp-ChR2-mcherry photo-stimulated in the ARC or AVPV of Kiss1-GFP mice. The majority of tested kisspeptin neurons in both regions receive direct excitatory input from PMVPACAP neurons (14/19 in ARC, 11/18 in AVPV/PeN), and a small number of non-kisspeptin neurons in each region (5/17 and 2/13 respectively) do as well. Arrows depict blue light pulse (473 nm, 5 ms epoch) administered 1 s apart during the first 4 s of an 8 s sweep, repeated for a total of 30 sweeps per recorded cell. Black line shows the average of all sweeps, and gray lines are individual sweeps. (E) Representative photomicrograph of coronal section showing GCaMP6f fluorescence in the AVPV/PeN specific to kisspeptin neurons of a diestrous female Kiss1-GCaMP6f mouse. (F) Traces showing the effect of 10 nM PACAP (grey bar) on GCaMP6f fluorescence levels (delta F/F) in three kisspeptin cells recorded simultaneously from the AVPV/PeN indicating PACAP causes different responses in different populations of neurons in this region. (G) Representative photomicrograph of coronal section showing GCaMP6f fluorescence in the caudal arcuate nucleus specific to kisspeptin neurons of a diestrous female Kiss1-GCaMP6f mouse. (H) Traces showing the effect of 10 nM PACAP (grey bar) on GCaMP6f fluorescence levels (delta F/F) in five kisspeptin cells recorded simultaneously from the caudal ARC indicating PACAP causes increased activity in a subpopulation of kisspeptin neurons.
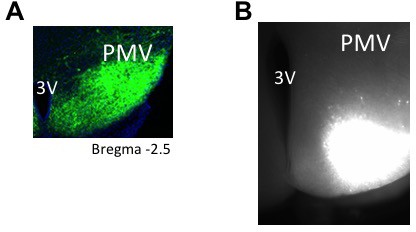
Representative images of PMV injection site for tracing and electrophyisiology experiments.
(A) Representative microphotograph depicting injection site for tracing experiments using AAV8.2-eF1a-DIO-syp-mCherry-WPRE (30 μm thick coronal section) restricted to PMV in PACAP-i-cre mice. (n = 3 mice). (B) Representative microphotograph depicting injection site for CRACM experiments using AAV8-hSyn-DIO-ChR2(H134R)-mCherry (300 μm thick coronal section) restricted to PMV in PACAP-i-cre mice (n = 5).
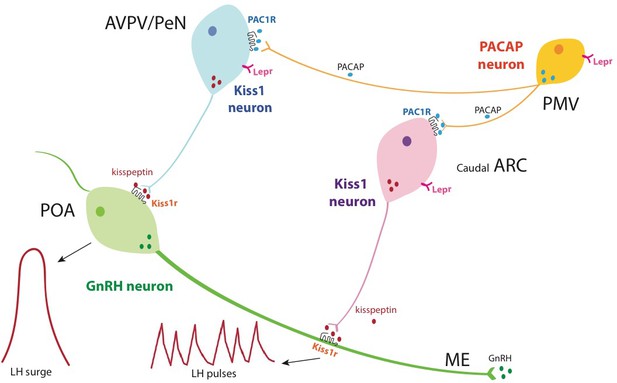
Schematic representation of the hierarchical inputs from PMVPACAP neurons to AVPV/PeN and ARC Kiss1 neurons.
Briefly, PMVPACAP neurons regulate reproductive function by directly modulating subpopulations of AVPV/PeNkisspeptin and caudal ARCkisspeptin neurons, which leads to the regulation of the preovulatory GnRH/LH surge and pulsatile GnRH release, respectively, through the action of kisspeptin at the level of GnRH neurons. Through the direct action of leptin, PMVPACAP neurons mediate in part the metabolic regulation of reproductive function.
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.35960.011





