Adult zebrafish Langerhans cells arise from hematopoietic stem/progenitor cells
Figures
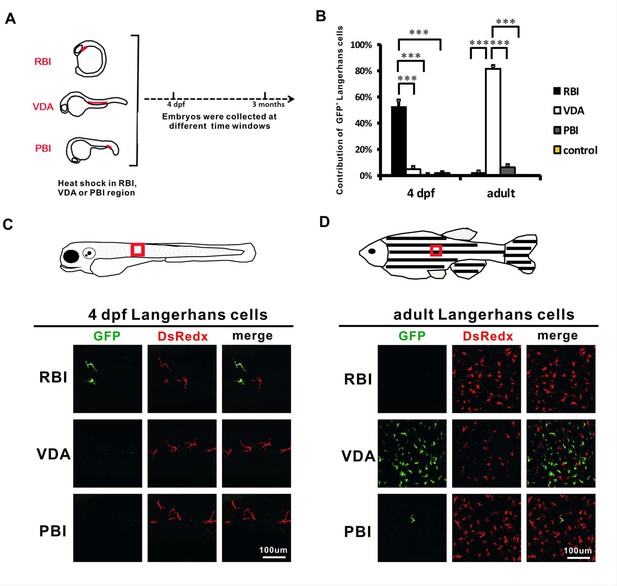
Adult LCs are largely derived from the VDA region in zebrafish.
(A) A schematic diagram indicates the RBI, VDA and PBI regions and the time point (4 dpf and 3 months) when the heat-shocked fish are analyzed. (B) Quantification of the percentage of GFP+ LCs derived from the RBI, VDA, PBI, and non-heat-shocked control at 4 dpf and adulthood. n = 13, 7, 10 and 6 for the RBI-, VDA-, PBI-, and non-heat-shocked control fish analyzed at 4 dpf respectively. n = 5, 5, 5 and 6 for the RBI-, VDA-, PBI-, and non-heat-shocked control fish analyzed at adulthood, respectively. Error bars represent mean SEM. ***p<0.001. (C) Anti-GFP staining shows that GFP+ LCs are detected in the RBI-labelled fish, but not VDA- and PBI-labelled fish at 4 dpf. The red box indicates the imaging region. (D) Anti-GFP staining indicates that GFP+ LCs are mainly detected in the VDA-labelled fish, but not the RBI- and PBI-labelled fish in adulthood. The red box indicates the imaging region.
-
Figure 1—source data 1
Quantification of GFP+ Langerhans cells at embryonic and adult stages.
- https://doi.org/10.7554/eLife.36131.005
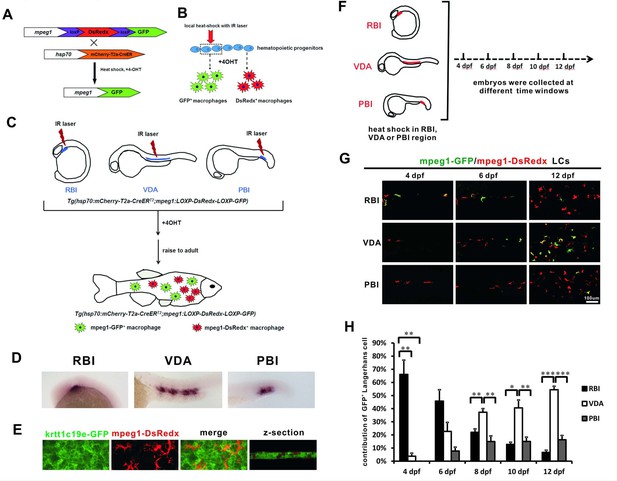
Experimental design of the IR-LEGO-CreER-loxP cell labelling system for LC fate mapping.
(A) The Tg(mpeg1:loxP-DsRedx-loxP-GFP) fish was crossed with the heat shock-inducible Tg(hsp70:mCherry-T2a-CreERT2) line. The resulted double transgenic embryos were applied to infrared heats-hock. Upon 4-OHT treatment, CreER mediated loxP recombination occurs. (B) Local heat-shock was introduced with IR laser to hematopoietic progenitors and 4-OHT was applied to induce loxP recombination. After cell differentiation, only the macrophages derived from the heat-shocked progenitors would express GFP, whereas the other macrophages remain DsRedx expression. (C) In the double transgenic embryos, IR laser was introduced to RBI, VDA or PBI, the three regions produce primitive myeloid cells, definitive hematopoiesis or intermediate EMPs respectively. After 4-OHT treatment, the embryos were raised to adult and the contribution of GFP+ and DsRedx+ LCs were examined. (D) Local heat-shock successfully induced CreER expression in RBI, VDA, and PBI. (E) mpeg1-DsRedx+ macrophages (red signals) locate within the krtt1c19e-GFP+ (green signals) basal epidermal layer as shown by the 3D view of the z-section. (F) A timeline shows that the fish heat-shocked in RBI, VDA and PBI regions were examined from 4 dpf to 12 dpf at 2 day intervals. (G) Imaging of the skin at 4 dpf, 6 dpf, and 12 dpf shows that VDA-derived GFP+ LCs gradually become the predominant population. The mpeg1-GFP and mpeg1-DsRedx signals were merged together, therefore both green and yellow LCs represent GFP+ cells. (H) Quantification of the percentage of RBI, VDA, and PBI derived LCs from 4 dpf to 12 dpf at 2 day intervals. n = 6, 5, and 5 for RBI, VDA, and PBI, respectively, (n = 4 for VDA at 10 dpf and 12 dpf). Error bars represent mean SEM. *p<0.05; **p<0.01; ***p<0.001.
-
Figure 1—figure supplement 1—source data 1
Quantification of GFP+ Langerhans cells at early developmental stages.
- https://doi.org/10.7554/eLife.36131.004
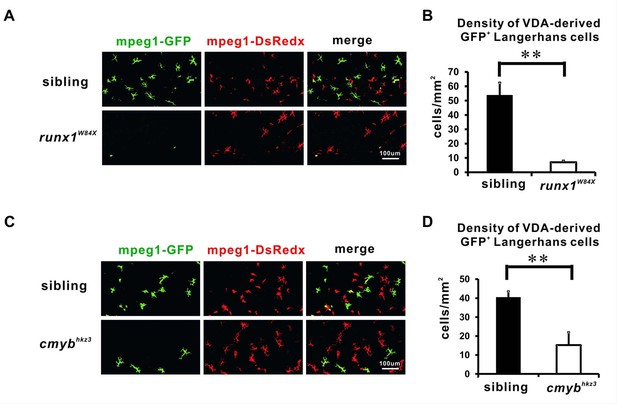
The VDA-derived LCs are reduced in Runx1 and cMyb mutants.
(A) Anti-GFP staining shows that the VDA-derived LCs are significantly reduced in 12 dpf runx1W84X mutants comparing with those in siblings. (B) Quantification of the density of the VDA-derived GFP+ LCs in 12 dpf runx1W84X mutants and siblings. n = 5 for siblings and n = 6 for mutants. Error bars represent mean SEM. **p<0.01. (C) Anti-GFP staining reveals a reduction of the VDA-derived LCs in 20 dpf cmybhkz3 mutants comparing with size matching siblings. (D) Quantification of the density of the VDA-derived GFP+ LCs in 20 dpf cmybhkz3 mutants and size matching siblings. n = 11 and 10 for siblings and mutants respectively. Error bars represent mean SEM. **p<0.01.
-
Figure 2—source data 1
Quantification of VDA derived LCs in Runx1 and cMyb mutants.
- https://doi.org/10.7554/eLife.36131.009
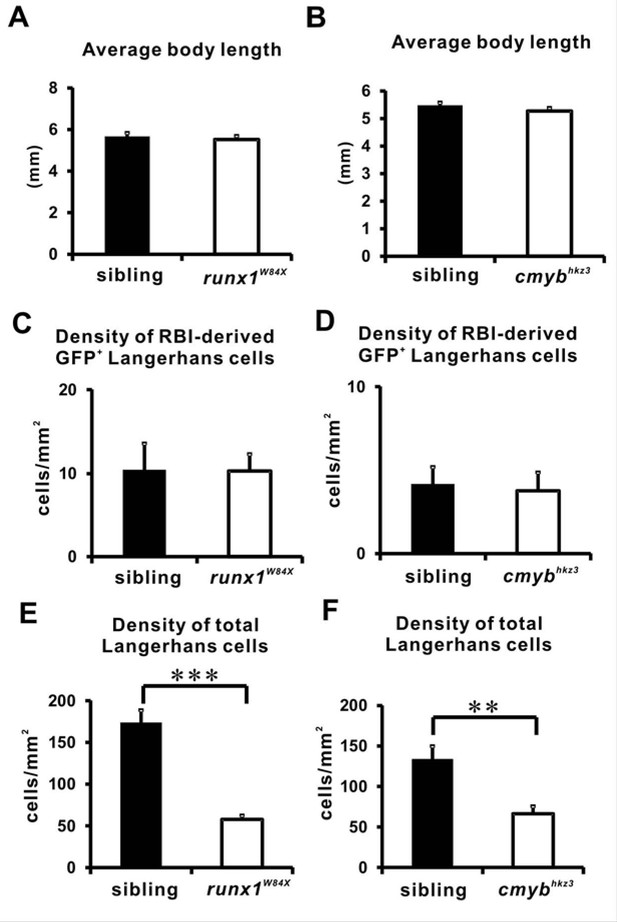
Quantification of body length and RBI derived LCs in Runx1 and cMyb mutants.
(A) Average body length of the runx1W84X mutant and its sibling. n = 5 for siblings and n = 6 for mutants. Error bars represent mean SEM. (B) Average body length of the 20 dpf cmybhkz3 mutant and its size matched sibling. n = 11 and 10 for siblings and mutants respectively. Error bars represent mean SEM. (C) Quantification of the density of the RBI-derived GFP+ LCs in the trunk of 12 dpf runx1W84X mutants and siblings. n = 6 for siblings and mutants. Error bars represent mean SEM. (D) Quantification of the density of the RBI-derived GFP+ LCs in the trunk of 20 dpf cmybhkz3 mutants and size matched siblings. n = 5 for siblings and n = 6 for mutants. Error bars represent mean SEM. (E) Quantification of the density of total LCs in the trunk of 12 dpf runx1W84X mutants and siblings. n = 5 for siblings and n = 6 for mutants. Error bars represent mean SEM. (F) Quantification of the density of total LCs in the trunk of 20 dpf cmybhkz3 mutants and size matched siblings. n = 11 and 10 for siblings and mutants, respectively. Error bars represent mean SEM.
-
Figure 2—figure supplement 1—source data 1
Quantification of body length and RBI derived LCs in Runx1 and cMyb mutants.
- https://doi.org/10.7554/eLife.36131.008
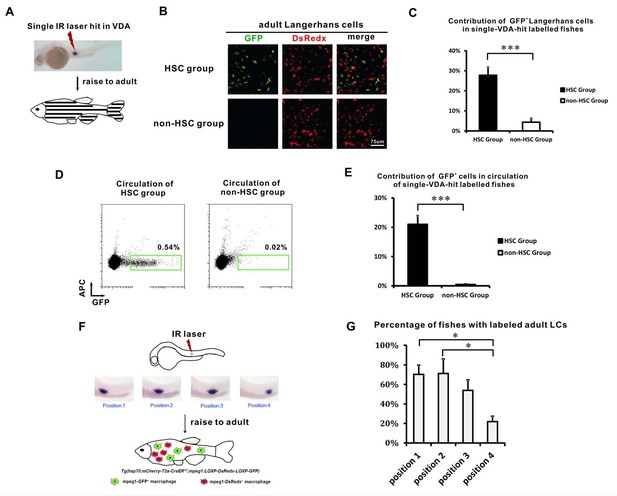
The VDA-derived LCs correlate with HSCs spatially.
(A) A schematic view of the single spot VDA heat-shocked experiment. A single IR laser hit is performed in the VDA region. The heat-shocked embryos are raised to adult (about 3 months) for analysis. (B) GFP+ LCs are mainly found in the HSC group but not in the non-HSC group of VDA labeling. (C) Quantification of the relative contribution of GFP+ LCs in HSC (n = 8) and non-HSC (n = 5) groups. Error bars represent mean SEM. ***p<0.001. (D) Flow cytometry shows GFP+ cells are mainly found in the circulation of HSC group but not in the circulation of non-HSC group. (E) Quantification of the relative contribution of GFP+ cells in the circulation of HSC (n = 8) and non-HSC (n = 5) groups. Error bars represent mean SEM. ***p<0.001. (F) A schematic view of the position restricted single spot VDA heat-shocked experiment. The VDA region from anterior to posterior is artificially divided into four positions (P1 to P4) and each position is labeled by a single IR laser hit. The embryos are raised to adult and the percentage of successful LCs labelling is analyzed for each labeled position. (G) The percentage of fish with widely labeled LCs in each position. Three independent experiments were performed. There are total 35 fish for position 1, 44 fish for position 2, 40 fish for position 3, and 38 fish for position 4. *p<0.05.
-
Figure 3—source data 1
Quantification data for Figure 3C, E, and G.
- https://doi.org/10.7554/eLife.36131.011
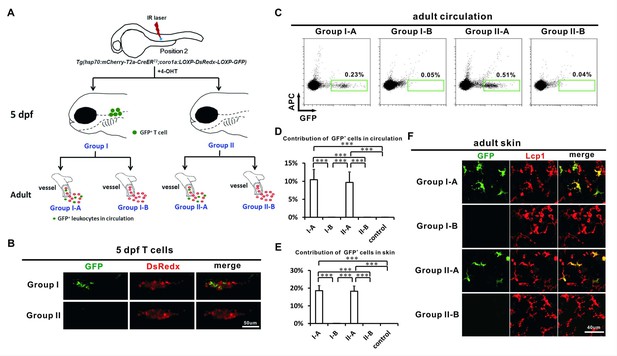
Adult LCs correlate with HSCs but not non-HSC progenitors.
(A) A schematic view of the experimental design for laser labeling of Tg(hsp70:mCherry-T2a-CreERT2;coro1a:loxP-DsRedx-loxP-GFP) embryos. A single IR laser hit was performed at Position 2 of VDA at about 24 hpf. After 4-OHT treatment, the heat-shocked embryos were raised to 5 dpf and the embryos were then separated into two groups according the GFP contribution to thymocytes. Group I embryos contain abundant GFP+ thymocytes, whereas Group II embryos hardly have GFP+ thymocytes. These two groups of embryos were raised to adult (over 3 months) and were subdivided into Group I-A, I-B, II-A and II-B according to the GFP contribution in circulation. (B) Confocal images show that Group I embryos, but not Group II embryos, contain abundant GFP+ thymocytes. (C) Flow cytometry shows that the circulation of Group I-A and II-A, but not I-B and II-B contain abundant GFP+ cells. (D) Quantification of the contribution of GFP+ cells in all fluorescent positive leukocytes in the circulation of Group I-A, I-B, II-A, II-B, and non-heat-shocked control group (n = 6 for each group). Error bars represent mean SEM. ***p<0.001. (E) Quantification of the contribution of GFP+ cells in all Lcp1 positive leukocytes in the skin of adult Group I-A, I-B, II-A, II-B, and non-heat-shocked control group (n = 5 for each group). Error bars represent mean SEM. ***p<0.001. (F) Confocal images show that GFP+ cells are mainly found on the skin of Group I-A and II-A, but not I-B and II-B. a significant portion of GFP+ cells showed ramified LC-like morphology, suggesting that they are LCs presumably.
-
Figure 4—source data 1
Quantification data for Figure 4D and E.
- https://doi.org/10.7554/eLife.36131.013
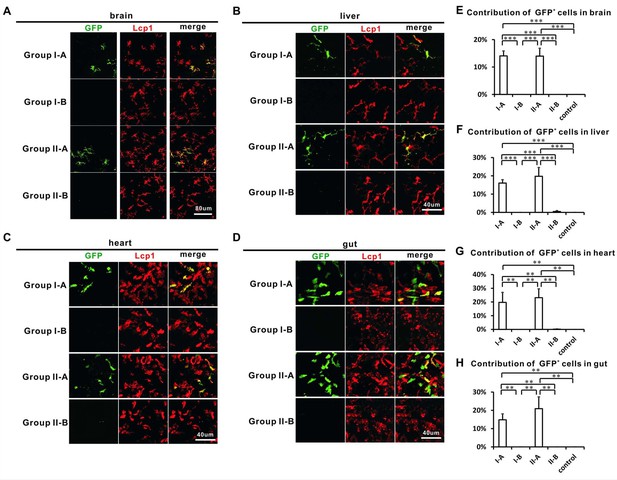
Adult tissue-resident macrophages in the brain, liver, heart and gut correlate with HSCs.
(A–D) Confocal images show that GFP+ cells are found in the brain (A), liver (B), heart (C) and gut (D) of Group I-A and II-A, but not I-B and II-B. Most GFP+ cells are Lcp1+ with the exception of gut GFP+ cells which only partially overlap with Lcp1 signals. (E–H) Quantification of the contribution of GFP+ cells in all Lcp1 positive leukocytes in the brain (E), liver (F), heart (G), and gut (H) of adult Group I-A, I-B, II-A, II-B, and non-heat-shocked control group (n = 4 for each group of brain and liver; n = 3 for each group of heart and gut). Error bars represent mean SEM. ***p<0.001; **p<0.01.
-
Figure 5—source data 1
Quantification data for Figure 5E, F, G, and H.
- https://doi.org/10.7554/eLife.36131.017
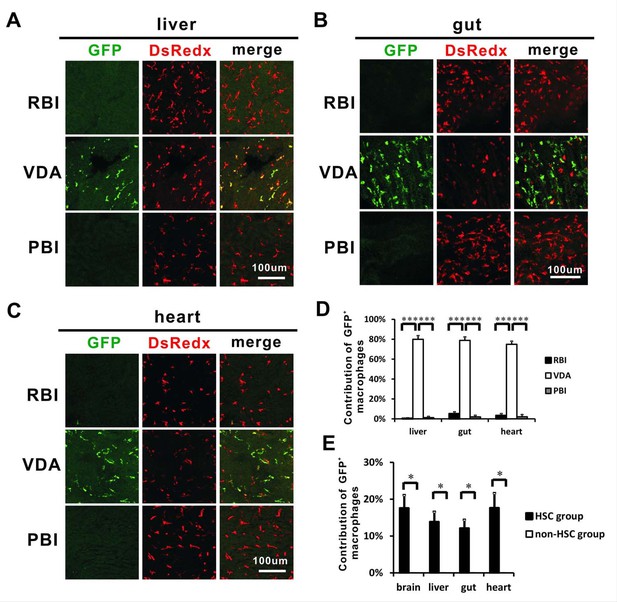
Other tissue-resident macrophages also correlate with HSCs.
(A) Anti-GFP staining indicates that GFP+ resident macrophages are mainly detected in the VDA-labelled fish, but not the RBI- and PBI-labelled fish in adult liver. (B) Anti-GFP staining indicates that GFP+ resident macrophages are mainly detected in the VDA-labelled fish, but not the RBI- and PBI-labelled fish in adult gut. (C) Anti-GFP staining indicates that GFP+ resident macrophages are mainly detected in the VDA-labelled fish, but not the RBI- and PBI-labelled fish in the adult heart. (D) Quantification of the percentage of GFP+ resident macrophages derived from the RBI, VDA, and PBI in adult liver, gut, and heart. n = 5 for each sample analyzed. Error bars represent mean SEM. ***p<0.001. (E) Quantification of the relative contribution of GFP+ cells in the adult liver, gut and heart of HSC (n = 4) and non-HSC (n = 3) groups. Error bars represent mean SEM. *p<0.05.
-
Figure 5—figure supplement 1—source data 1
Quantification data for Figure 5—figure supplement 1D and E.
- https://doi.org/10.7554/eLife.36131.016
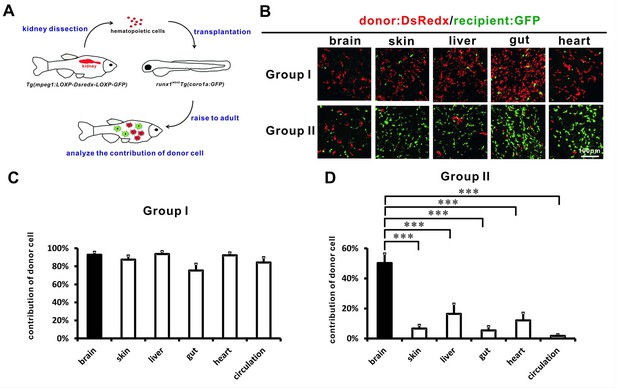
Adult tissue-resident macrophages are largely associated with HSCs during transplantation.
(A) A schematic view of the cell transplantation experiment. Hematopoietic cells collected from the whole kidney marrow of adult Tg(mpeg1:loxP-DsRedx-loxP-eGFP) fish are transplanted into the circulation of 2 dpf Tg(coro1a:eGFP);runx1W84X embryos. The recipients are raised to adulthood for analysis. (B) Donor Dsredx+ cells were predominant in the brain, skin, liver, gut, and heart of Group I fish. In Group II fish, donor cells were only significant in the brain but not in other tissues. (C) Quantification of the relative contribution of donor DsRedx+ cells versus total fluorescent cells (Dsredx+ and GFP+) in brain, skin, liver, gut, heart, and circulation in Group I fish (n = 8 for brain, skin, liver, gut, and circulation; n = 7 for heart). Error bars represent mean SEM. Donor DsRedx+ cells were predominant in all tissues and in circulation of Group I fish. (D) Quantification of the relative contribution of donor cells (Dsredx+) versus total fluorescent cells (Dsredx+ and GFP+) in brain, skin, liver, gut, heart, and circulation in Group II fish (n = 16 for brain, skin and circulation; n = 15 for liver and gut; n = 14 for heart). Error bars represent mean SEM. ***p<0.001. In Group II fish, although donor cells were significant in the brain, they were drastically decreased in other tissues.
-
Figure 6—source data 1
Quantification data for Figure 6C and D.
- https://doi.org/10.7554/eLife.36131.019
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| strain, strain background (Danio rerio) | Tg(mpeg1:loxP-DsRedx-loxP-GFP) | doi: 10.1016/j.devcel.2016.06.018. | ||
| strain, strain background (Danio rerio) | Tg(coro1a:loxP-DsRedx-loxP-GFP) | doi: 10.1016/j.devcel.2015.08.018. | ||
| strain, strain background (Danio rerio) | runx1W84X mutant | doi: 10.1242/dev.029637. | ||
| strain, strain background (Danio rerio) | cmybhkz3 mutant | doi: 10.1182/blood-2011-03-342501. | ||
| antibody | Anti-GFP | Abcam | ab6658 | 1:400 Overnight 4°C |
| antibody | Anti-DsRedx | Clontech | 632496 | 1:100 Overnight 4°C |
| antibody | anti-Lcp1 | doi: 10.1242/dev.029637. | 1:400 Overnight 4°C |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.36131.020





