Inhibition gates supralinear Ca2+ signaling in Purkinje cell dendrites during practiced movements
Figures
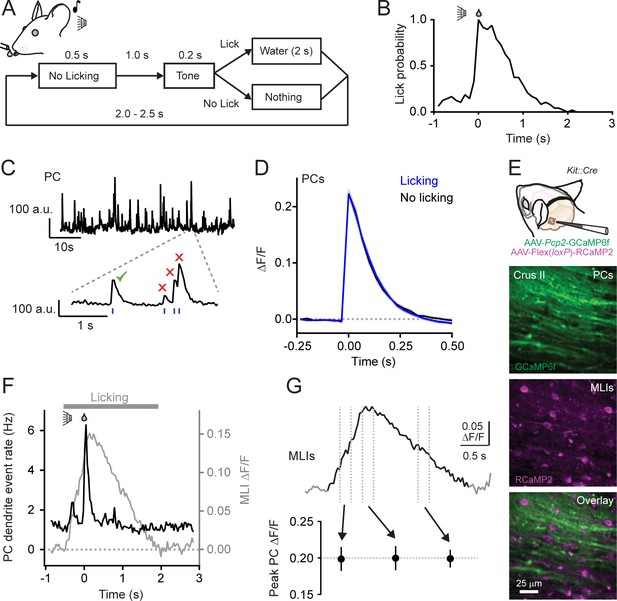
Climbing fiber-evoked Ca2+ signals in PCs during behavior.
(A) Head-fixed mice were trained to lick water from a port, cued by an audible tone, using the procedure shown. (B) Across-trial distribution of lick probability, aligned to the delivery of water, for a trained mouse. (C) Continuous record of Ca2+ activity in a PC dendrite. Expanded area shows algorithmically identified climbing fiber-evoked events (blue tick marks). Isolated Ca2+ events, indicated by the checkmark, were collected for analysis. (D) Average of climbing fiber-evoked Ca2+ events in PC dendrites occurring during water consumption (blue) or in the absence of licking (black). Measurements were obtained from 11 to 51 PCs in each of 11 mice; 211 cells total. (E) Genetic targeting of PCs and MLI using AAVs with GCaMP6f under control of the Pcp2 promoter and Cre-dependent RCaMP2 in Kit::Cre mice. In vivo images are from an infected area of Crus II. (F) The average frequency of climbing fiber-evoked Ca2+ events in PC dendrites (11 to 19 PCs in each of 5 mice; 82 cells total) plotted against the response in MLIs, acquired simultaneously in a subset of recordings (3 mice). (G) Trial-averaged measurement of MLI activity during cued licking. The peak amplitudes of Ca2+ events in PCs, plotted below, that correspond to three different phases of MLI activation during the task (4 to 19 PCs in each of 6 mice; 79 cells total; p=0.99, ANOVA test).
-
Figure 1—source data 1
Source data for panels D and G.
- https://doi.org/10.7554/eLife.36246.008
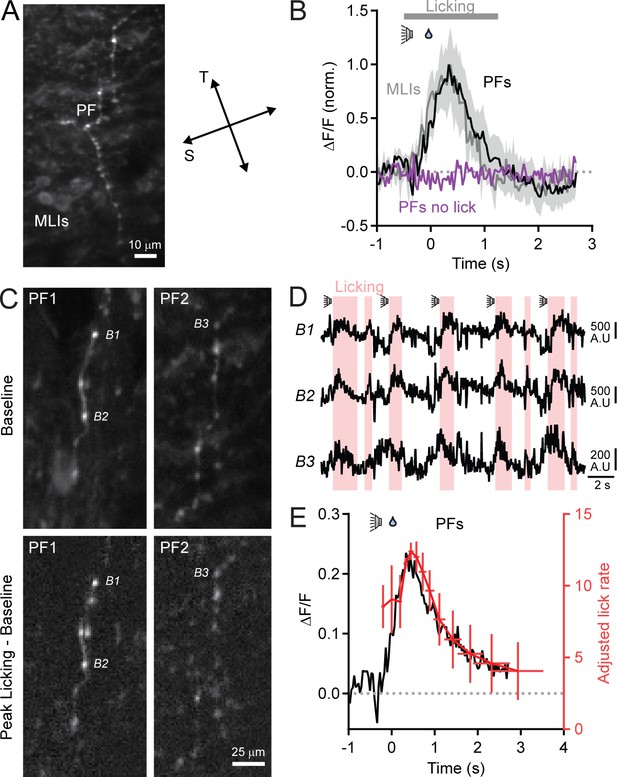
Ca2+ activity measurements in parallel fibers.
(A) 2pLSM image from Crus II of an awake mouse previously injected with AAV containing GCaMP6f under control of the synapsin promoter. GCaMP6f expression is apparent in both parallel fibers (PFs) and MLIs. Parallel fibers were identifiable because branches ran unbifurcated over distance (>10 μm) in a transverse (T) direction whereas MLI processes projected stochastically with branches predominately in a sagittal orientation (S). (B) Normalized Ca2+ activity measurements from parallel fibers (black) and MLIs (gray) recorded simultaneously during cued licking. Error bars represent SEM (3 to 5 PFs and 5 to 15 MLIs in each of 2 mice). No discernable change in activity was apparent in PFs when the animal did not lick in response to the sound cue (purple). Similar results were obtained for MLIs, but were excluded for clarity. (C) Top row: baseline fluorescence images for two separate parallel fibers (PFs) in the molecular layer. Bottom row: difference images calculated by subtracting resting fluorescence from the evoked response during the peak phase of cued licking. Images are the averages of many trials. Three boutons are labeled. (D) Raw fluorescence traces for the three identified boutons. The highlighted regions indicate bouts of licking. (E) Comparison of average parallel fiber activity (black trace) and adjusted lick rate (red trace). Error bars represent SEM. Data were obtained from 1 to 5 fibers from each of 3 mice; nine total fibers.
-
Figure 1—figure supplement 1—source data 1
Source data for panels B and E.
- https://doi.org/10.7554/eLife.36246.004
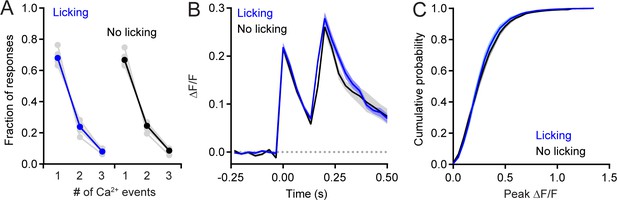
Analysis of non-isolated PC dendritic Ca2+ events.
(A) Comparison of the fraction of responses composed of single isolated events or those containing overlapping events, grouped closely in time. Responses were categorized whether they occurred during water consumption (blue) or in the absence of licking (black). Data from individual animals are shown in gray. Significant differences were not found between the two behavioral states (p=0.26 for isolated events, or 0.57, and 0.18, for two or three overlapping Ca2+ events, respectively; paired Student’s t-test). (B) Averages of responses containing two dendritic Ca2+ events, separated by 150–200 ms. Neither peak was significantly different between the two conditions (p=0.91 and 0.28, respectively; paired Student’s t-test). (C) Cumulative probability of amplitudes for all Ca2+ events occurring during water consumption (blue, 18,320 events) and those in the absence of licking (black, 5,839 events). Means were not significantly different (p=0.57, paired Student’s t-test). All measurements taken from 11 to 19 PCs in each of 7 mice; 100 cells total.
-
Figure 1—figure supplement 2—source data 1
Source data for panels A-C.
- https://doi.org/10.7554/eLife.36246.006
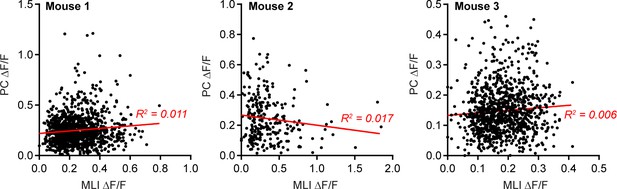
PC Ca2+ event amplitudes do not co-vary with the level of MLI activity.
Examples from three mice plotting the amplitudes of all identified PC Ca2+ events (each point is an individual dendritic Ca2+ event) and the corresponding Ca2+ activity measurement in surrounding MLIs acquired at the same time. The data were fit with linear regressions.
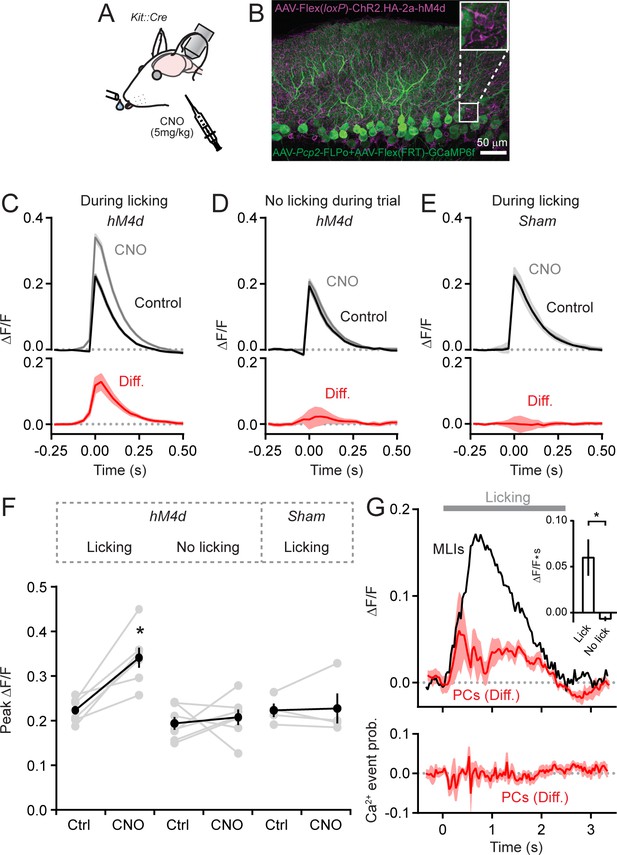
MLIs suppress climbing fiber-evoked dendritic Ca2+ signals in PCs during licking.
(A) Following an audible cue, head-fixed mice licked from a port during in vivo imaging with 2pLSM. In some sessions, CNO was administered by intraperitoneal injection prior to the start of the task. (B) Image of fixed tissue showing PCs transduced with GCaMP6f and MLIs expressing HA-tagged hM4d (inset is a magnified view of labeled MLI somata). For this set of experiments, PCs were transduced with AAV containing the recombinase FLPo under control of the PC-specific Pcp2 promoter in combination with an FLPo-dependent AAV containing GCaMP6f to generate high-level expression of the Ca2+ indicator. (C–E) Average dendritic Ca2+ events in PCs recorded in control or in sessions with CNO (11 to 35 PCs each from 7 mice, 100 and 156 cells total; control and CNO, respectively). Isolated Ca2+ events were sorted based on whether or not they occurred in correspondence with licking. A separate group of sham mice lacked expression of hM4d were also tested (10 to 51 PCs each from 4 mice, 111 and 100 cells total; control and CNO, respectively). Difference signals are shown below in red. (F) Summary plot of the effect of chemogenetic MLI activity suppression on the amplitude of isolated dendritic Ca2+ events in PCs. Black, group average (mean ± SEM); gray, individual mice (N = 7). Asterisk indicates a significant difference from all other conditions (p<0.01; ANOVA with Tukey’s post-hoc multiple comparison test; all other comparisons were insignificant). (G) Top plot, the difference in trial-averaged PC dendritic Ca2+ activity between sessions in control and in CNO. Note the correspondence of the difference signal in PCs and the licking-evoked activation of MLIs measured simultaneously in a subset of experiments. Inset: the mean integrated Ca2+ activity during water consumption (lick) and in the absence of licking (no lick). Asterisk indicates a significant difference (p=0.025; paired Student’s t-test). Bottom plot, the probability of climbing fiber-evoked Ca2+ events in PCs was unchanged by molecular layer disinhibition, relative to that in control (11 to 35 PCs each from 6 mice, 145 and 97 cells total).
-
Figure 2—source data 1
Source data for panels C-E and G.
- https://doi.org/10.7554/eLife.36246.016
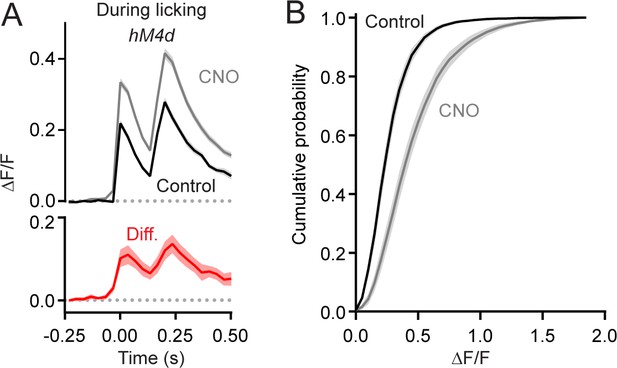
MLI-mediated suppression of non-isolated PC Ca2+ events.
(A) Average PC dendritic Ca2+ responses composed of two climbing fiber-evoked events in close temporal apposition (within 150–200 ms). Peak amplitudes were significantly larger in CNO than in control conditions (p=0.0029 and 0.0049, respectively, paired Student’s t-test). (B) Cumulative probability of amplitudes for all Ca2+ events (including those in overlapping responses) recorded during water consumption in control sessions (black line, 18,348 events) and those in CNO (gray line, 26,509 events). The means were significantly different (p=0.0009, Student’s t-test). All measurements taken from 11 to 35 PCs each from 7 mice, 100 and 156 cells total; control and CNO, respectively.
-
Figure 2—figure supplement 1—source data 1
Source data for panels A and B.
- https://doi.org/10.7554/eLife.36246.011
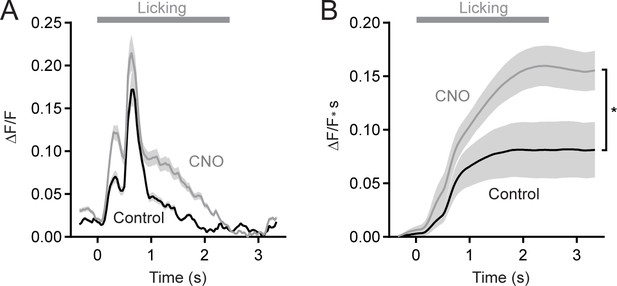
Chemogenetic suppression of MLIs increases trial-averaged Ca2+ activity in PC dendrites.
(A) Trial-averaged Ca2+ activity in PCs dendrites during cued bouts of water consumption. Measurements were obtained in control or during chemogenetic suppression of MLI activity. (11 to 35 PCs each from 6 mice, 97 and 145 cells total in control and CNO, respectively). (B) Comparison of the integral of trial-averaged Ca2+ activity (area expressed as cumulative signal over time) with and without molecular layer disinhibition. Asterisk denotes a significant difference in final amplitude (p=0.039; paired Student’s t test).
-
Figure 2—figure supplement 2—source data 1
Source data for panels A and B.
- https://doi.org/10.7554/eLife.36246.013
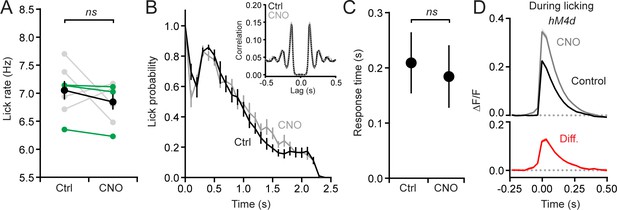
Chemogenetic suppression of MLIs in Crus II does not affect licking rates.
(A) Average lick rate during cued bouts of licking in control sessions and those with chemogenetic disinhibition of the molecular layer (p=0.32; paired Student’s t-test; N = 7 mice). In all of our experiments, hM4d was expressed unilaterally in left Crus II. The mean is in black, with individual mice in gray (>2% change) and green (<2% change). (B) The distribution of lick probability across time for bouts of licking; the average responses are aligned to the first lick after the cue. Control is in black, CNO in gray. The distributions are not significantly different (p=0.92; Kolmogorov-Smirnov test). Inset: autocorrelation of lick timing across bouts of water consumption. Rhythmicity was the same in both conditions. The peak at lag = 0 was removed for clarity. (C) Response time from the tone cue to the first lick in control and during chemogenetic suppression of MLIs. Measurements from 960 control trials and 956 CNO trials from seven mice. (p=0.549; paired Student’s t test). (D) Average climbing fiber-evoked Ca2+ events in PCs from three mice that showed almost no change in lick rate with molecular layer disinhibition (<2% change; mice indicated in green in panel A). These data confirm that behavioral differences cannot account for the observed changes in PC Ca2+ responses with MLI activity suppression.
-
Figure 2—figure supplement 3—source data 1
Source data for panels B and C.
- https://doi.org/10.7554/eLife.36246.015
Licking behavior in control and the disinhibited condition.
Left (hM4d alone) and right (hM4d + CNO) showing example licking behavior. Images were collected at 2000 Hz and slowed down 10-fold for replay. Total time is 1.5 s.
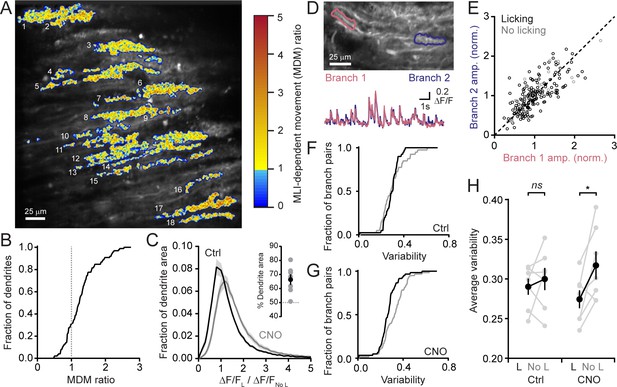
MLIs broadly influence climbing fiber-evoked Ca2+ signaling in PCs.
(A) The across-session change in trial-averaged PC Ca2+ activity, colored-coded based on the extent to which chemogenetic disinhibition affected responses during movement (MDM ratio; see Materials and methods). Each algorithmically identified PC dendrite is numbered. (B) Cumulative probability histograms of the effect of disinhibition on trial averaged Ca2+ activity in identified PCs (2 to 15 PCs each from seven mice, 50 cells total). Dotted line demarcates a threshold level of change indicative of enhancement with disinhibition (MDM ratio >1). (C) Histogram of average Ca2+ activity measurements, determined for each dendritic pixel across the PC population, during licking (L) divided by that observed in the absence of licking (No L), in both control sessions (black) as well as after administration of CNO (gray). For all mice (N = 7), the distributions were significantly different (p<0.0001, Kolmogorov-Smirnov test). The inset shows the summary of ROC analysis on these distributions, obtained for each mouse, where area under the curve was used to calculate the percentage of pixels that showed an effect with chemogenetic disinhibition (gray, individual mice; black, the mean ± SEM). (D) In the image, two segments of a dendrite from distinct branches of the same PC are outlined. Measurements of Ca2+ activity in these segments are shown superimposed in the traces below. (E) Comparison of the amplitudes of simultaneous, inter-branch Ca2+ events for many climbing fiber-evoked responses for the two branches shown in example in panel D. Events were sorted based on whether they occurred during water consumption (black) or in the absence of licking (gray). Unity is marked by the dashed line. (F,G) Cumulative probability of the inter-branch variance of climbing fiber Ca2+ event amplitudes (see Materials and methods). Events were sorted depending on their correspondence with water consumption (black) or the absence of licking (gray). Distributions were not different in control sessions (N = 74 pairs; range: 4 to 20 pairs each from seven mice; p=0.57, Kolmogorov-Smirnov test) but were in the disinhibited condition with CNO (N = 114 pairs; range 12 to 28 pairs each from seven mice; p=0.0013, Kolmogorov-Smirnov test). (H) The effect of molecular layer disinhibition on average inter-branch variability of Ca2+ event amplitudes in PCs. In control sessions, the variance was similar whether or not events occurred during licking movements (p=0.50; paired Student’s t-test). In contrast, a modest, but significant drop in variability occurred during movement with disinhibition (p=0.029; paired Student’s t-test). Black, group average (mean ± SEM); gray, individual mice.
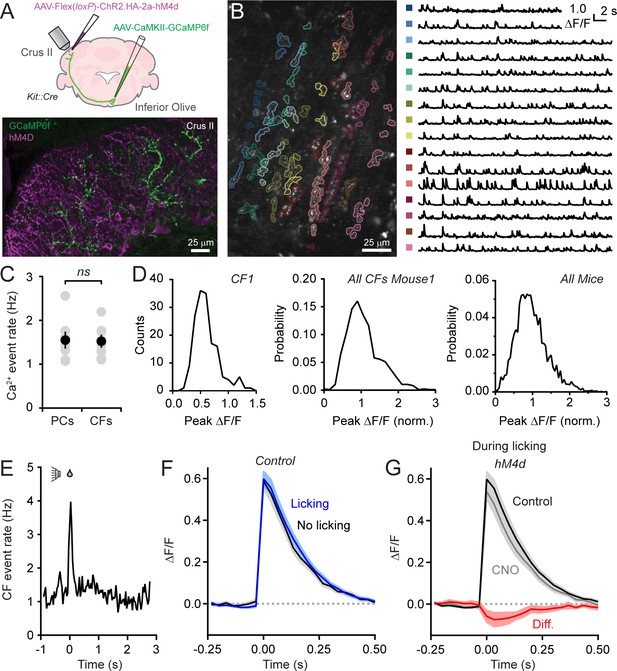
Disinhibition does not affect presynaptic climbing fiber activity.
(A) AAVs containing genetically encoded activity reporters and effectors were injected in the inferior olive and lobule Crus II of Kit::Cre mice, respectively. Image from fixed tissue showing GCaMP6f expression in climbing fibers and HA-tagged hM4d in MLIs. (B) In the image, individual climbing fibers were identified using automated segmentation routines. Traces show activity measurements from color-coded climbing fibers. (C) Ca2+ event rates in PC dendrites and climbing fibers, measured in separate cohorts of mice (11 to 19 PCs and 2 to 6 climbing fibers each from 7 mice, 100 and 29 total, respectively). Black circles, mean ± SEM; gray circles, measurements from individual mice (p=0.92, Student’s t-test). (D) Distribution of Ca2+ event amplitudes for an individual climbing fiber, all climbing fibers in a single mouse (N = 6), and for all mice (2 to 12 climbing fibers each from 7 mice, 36 fibers total). Data were normalized to facilitate comparisons across climbing fibers. (E) The frequency of Ca2+ events in climbing fibers during cued licking (average of 3 mice). (F) Average of isolated Ca2+ events in climbing fibers collected either during the consumption of water (blue) or in the absence of licking (black). Measurements obtained from 4 to 12 climbing fibers each from 5 mice, 38 fibers total. (G) Ca2+ events recorded in climbing fibers both in control and during sessions with chemogenetic MLI activity suppression. Events were collected only during periods of water consumption (4 to 9 climbing fibers each from 5 mice, 26 fibers total). The difference signal is shown in red.
-
Figure 4—source data 1
Source data for panel C.
- https://doi.org/10.7554/eLife.36246.020
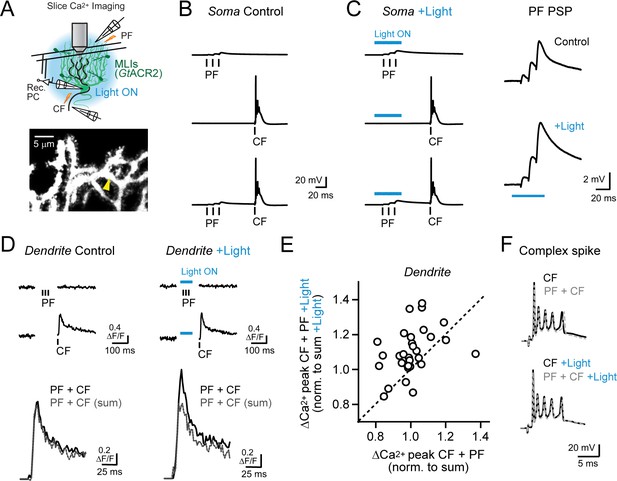
Feed-forward inhibition attenuates supralinear Ca2+ signaling in PC dendrites.
(A) In acute slices, parallel fibers were stimulated in conjunction with climbing fibers during whole-cell patch recording from PCs in lobule Crus II. Ca2+ imaging was performed using 2pLSM in PC spiny dendrites as shown in the fluorescence image. (B) Evoked responses in a PC either to the parallel fiber tetanus (3 pulses at 100 Hz), the climbing fiber stimulus, or their conjunctive pairing (50 ms interval). (C) In alternate trials, MLIs expressing GtACR2 were photoinhibited using wide-field illumination with blue light (λ = 461 nm; 40 ms; 6.6 mW/mm2), coincident with the parallel fiber tetanus. Average parallel fiber-evoked PSPs in control and during optogenetic suppression of feed-forward inhibition are enlarged on the right. (D) Left, the average climbing fiber-evoked Ca2+ signal in a PC dendrite (location demarcated by the yellow arrowhead in the morphological image in panel A) produced following conjunctive stimulation with parallel fibers. The estimated summed response, shown in gray, for parallel fiber and climbing fiber transients evoked in isolation on separate trials (Ca2+ activity traces shown above). Right, climbing fiber-evoked Ca2+ signals from the same dendritic location but with feed-forward inhibition suppressed by optogenetics during the parallel fiber tetanus. (E) The change in amplitude of climbing fiber-evoked Ca2+ signals with conjunctive stimulation of parallel fibers, measured in the same PC dendrite, in trials either with or without optogenetic suppression of MLI-mediated feed-forward inhibition. Data are normalized to the estimated, summed response of parallel fibers and climbing fibers for each condition. Each point is a measurement from a different dendritic branch (3 to 10 sites for each of 5 PCs, 31 sites total) with unity demarcated by the dashed line. (F) Somatic complex spikes evoked by the climbing fiber stimulus, both in isolation as well as in conjunction with parallel fiber activation. Responses in the same PC in control and with MLIs photo-inhibited during the parallel fiber tetanus.
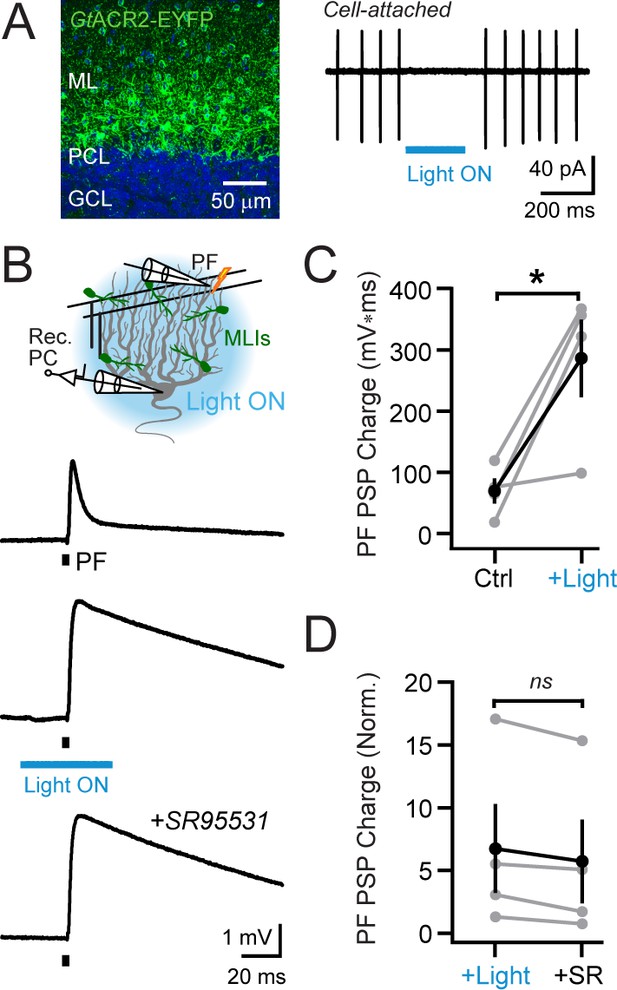
Optogenetic elimination of MLI-mediated feed-forward inhibition.
(A) Confocal image from the cerebellum of a Kit::Cre mouse injected with AAV containing Cre-dependent GtACR2-YFP showing transduction in MLIs (DAPI counterstain in blue). During a cell-attached recording from an MLI expressing GtACR2, blue light illumination (λ = 461 nm; 200 ms; 6.6 mW/mm2) abruptly arrested spontaneous firing, a reproducible effect across cells (30.5 ± 4.8 and 1.8 ± 0.9 Hz firing, control and during light, respectively; N = 6; p=0.003; paired Student’s t-test). (B) In a PC whole-cell recording, stimulation of parallel fibers by a single electrical pulse evoked a depolarizing PSP (top trace; average response). In interleaved trials, feed-forward inhibition was suppressed by optogenetic MLI activity suppression coincident with the parallel fiber stimulus (middle trace). Or, at the end of the experiment, inhibition was blocked by bath application of the GABAA receptor antagonist SR95531 (20 μM; bottom trace). (C) The effect of optogenetic suppression of feed-forward inhibition on the integral of the parallel fiber-evoked PSP. Gray, individual cells (N = 4); black, group average (mean ± SEM). (D) Within-cell comparison of the effect of MLI activity suppression, by GtACR2 or pharmacological block of GABAA receptors, on the integral of the parallel fiber-evoked PSP. Data are normalized to control responses to parallel fiber stimuli alone, obtained for each cell (N = 4).
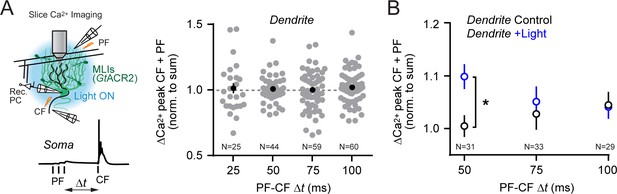
Temporal dynamics of conjunctive parallel fiber-climbing fiber Ca2+ signaling in PC dendrites.
(A) The timing between the parallel fiber tetanus and the climbing fiber stimulus was systematically varied. Supralinear enhancement of climbing fiber-evoked Ca2+ transients by preceding parallel fiber activity was not observed in PC dendrites across a range of test intervals (p>0.05 for all comparisons; 1-way ANOVA with Tukey’s post hoc multiple comparison test). (B) The time dependence of supralinear enhancement of climbing fiber-evoked Ca2+ signals, induced when MLI-mediated feed-forward inhibition was suppressed by GtACR2 activation during the parallel fiber tetanus (*p<0.05; 2-way ANOVA with Bonferroni’s post hoc multiple comparison test). Data from multiple dendrites sites (indicated in figure) each from six different PCs.
-
Figure 5—figure supplement 2—source data 1
Source data for panel B.
- https://doi.org/10.7554/eLife.36246.024
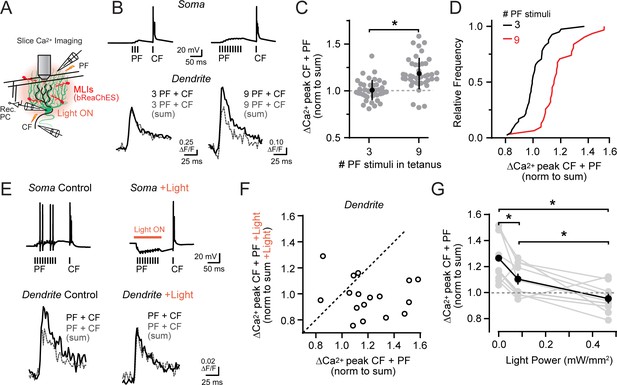
Activity-dependent recovery of supralinear climbing fiber Ca2+ signaling is sensitive to MLI inhibition.
(A) Acute slice recording configuration. In a subset of experiments, bReaChES was expressed in MLIs by Cre-dependent AAV in Kit::Cre mice. (B) Comparison of average climbing fiber-evoked Ca2+ signals in two different PCs with the conjunctive parallel fiber tetanus including either 3 or 9 stimuli (100 Hz). The summed response of the parallel fiber and climbing fiber transients, evoked in isolation on separate trials, is shown in gray. (C) Across-cell comparison shows that increasing the number of stimuli in the parallel fiber tetanus results in a supralinear enhancement of climbing fiber Ca2+ signals in PC spiny dendrites. Individual dendritic recording sites are indicated in gray (N = 41 and 42 dendritic sites from 6 and 4 cells; 3 and 9 stimuli, respectively) with mean data in black (±SEM; p<0.0001; Student’s t-test). (D) In the cumulative probability histogram, supralinear climbing fiber Ca2+ signaling was observed in a majority of PC dendrites when stimulated with a longer lasting parallel fiber tetanus. (E) Climbing fiber-evoked Ca2+ signals at the same PC dendritic site. In interleaved trials, optogenetic activation of MLIs (λ = 596 nm; 40 ms; 0.93 mW/mm2) occurred during the parallel fiber tetanus. (F) Relationship between the change in amplitude of climbing fiber-evoked Ca2+ signals with conjunctive activity of parallel fibers, in trials either with or without optogenetic activation of MLIs during the parallel fiber tetanus (3 to 7 dendritic sites from 4 cells, 17 sites total). Data are normalized to the estimated, summed response of the parallel fiber and climbing fiber transients for each condition recorded at the same PC dendritic location. Dashed line is unity. (G) The effect of varying optogenetic MLI-mediated inhibition on the ability of parallel fibers to produce supralinear climbing fiber Ca2+ signals in PC dendrites. Mean data (black symbols ± SEM) are from matched comparisons at the same dendritic recording site (4 to 5 sites from 2 cells, 9 dendrites total; p<0.05; Repeated measures 1-way ANOVA with Tukey’s post hoc multiple comparison test). Gray symbols are individual measurements.
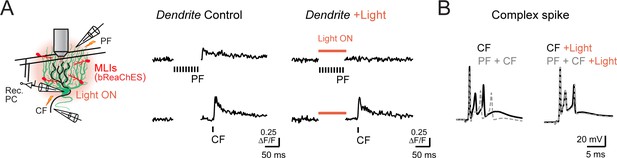
Influence of optogentic MLI activation on PC dendritic and somatic excitation.
(A) Left: diagram of recording configuration. Right: average Ca2+ activity responses in a PC dendrite (corresponding to the same site as Figure 6E) from trials with a parallel fiber tetanus (PF; above) or to climbing fiber stimulation (CF; below). Measurements, obtained in alternating trials, were in control conditions or with optogenetic activation of bReaChES-expressing MLIs (left and right, respectively). (B) Somatic recordings of complex spikes from the same PC. Shown superimposed are responses measured to climbing fiber stimulation alone or in conjunction with a preceding parallel fiber tetanus (nine stimuli). Measurements were obtained in control or with preceding MLI optogenetic activation (above and below, respectively) that corresponded to the timing of the parallel fiber stimulus.
Tables
| Reagent type or resource | Designation | Source or reference | Additional information |
|---|---|---|---|
| strain, (Mus Musculus) | Kit::Cre | Amat et al., 2017 | on C57Bl/6 background |
| transfected construct | AAV1-Pcp2.4-GCaMP6f | University of North Carolina | custom |
| transfected construct | AAV1-Pcp2.4-FLPo | University of North Carolina | custom |
| transfected construct | AAV1-CAG-Flex(FRT) rev-RCaMP2 | University of North Carolina | custom |
| transfected construct | AAV1-CAG-Flex(loxP) rev-RCaMP2 | University of Pennsylvania | custom |
| transfected construct | AAV1-CAG-Flex(FRT) rev-GCaMP6f | University of North Carolina | custom |
| transfected construct | AAV1-CAG-Flex(loxP) rev-ChR2.HA-2a-hM4d | ViGene | custom |
| transfected construct | AAV1-Syn-GCaMP6f | University of Pennsylvania | AV-1-PV2822 |
| transfected construct | AAV1-CaMKIIα-GCaMP6f | University of Pennsylvania | AV-1-PV2822 |
| transfected construct | AAV1-EF1α-Flex(loxP) rev-GtACR2.eYFP | ViGene | custom |
| transfected construct | AAV5-EF1α-Flex(loxP) rev-bReachES-TS-YFP | University of North Carolina | shelf |
| antibody | anti HA | Abcam | #ab9110 |
| software, algorithm | Prism | GraphPad | Statistical analysis |
| software, algorithm | Matlab | Mathworks | Image analysis |
| software, algorithm | ImageJ | NIH | Image analysis |
| software, algorithm | bControl | Carlos Brody, Princeton | Behavior control |
| software, algorithm | ScanImage | Vidrio Technologies | Microscope control |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.36246.027





