Oligodendrocyte-encoded Kir4.1 function is required for axonal integrity
Figures
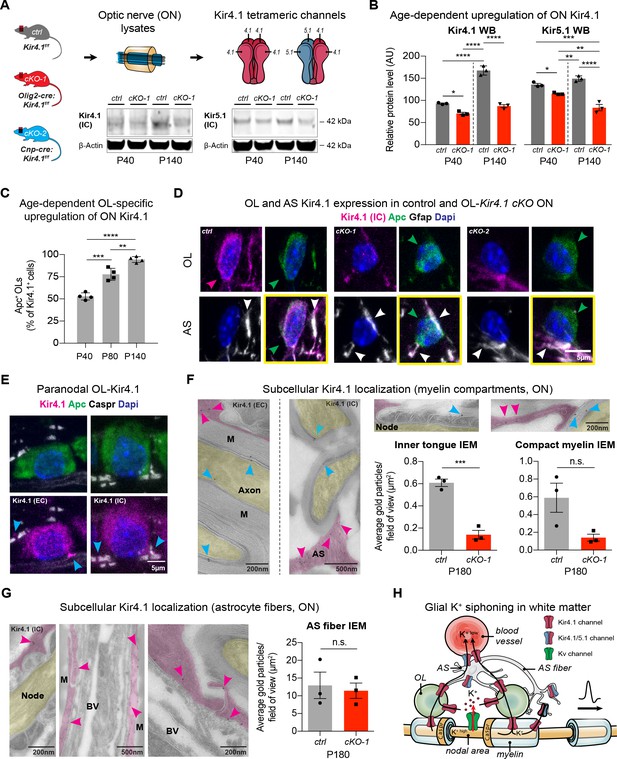
OL-Kir4.1 is upregulated during postnatal development and localized to peri-axonal spaces.
Kir4.1 ON protein levels were upregulated between age P40 and P140, whereas Kir5.1 protein levels did not change during aging (A–B). Note substantial loss of Kir4.1 protein in Olig2-cre driven Kcnj10 cKO (cKO-1) mice at P40, which became more apparent at P140; Kir5.1 protein was also reduced in cKO-1 ONs at P40 and P140 (control and cKO-1: n = 3 for all time points) (A–B). Quantification of Kir4.1+ Apc+ OLs confirmed age-dependent upregulation of OL-Kir4.1 channels between P40 and P140 (n = 4 for all time points) (C). One-way ANOVA with Tukey’s multiple comparison tests were performed in B and C; *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001. Kir4.1 channels were lost from both ON OL cell bodies in cKO-1 and Cnp-cre driven Kcnj10 cKO (cKO-2) mice versus controls (D). Note that Kir4.1+ OL are marked by magenta-colored arrowhead; Apc+ OLs are indicated by green arrowheads. Note AS Kir4.1 immunoreactivity and contacts of Kir4.1+ AS fibers with OLs (white arrowheads). Merged images are shown in panels highlighted by yellow surroundings (D). Kir4.1 was strongly expressed in OLs along spinal fiber tracts; note that cyan-colored arrowheads mark juxta-axonal Kir4.1 IR (E). Kir4.1 immunogold electron microscopy (IEM) labeling revealed presence of gold particles at inner and outer myelin tongue (cyan-colored arrowheads) and within AS fibers (magenda-colored arrowheads) adjacent to myelin sheaths (M = myelin) and blood vessels (BV = blood vessel; ctrl: n = 3, cKO-1: n = 3; F–G). Axon structures are highlighted in yellow, AS fibers are highlighted in magenta. Note decrease in inner tongue (F) but not compact myelin (F) or AS fiber (G) IEM labeling in cKO-1 ON tissue versus controls. Cartoon highlights proposed mechanism of glial K+ siphoning from axons during saltatory conduction towards blood vessels via a network of axonal Kv and glial Kir4.1 channels (H). Mann-Whitney tests were performed in F–G; ***p≤0.001, p=0.06 (F, compact myelin IEM), p=0.74 (G, AS fiber IEM). Data are presented as mean ±s.e.m in B–C and F–G.
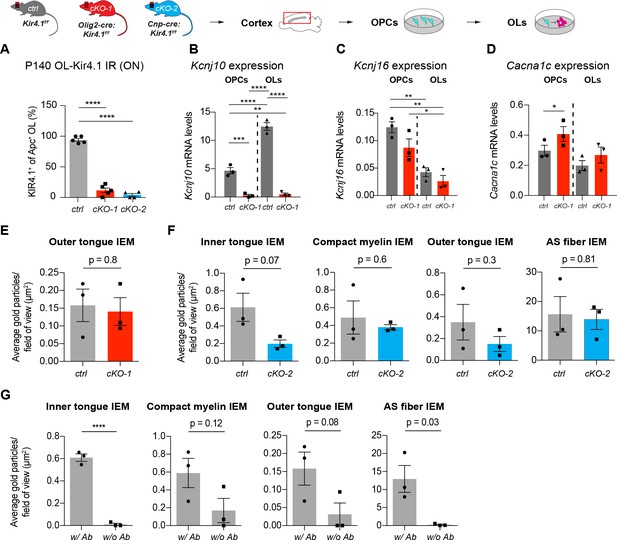
Validation of OL-encoded Kcnj10 cKO efficiency
Kir4.1 channels were efficiently ablated from ON OLs in cKO-1 (n = 5) and cKO-2 (n = 4) mice versus control ONs (n = 5; A). One-way ANOVA with Tukey’s multiple comparisons test was performed in A; ****p≤0.0001. Kcnj10 was upregulated during OL differentiation, and expression significantly suppressed in purified and immunopanned OPCs (ctrl: n = 3, cKO-1: n = 3) and OLs (ctrl: n = 3, cKO-1: n = 3) from cKO-1 mice (B). Conversely, Kcnj16 was not downregulated in OPCs (ctrl: n = 3, cKO-1: n = 3) and OLs (ctrl: n = 3, cKO-1: n = 3) from cKO-1 mice in vitro, however, note Kcnj16 downregulation during OPC-OL maturation (C). Cacna1c mRNA levels were increased in cultured OPCs (ctrl: n = 3, cKO-1: n = 3) but not OLs (ctrl: n = 3, cKO-1: n = 3) from cKO-1 mice suggesting a partial activation of Cav1.2 channels in OPCs (D). One-way ANOVA with Tukey’s multiple comparison tests were performed in B–D; *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001. No difference in outer tongue Kir4.1 IEM labeling between controls and ON cKO-1 tissue (E). Trend towards decreased Kir4.1 IEM labeling in cKO-2 ON tissue as compared to controls with respect to myelin compartments but not AS fibers (F). Background IEM labeling without primary antibody confirmed specificity of Kir4.1 IEM antibody labeling of myelin compartments and astrocyte fibers (G). Mann-Whitney tests were performed in E–G; ****p≤0.0001, p=0.8 (E, outer tongue IEM), p=0.07 (F, inner tongue IEM), p=0.6 (F, compact myelin IEM), p=0.3 (F, outer tongue IEM), p=0.81 (F, AS fiber IEM), p=0.12 (G, compact myelin IEM), p=0.08 (G, outer tongue IEM), p=0.03 (G, AS fiber IEM). Data are presented as mean ±s.e.m in A–G.
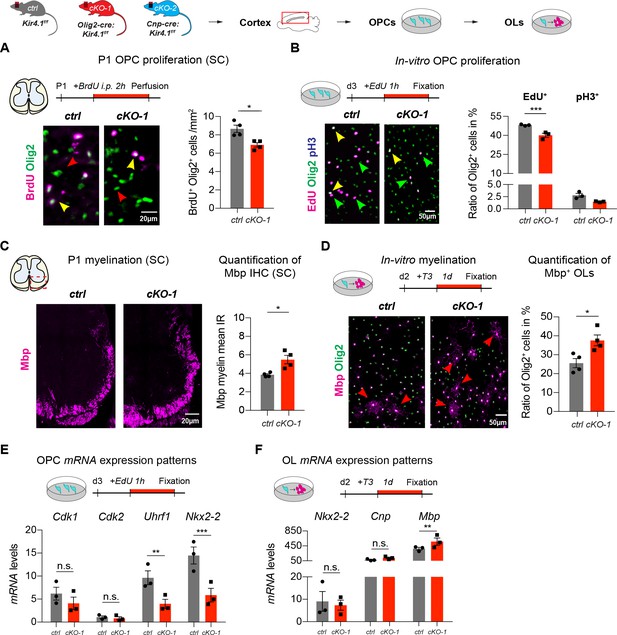
Early developmental changes in OL-encoded Kcnj10 loss-of-function
Kcnj10-deficient OPCs exhibited less BrdU incorporation in spinal cord tissue of P1 mice suggesting precocious exit from the cell cycle (ctrl: n = 4, cKO-1: n = 4; A). Likewise, purified and immunopanned Kcnj10-deficient OPCs exhibited less EdU incorporation but no difference in the mitosis marker phospho-histone H3 (pH3) in-vitro (ctrl: n = 3, cKO-1: n = 3; B). cKO-1 mice showed enhanced myelination in spinal cord WM at P1 by Mbp IHC (ctrl: n = 4, cKO-1: n = 4; C), and Kcnj10-deficient OLs showed more myelination during differentiating culture conditions in-vitro (ctrl: n = 4, cKO-1: n = 4; D). Mann-Whitney tests were performed in A–D; *p≤0.05, ***p≤0.001. Transcript levels for Cdk1 and Cdk2 were not different between control and Kcnj10-deficient OPCs in-vitro, whereas mRNA levels for Uhrf1 and Nkx2-2 were reduced in Kcnj10-deficient OPCs (ctrl: n = 3, cKO: n = 3; E). Note transcript levels for Nkx2-2 and Cnp were not different between control and Kcnj10-deficient OLs in-vitro after switching to differentiating culture conditions, however, Mbp mRNA levels increased in Kir4.1-deficient OPCs (ctrl: n = 3, cKO-1: n = 3; F). Multiple t tests were performed in E–F; **p≤0.01, ***p≤0.001; E: p=0.25 (Cdk1), p=0.88 (Cdk2) and F): p=0.97 (Nkx2-2), p=0.42 (Cnp). Data are presented as mean ±s.e.m in A–F.
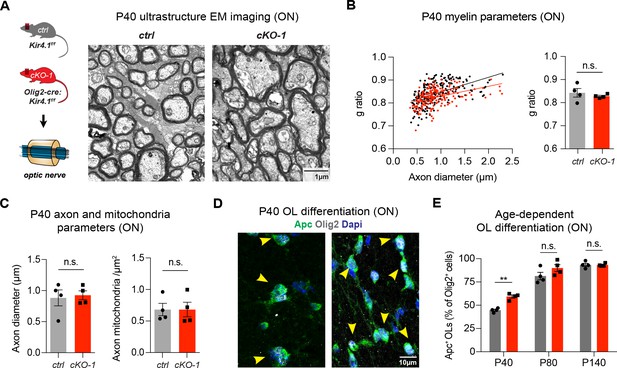
OL-Kir4.1 regulates early OL differentiation but is dispensable for myelination.
Early developmental loss of OL-Kcnj10 did not affect myelin sheath thickness or axon diameters in ONs from animals at P40 (210 axons from 4 control mice, 202 axons from 4 cKO-1 mice; A–C). Densities of intra-axonal mitochondria were not different between control and cKO-1 ONs at P40 (81 axons from 4 control mice, 77 axons from 4 Kcnj10 cKO-1 mice; C). Mann-Whitney test was performed in B–C; p=0.49 (g-ratios, B), p=0.89 (axon diameter, C) and p=0.89 (mitochondria density, C). Immunostaining for Olig2 (pan-lineage marker for OPC/OL cells) and Apc (OL maturation marker) demonstrated precocious OL differentiation in cKO-1 ONs at P40 versus P80 and P140 (D–E). Two-way ANOVA with Sidak’s multiple comparison test was performed in E; **p≤0.01. Data are presented as mean ±s.e.m in B, C and E.
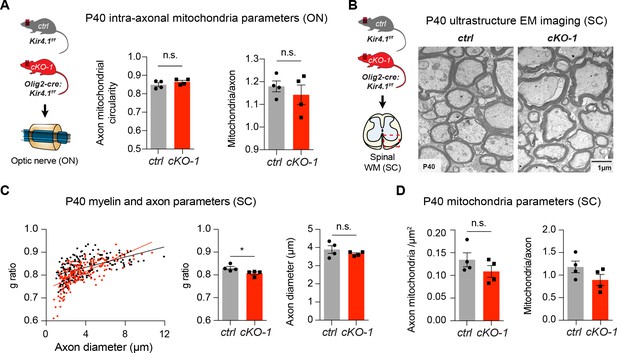
Early white matter changes in OL-encoded Kcnj10 loss-of-function
Intra-axonal mitochondria did not differ with respect to circularity and numbers per individual axon between ON tissue from control and cKO-1 mice (210 axons from 4 control mice, 202 axons from 4 cKO-1 mice; A). Mann-Whitney test was performed in A; p=0.31 (mitochondria circularity) and p=0.69 (mitochondria counts/axon). In line with ON results, spinal cord WM axon diameters and intra-axonal mitochondrial parameters (density, numbers/axon) were not different between cKO-1 mice and control littermates at P40 (191 axons from control mice, 193 axons from 4 cKO-1 mice; (B–D). Of note, g-ratios were slightly smaller in cKO-1 mice corresponding to thicker myelin sheaths in spinal cord axons at P40. Mann-Whitney tests were performed in A and C–D; *p≤0.05, p=0.31 (mitochondrial circularity, A), p=0.2 (mitochondria counts/axon, A), p=0.68 (axon diameters, C), p=0.49 (mitochondria density, D), p=0.2 (mitochondria counts/axon, D). Data are presented as mean ±s.e.m in A and C–D.
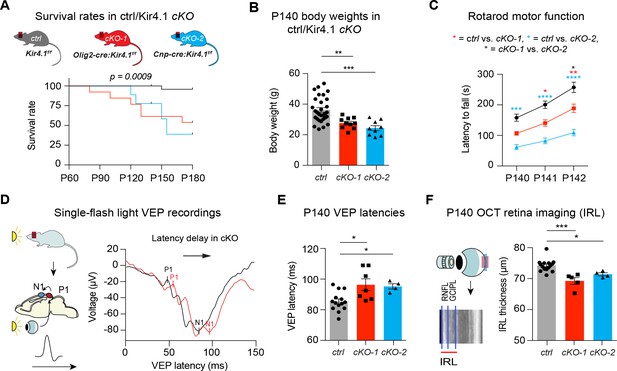
OL-Kir4.1 controls motor performance and visual function in adult mice.
Mice lacking OL-Kir4.1 channels had increased mortality with survival rates of 96% in the control group (n = 29), 54% in cKO-1 (n = 13) and only 33% in cKO-2 (n = 9) mice at P180 (A). Log-rank (Mantel-Cox) test was performed and p-value shown in A. Kcnj10 cKO-1 (n = 10) and cKO-2 (n = 9) mice were significantly smaller than control littermates (n = 30) at P140 (B). Kruskal-Wallis with Dunn’s multiple comparisons test was performed in B; **p≤0.01, ***p≤0.001. Motor dysfunction with reduced rotarod performance has been observed in both cKO-1 (n = 7) and cKO-2 (n = 7) mice as compared to controls (n = 21) (C). Two-way ANOVA with Tukey’s multiple comparisons test was performed in C; *p≤0.05, **p≤0.01, ***p≤0.001, ****p≤0.0001. Visual function was measured by single-flash light VEP recordings from control and Kcnj10 cKO mice (D–E). VEPs were delayed in cKO-1 (n = 7) and cKO-2 (n = 4) mice versus controls (n = 14) (E). Kruskal-Wallis with Dunn’s multiple comparisons test was performed in E; *p≤0.05. Retina integrity was measured by OCT imaging at P140 and revealed IRL thinning in cKO-1 (n = 5) and cKO-2 (n = 4) mice as compared to controls (n = 15; F). Kruskal-Wallis with Dunn’s multiple comparisons test was performed in F; *p≤0.05, ***p≤0.001.
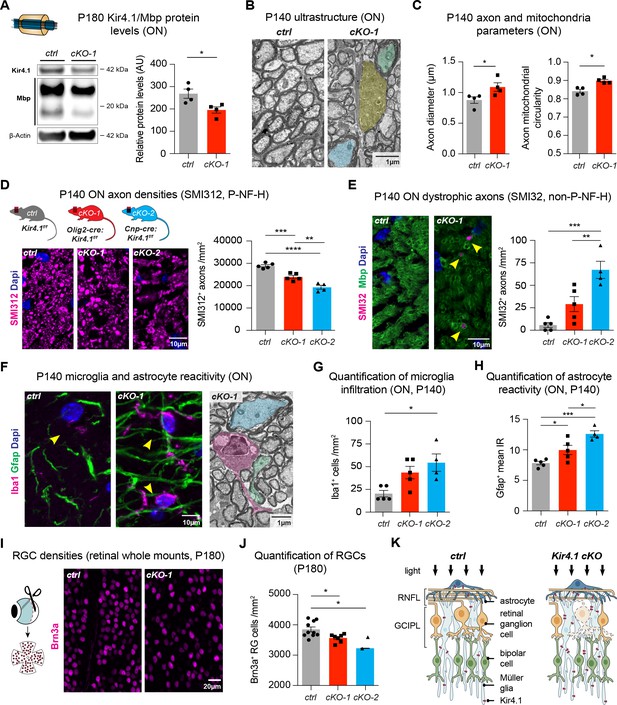
OL-Kir4.1 has a critical role in WM integrity and long-term maintenance.
Myelin basic protein (Mbp) was decreased in ON lysates from cKO-1 (n = 4) mice versus controls (n = 4) at P180 (A). Mann-Whitney test was performed in A; *p≤0.05. Transmission electron microscopy demonstrated WM pathology with presence of degenerating (highlighted in yellow) and damaged axons of mild (highlighted in blue) and more pronounced severity (green highlight) at P140 (B). Axons were larger in cKO-1 versus control ONs at P140 (146 axons from 4 control mice, 139 axons from 4 cKO mice; C). Intra-axonal mitochondria were more circular as a proxy for swelling and dysfunction in cKO-1 mice ONs as compared to controls at P140 (86 axons from four control mice, 73 axons from 4 cKO mice; C). Mann-Whitney tests were performed in C; *p≤0.05. Numbers of physiological SMI312+ (phosphorylated neurofilaments) axon profiles were reduced in ONs from cKO-1 (n = 5) and cKO-2 (n = 4) versus controls (n = 5; D), and numbers of dystrophic SMI32+ (non-phosphorylated neurofilaments) axons were increased in ONs from cKO-2 (n = 4) versus control (n = 5) and cKO-1 (n = 5) mice (E). Kruskal-Wallis with Dunn’s multiple comparisons tests were performed in D and E; **p≤0.01, ***p≤0.001, ****p≤0.0001. Iba1+ microglia activation was a common feature in ONs from cKO-1 (n = 5) and cKO-2 (n = 4) versus control (n = 5) mice (F–G). Kruskal-Wallis with Dunn’s multiple comparisons test was performed in G; *p≤0.05. Note microglial cell (highlighted in magenta) adjacent to dystrophic axons in cKO-1 ON (F). Astrogliosis as indicated by increased Gfap IR was enhanced in cKO-1 (n = 5) and cKO-2 (n = 4) ONs versus controls (n = 5) (H). One-way ANOVA with Tukey’s multiple comparison test was performed in H; *p≤0.05, ***p≤0.001. Reduced densities of Brn3a+ RGCs in cKO-1 (n = 7) and cKO-2 (n = 2) versus control mice (n = 10) were indicative of retrograde retinal neurodegeneration (I–J). Kruskal-Wallis with Dunn’s multiple comparisons test was performed in J; *p≤0.05. Cartoon highlights pathological changes in the retina of Kcnj10 cKO versus control mice with retrograde ‘dying back’ degeneration of RGCs and compensatory upregulation of Kir4.1 in retinal glial cells of Kcnj10 cKO mice (K). Data are presented as mean ±s.e.m in A, C, D–E, G–H and J.
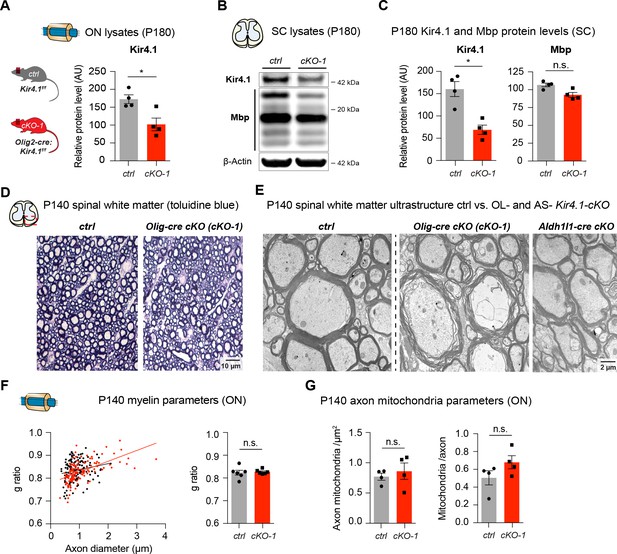
Long-term white matter pathologies in chronic OL-encoded Kcnj10 loss of function
Note Kir4.1 protein levels were significantly reduced in ONs from cKO-1 mice at P180 (ctrl: n = 4, cKO-1: n = 4; A). Likewise, Kir4.1 protein levels were substantially decreased in spinal cord tissue from cKO-1 mice at P180 (B–C); note that Mbp protein levels are slightly reduced, but not significantly different between control and OL-Kcnj10 cKO-1 animals (ctrl: n = 4, cKO-1: n = 4; B–C); Mann-Whitney tests were performed in A and C; *p≤0.05, p=0.06 (Mbp, C). Representative toluidine blue staining of control and cKO-1 spinal WM at P140 (D); note disorganized WM tracts in Kcnj10 cKO mice. By electron microscopy, dystrophic myelin and altered axonal integrity was observed in spinal WM tracts of OL-specific cKO-1 but not in control or AS-specific Kcnj10 cKO (Aldh1l1-cre) mice (E). At P140, g-ratios (146 axons from 4 control mice, 139 axons from 4 cKO-1 mice), densities and counts of intra-axonal mitochondria (73 axons from 4 control mice, 86 axons from 4 cKO-1 mice) were not altered in ONs between control and cKO-1 mice (F–G). Mann-Whitney tests were performed in F–G; p=0.18 (g-ratios, F), p=0.23 (mitochondrial densities, G) and p=0.20 (mitochondria/axon, G).
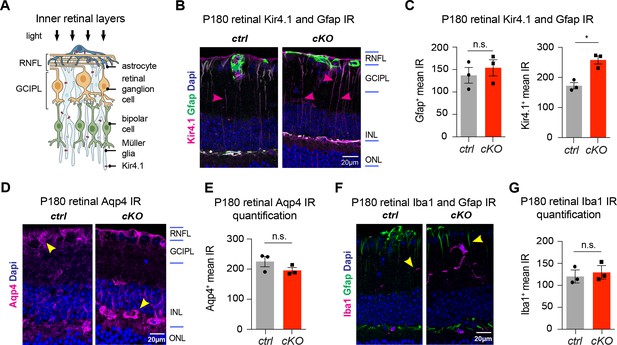
Long-term retinal changes in chronic OL-encoded Kcnj10 loss-of-function
Cartoon shows cross-section of inner retinal layers with glial cells expressing Kir4.1 (A); note that Kir4.1 immunoreactivity was increased in inner retinal layers of cKO-1 mice at P180 (B–C). Conversely, IRs for Gfap (B–C), Aqp4 (D–E) and Iba1 (F–G) were not different in retinal layers between control and cKO-1 mice at P180 (ctrl: n = 3, cKO: n = 3; A–F). Mann-Whitney tests were performed in C, E and G; *p≤0.05, p=0.1 (Gfap, C), p=0.2 (Aqp4, E), p=0.7 (Iba1, G). Data are presented as mean ±s.e.m in C, E and G.
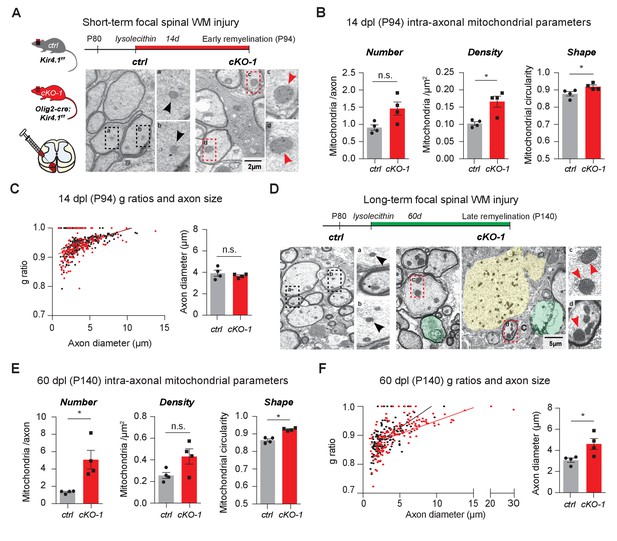
OL-Kir4.1 is dispensable for remyelination, but critical for long-term axon maintenance after WM demyelinating injury.
OL-Kir4.1 function was studied in short- (A–C) and long-term remyelination (D–F) after lysolecithin-induced focal demyelination to ventrolateral spinal WM tracts. Mice were euthanized and perfused at two survival time points corresponding to days post lesioning (dpl, n = 4 for each time point and genotype): 14 dpl (corresponding to P94, representing new myelin sheath formation) and 60 dpl (corresponding to P140, full remyelination). Densities of intra-axonal mitochondria were increased in cKO-1 (176 axons from 4 mice) versus control animals (230 axons from 4 mice) at 14 dpl and circularity/swelling of intra-axonal mitochondria was higher in cKO-1 (79 axons from 4 mice) versus controls (141 axons from four mice; A-B). Note high-magnification images of representative mitochondria in A indicating enlarged mitochondria in axons from cKO-1 versus control lesioned tissue. Conversely, loss of OL-Kir4.1 did not affect g-ratios and axon diameters in cKO-1 (176 axons from 4 mice) versus control mice (230 axons from four mice) (C). At 60 dpl, cKO-1 mice exhibited pronounced WM damage during long-term remyelination with presence of enlarged and degenerating axons (highlighted in green and yellow) as well as increased numbers of swollen intra-axonal mitochondria (D). Numbers of intra-axonal mitochondria were increased in cKO-1 versus control mice 60 dpl but densities of mitochondria were not different due to enlargement of lesion axons and thus relative lower mitochondria densities in cKO-1 axons; intra-axonal mitochondria were more circular in cKO-1 (90 axons from 4 mice) mice as compared to controls (88 axons from 4 mice) at 60 dpl (E). Remyelination was efficient and not different between cKO-1 (139 axons from 4 mice) and control mice (152 axons from 4 mice) at 60 dpl, however, enlarged axons were observed in cKO-1 mice as compared to controls (F). Mann-Whitney tests were performed in B–C and E–F; *p≤0.05, p=0.69 (C). Data are presented as mean ±s.e.m in B–C and E–F.
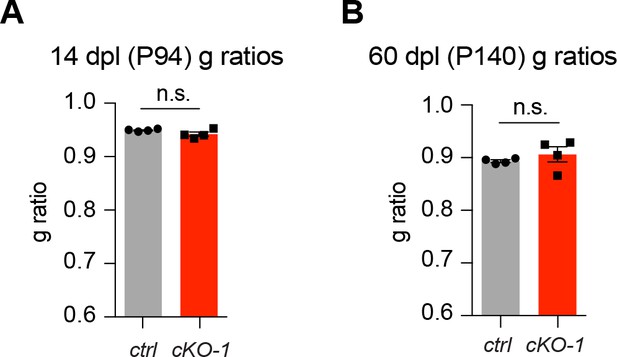
Short- and long term remyelination efficiencies in chronic OL-Kcnj10 loss-of-function
No differences in g-ratios were observed in early (14dpl) and late (60dpl) remyelinating white matter lesions from control and cKO-1 mice.
Mann-Whitney tests were performed in A and B; p=0.34 (A and B). Data are presented as mean ±s.e.m in A and B.
Videos
Ataxia and motor dysfunction are progressive symptoms in OL-Kcnj10 cKO mice.
Video shows gait ataxia in OL-Kcnj10 cKO mouse (left) as compared to littermate control (right) at P140.
Seizures are common and progressive in adult OL-Kcnj10 cKO mice.
Video shows generalized seizure in OL-Kcnj10 cKO mouse at P140.
Hind limb clasping is characteristic in adult OL-Kcnj10 cKO mice.
Video 3 shows hind limb clasping as typical sign of motor dysfunction in OL-Kcnj10 cKO mice compared to Video 4 without presence of hind limb clasping in a control mouse at P140.
Hind limb clasping is not typical in normal adult mice.
Video 4 shows that hind limb clasping is not typical in an adult control mice.
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.36428.017





