In vivo detection of optically-evoked opioid peptide release
Figures
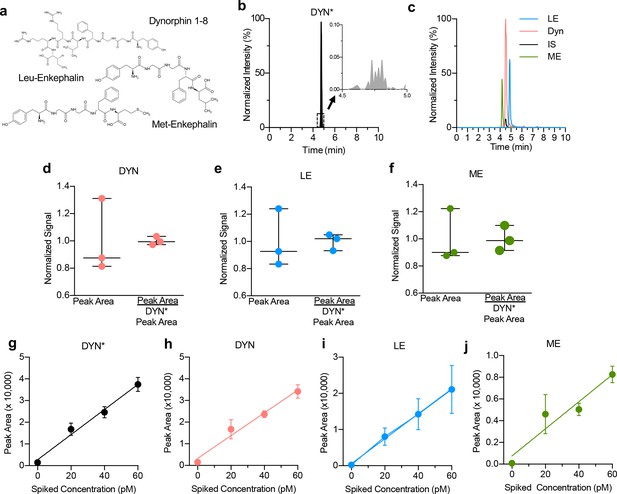
Optimization of opioid peptide detection parameters.
(a) Chemical structures of Dynorphin A 1–8, Met-Enkephalin and Leu-Enkephalin (b) Isotopically labeled dynorphin as an internal standard for quantitative analysis. High concentration injections of DYN* did not show significant traces of endogenous dyn (inset trace). A 500 pM sample of DYN* was injected while monitoring both the endogenous dyn (491 → 435 m/z) and isotopically labeled DYN* (495 → 438 m/z) mass-to-charge transitions. (c) Nano LC-MS chromatograms of 100 pM standards. Reconstructed ion chromatogram of ME, dyn, DYN*, and LE. (d) Addition of isotopically labeled DYN* results in better quantification of dyn A1-8, (e) Leu-Enkephalin and (f) Met-Enkephalin. (g–j) No effect of ionization suppression from the matrix, as shown for DYN*, Dyn A1-8, Leu-Enkephalin and Met-Enkephalin, respectively. Bulk dialysate was collected and spiked with known amounts of standard. This resulted in a linear response that corresponded with the signal increase from the original sample analyte plus the additional analyte, showing no effect of ionization suppression from the matrix. Four replicates per sample; data shown as average ± SD.
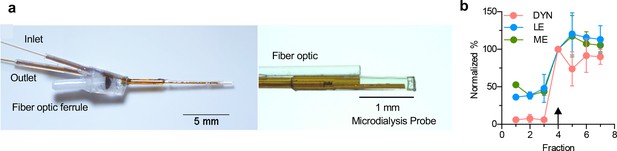
Design and function of opto-dialysis probe.
(a) Images of the optodialysis probe. (b) Trace of an in vitro step change from 100 pM of DYN, LE, and ME stock solution to a solution of 1 nM Dyn and 400 pM LE and ME. The arrow indicates the first fraction in which the peptide was expected to change. Data were normalized to fraction 4, the fraction expected to reflect elevated concentration stock change. Data shown as average ± SD, n = 4 probes.
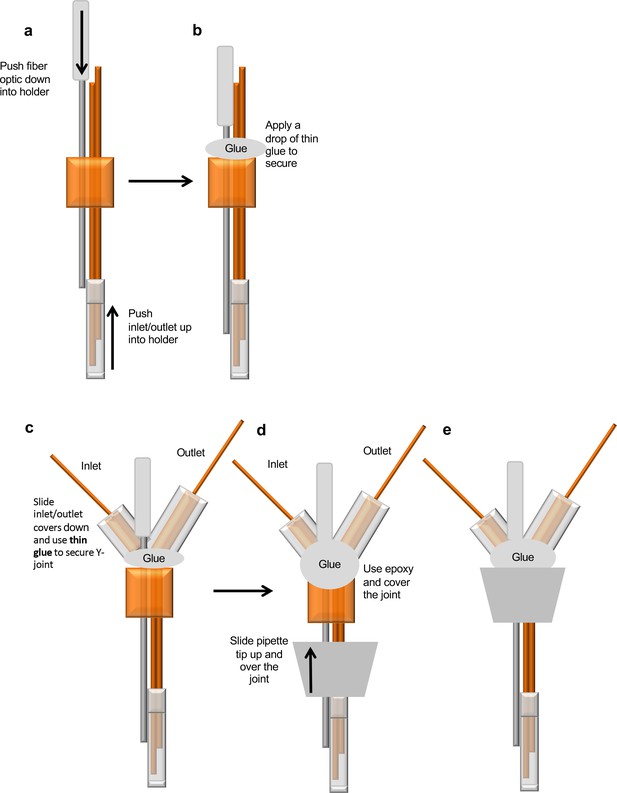
Step-by-step illustration of the custom-made integrated optogenetic-dialysis probes measure peptides and small molecules in freely moving animals.
Also see supplementary file 1.
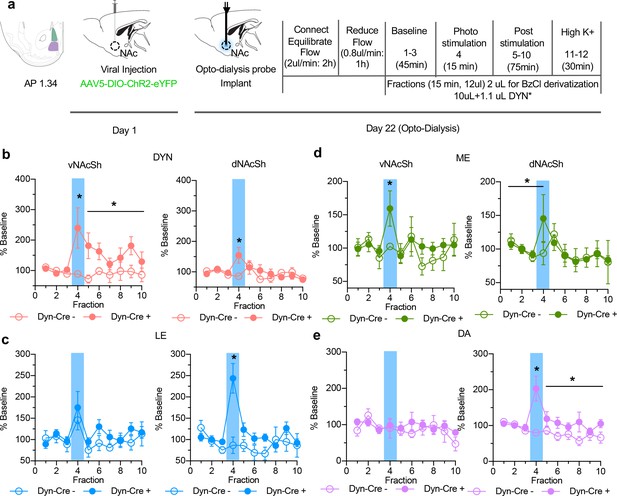
In vivo changes in opioid peptide release following photostimulation of dynorphin cells.
(a) Timeline of experimental procedure outlining viral injection, probe implantation and dialysate collection. (b) Extracellular opioid peptide release shown as % baseline in vNAcSh dyn1-8, (left panel, n = 8), dNAcSh dyn1-8 (right panel, n = 6). (c) vNAcSh Leu-Enkephalin (left panel, n = 4), dNAcSh Leu-Enkephalin (right panel, n = 6). (d) vNAcSh Met-Enkephalin (left panel, n = 7) and dNAcSh Met-Enkephalin (right panel, n = 7). (e) Small molecules simultaneously collected and shown as % baseline in vNAcSh Dopamine (left panel, n = 7), dNAcSh Dopamine (right panel, n = 7).
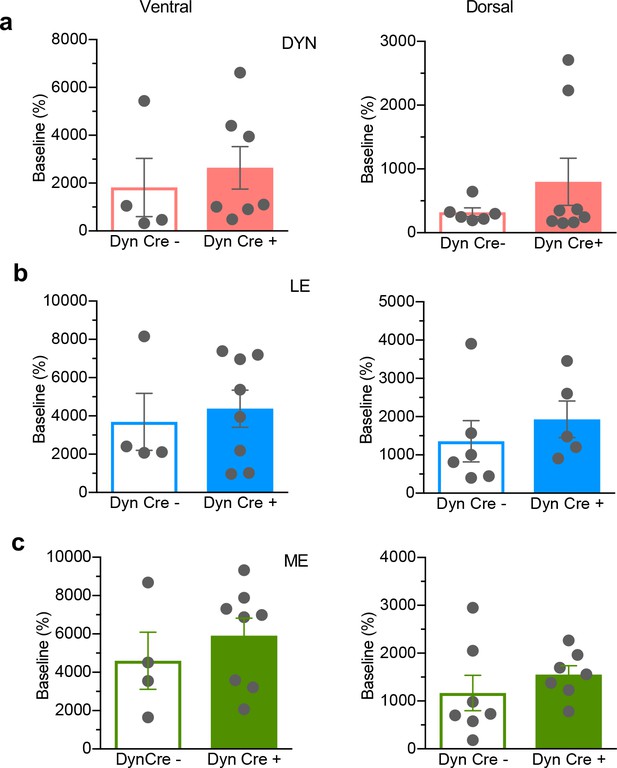
In vivo K + depolarization.
Reliable depolarisation following K + was detected for (a) vNAcSh dyn1-8, (left panel, n = 8), dNAcSh dyn1-8 (right panel, n = 6). (b) vNAcSh Leu-Enkephalin (left panel, n = 4), dNAcSh Leu-Enkephalin (right panel, n = 6). (c) vNAcSh Met-Enkephalin (left panel, n = 7) and dNAcSh Met-Enkephalin (right panel, n = 7). Data show as mean ± SEM.
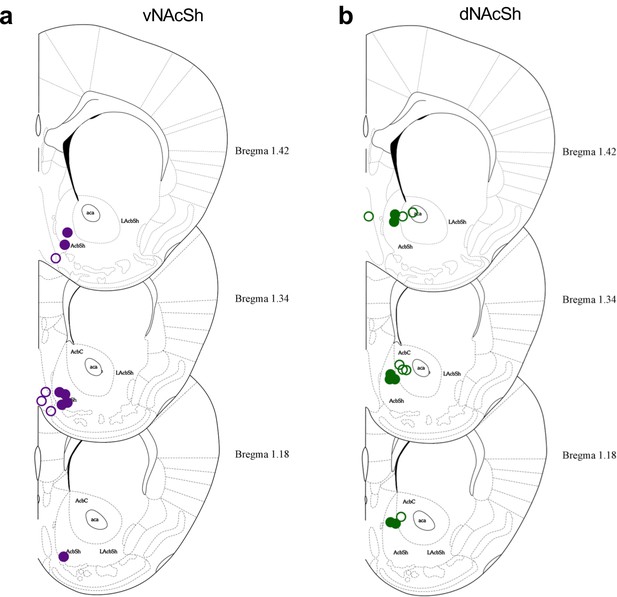
Hits maps showing placement of opto-dialysis probes (a) vNAcSh, closed purple circles represent correct hits, open circles represent misses.
(b) dNAcSh, closed green circles represent correct hits, open circles represent misses.
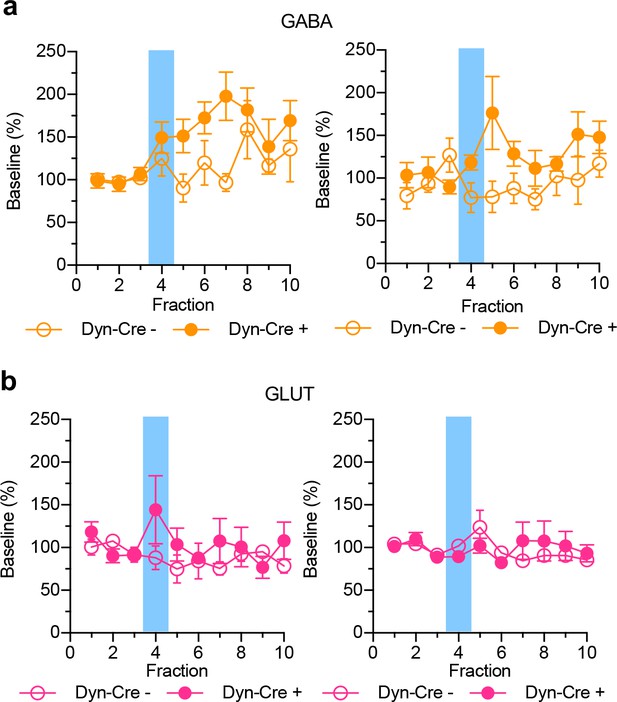
Small molecules simultaneously collected and shown as % baseline in (a) vNAcSh GABA (left panel, n = 6), dNAcSh GABA (right panel, n = 6).
(b) vNAcSh Glutamate (left panel, n = 7) and dNAcSh Glutamate (right panel, n = 7).
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers |
|---|---|---|---|
| Antibody | Neurotrace | Invitrogen | RRID:SCR_008410 |
| Peptide, recombinant protein | Dynorphin A 1–8 (dyn) | Bachem 4005845 | |
| Peptide, recombinant protein | Leu-Enkephalin (LE) | Bachem 4006097 | |
| Peptide, recombinant protein | Met-Enkephalin (ME) | Sigma M6638 | |
| Chemical compound, drug | Vectashield | Vector Labs | |
| Software, algorithm | Thermo Xcalibur QuanBrowser | ThermoFisher | RRID:SCR_008452 |
| Other | pAAV-EF1α-double floxed- hChR2(H134R)-eYFP-WPRE- HGHpA | Addgene | RRID:SCR_002037 |
| Other | Agilent 1100 HPLC pump | Agilent Technologies | RRID:SCR_013575 |
| Other | linear ion trap | LTQ XL, Thermo Scientific | RRID:SCR_014992 |
| Other | Accela UHPLC system/TSQ Quantum Ultra triple quadrupole mass spectrometer | ThermoFisher | RRID:SCR_008452 |
Additional files
-
Supplementary file 1
Opto-dialysis probe fabrication
- https://doi.org/10.7554/eLife.36520.009
-
Transparent reporting form
- https://doi.org/10.7554/eLife.36520.010





