Acute control of the sleep switch in Drosophila reveals a role for gap junctions in regulating behavioral responsiveness
Figures
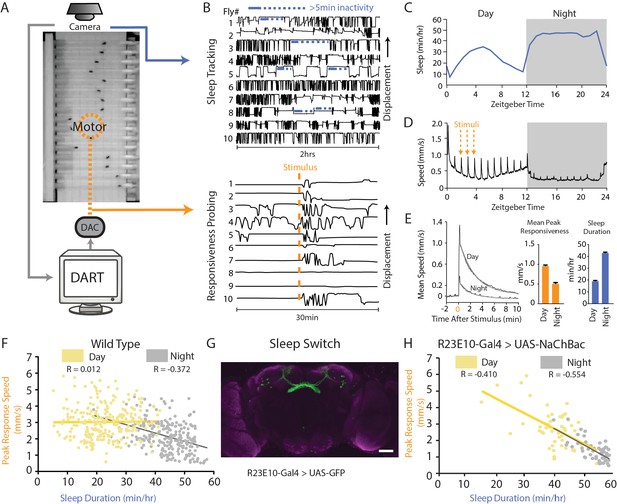
The sleep switch modulates behavioral responsiveness.
(A) Flies in glass tubes were filmed from above and DART was used to track activity and to test behavioral responsiveness using a mechanical vibration. (B) Sleep duration was measured using 5 min inactivity criteria (top panel). Behavioral responsiveness was tested by quantifying the change in fly activity following a vibration stimulus. Following the stimulus (orange line), flies increase their locomotion speed as shown by their displacement in the tube (bottom panel). (C) Mean sleep duration (min/hr) is tracked over a circadian cycle. (D) Fly activity (speed, mm/sec) is plotted for a 24-hr day/night (white and grey, respectively) cycle during which a five-pulse 0.2 s 2.4 g vibration is delivered once per hour. Spikes in activity show timing of the stimuli, and the orange lines highlight three examples. (E) The mean response (speed, mm/s) for all stimuli during the day or night (left panel, black line). Shown in grey is a fitted curve for this average response (see 'Materials and methods'), the peak of which is a measure of the magnitude of response to the stimulus (middle panel). Responsiveness is greater during the day and lower during the night, whereas sleep duration is decreased during the day and increased at night (right panel). (F) Correlation between the peak response speed (mm/s) and sleep duration (min/hr) for wildtype (w2202) flies (n = 225) during the day (yellow R = 0.012, p=0.84) and the night (grey R = −0.372, p<0.0001). (G) R23E10-Gal4 neurons were chronically activated by expressing NaChBac, a bacterial sodium channel. Scale bar = 50 μm, the genotype in this image is R23E10-Gal4/+;UAS-2eGFP/+. (H) Correlation between responsiveness and sleep duration following activation of the dFB (R23E10-Gal4/UAS-NaChBac, n = 51) during daytimes (yellow R = −0.410, p<0.001) and nighttimes (grey R = −0.554, p<0.0001). See also Figure 1—figure supplement 1.
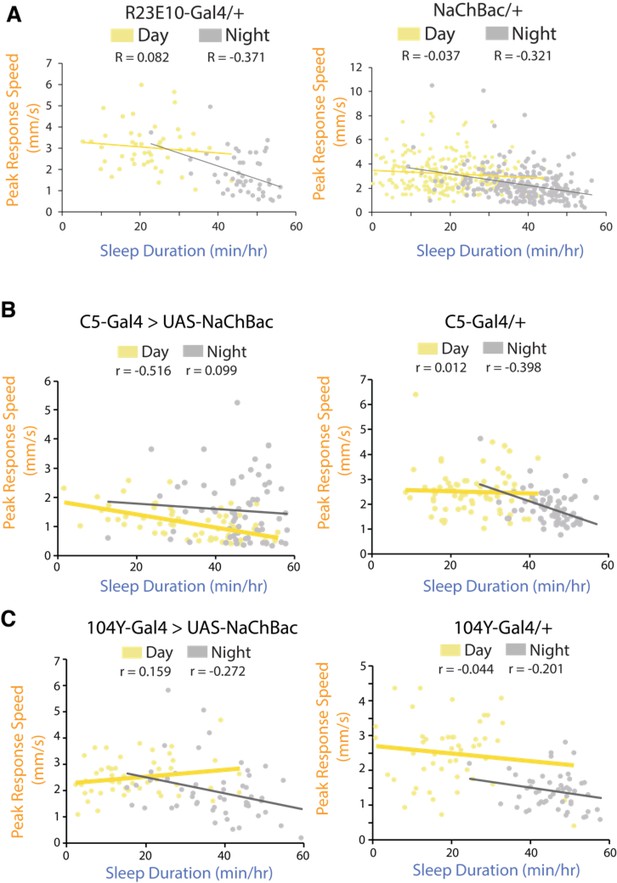
Correlation between responsiveness and sleep duration following activation of the dFB in various Gal4 drivers during the day (yellow) and night (grey).
(A) Left, correlation between the peak response speed (mm/s) and sleep duration (min/hr) for R23E10-Gal4/+control flies (n = 49) during the day (yellow R=- 0.082, p=0.57) and night (gray R = −0.371, p<0.01). Right, correlation between the peak response speed and sleep duration for UAS-NaChBac/+control flies (n = 250) during the day (yellow R = −0.073, p=0.24) and night (gray R = −0.321 P<0.0001). (B) C5-Gal4/UAS-NaChBac (n = 63, day p<0.0001, night p=0.435) and C5-Gal4/+control (n = 68, day p=0.921, night p<0.001). (C) 104Y-Gal4/UAS-NaChBac (n = 51, day p=0.267, night p=0.055) and 104Y-Gal4/+control (n = 51, day p<0.05 night p=0.157).
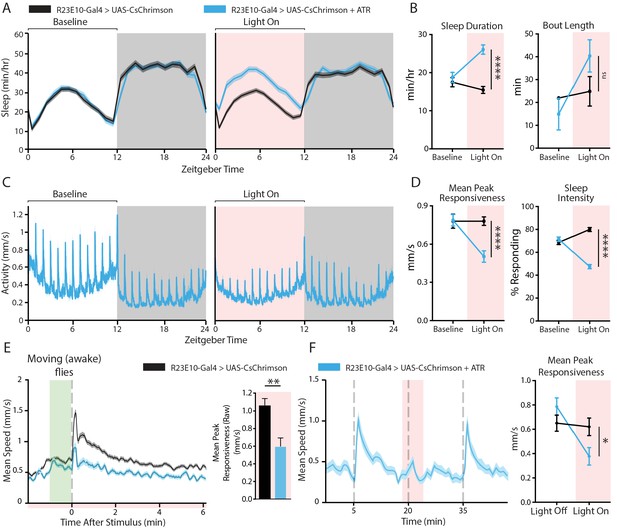
Behavioral effects of acutely activating R23E10 neurons.
(A–D) Effects on sleep and responsiveness following the activation of R23E10 neurons (blue, UAS-CsChrimson/+;R23E10-Gal4/+ with ATR, n = 102) compared to those in control flies (black, no ATR feeding, n = 118). Error bars and shading indicate standard errors of the mean (SEMs) and asterisks indicate significance (****p<0.0001, ns = not significant, t-tests). (A) Mean sleep duration (min/hr) during the period 24 hr before red light activation (left), then during the next 24 hr when red light is delivered for 12 hr during the day (pink shading, right). (B) Comparison of the 12-hr day period without red light (baseline) to the period of red-light activation in terms of sleep duration and bout length. (C) Mean activity (mm/s) for R23E10 activation for the time periods in (A). Spikes in activity show timing of hourly vibration stimuli. (D) Comparison between the 12-hr day period without red light (baseline) and the period of red-light activation for peak responsiveness and sleep intensity. (E) Left, average stimulus response for UAS-CsChrimson/+;R23E10-Gal4/+ with ATR (blue, n = 50) compared to control flies (black, no ATR feeding, n = 48) during red-light activation (Figure 2A–D red shading) in flies that moved in the minute prior to the stimulus (i.e. awake flies). Right, summary histogram (average ± SEM). **p<0.01, t-test. (F) Example activity trace of flies responding to stimuli 15 min apart (gray dashed lines): 1 min CsChrimson activation (red shading) prior to the stimulus is alternated with trials without red light (left panel). One minute of dFB activation is sufficient to decrease responsiveness (right panel, UAS-CsChrimson/+;R23E10-Gal4/+ with ATR n = 83, control n = 80, *p<0.05, t-test). See also Figure 2—figure supplements 1, 2 and 3.
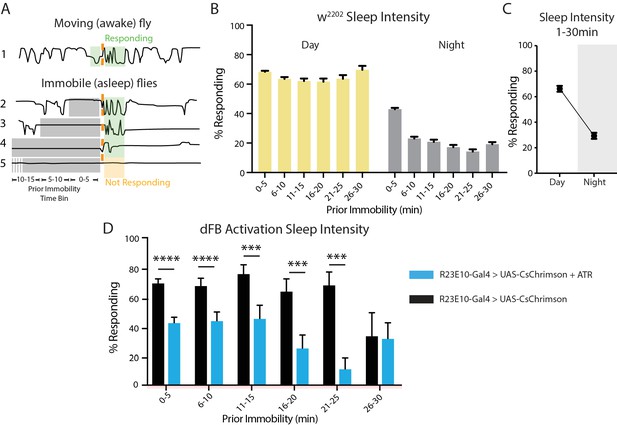
Measuring sleep intensity.
(A) Schematic showing fly activity prior to and in response to a mechanical stimulus (orange dashed line). Flies can be inactive (grey shading) or active (green shading) prior to stimulus delivery. Flies that are inactive for longer than 5 min are classified as asleep, and the proportion of sleeping flies that respond to the stimulus (green shading) compared to those not responding (orange shading) is a measure of sleep intensity. (B) Sleep intensity for w2202 flies during the day (yellow) and night (grey) for inactivity bins of 5 min (n = 250, error bars are SEM). (C) During the night, flies are less likely to respond to a stimulus than they are during the day and are therefore sleeping more intensely. (D) Sleep intensity during red-light activation of dFB neurons in UAS-CsChrimson/+;R23E10-Gal4/+flies fed ATR (blue, n = 102) compared to non-ATR-fed controls (black, n = 118). ****p<0.0001, ***p<0.001, t-test corrected for multiple comparisons (using the Bonferroni correction).

Acute effects in awake flies.
(A) Mean speed of all flies for the minute prior to stimulation during acute CsChrimson activation. n.s. represents not significantly different in a t-test. (B) Average stimulus response for UAS-CsChrimson/+;R23E10-Gal4/+with ATR (blue, n = 83) compared to control flies (black, no ATR feeding, n = 80) in flies that moved in the minute prior to the stimulus (i.e. awake flies), with the red light off. (C) Average stimulus response for the same flies as those in (B) that moved in the minute prior to the stimulus (i.e. awake flies), with the red light on. (D) Summary histogram of mean peak responsiveness data in (B) and (C) (average ± SEM). *p<0.05, t-test.
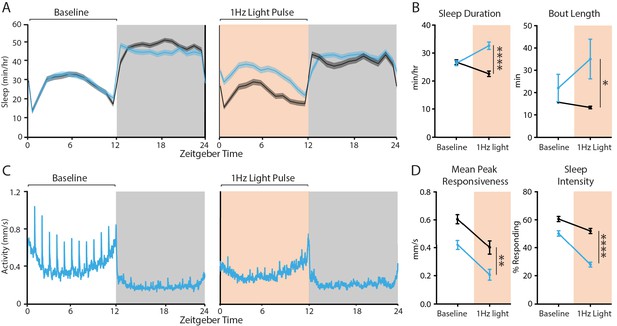
1 Hz optogenetic activation of the dFB.
(A)-(D) Effects on sleep and responsiveness following 1 Hz activation of R23E10 neurons (blue, UAS-CsChrimson/+;R23E10-Gal4/+ with ATR, n = 115) compared to control flies (black, no ATR feeding, n = 115). Error bars and shading indicate SEM and asterisks indicate significance (*p<0.05, ****p<0.0001, t-tests). (A) Mean sleep duration (min/hr) during the 24 hr before red-light activation, then during the next 24 hr when red light is delivered for 12 hr during the day (red shading). (B) Comparison of the 12-hr day period without red light (baseline) to the period of red-light activation in terms of sleep duration and bout length. (C) Mean activity (mm/s) for R23E10 activation for the same time periods as in (C). Spikes in activity show the timing of hourly vibration stimuli. (D) Comparison between the 12-hr day period without red light (baseline) and the period of red-light activation in terms of peak responsiveness and sleep intensity. Error bars and shading indicate SEM and asterisks indicate significance (**p<0.01, ****p<0.0001, t-tests).
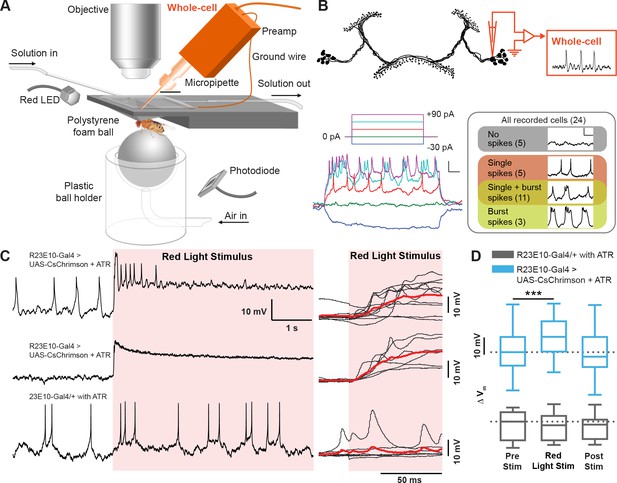
Electrophysiological effects of acutely activating R23E10 neurons.
(A) Setup for recording in vivo adult Drosophila electrophysiology with whole-cell patch clamp (orange). (B) dFB neuron schematic showing whole-cell recordings targeted to R23E10-Gal4 cell bodies. Injecting current in a stepwise manner causes firing in these neurons (bottom left panel), which are heterogeneous in their endogenous firing patterns (bottom right panel). Scale bars indicate 10 mV and 100 ms. (C) Example traces (left) of a CsChrimson-expressing (UAS-CsChrimson/+;R23E10-Gal4/+ with ATR) spiking cell (top) and non-spiking cell (middle), and of a non-CsChrimson-expressing (R23E10-Gal4/+ with ATR) cell (bottom) when exposed to constant red light (red shading). Superimposed traces of the corresponding types from multiple cell recordings (right panel, top to bottom: n = 10, n = 6, n = 6). Solid red lines indicate mean values. (D) Boxplots show median membrane potentials for CsChrimson-expressing cells (blue) and non-CsChrimson expressing cells (gray, n = 6) before, during and after constant light stimulation (***p<0.001, Friedman test with Dunn’s multiple comparisons to pre-stimulus condition). See also Figure 3—figure supplements 1 and 2.
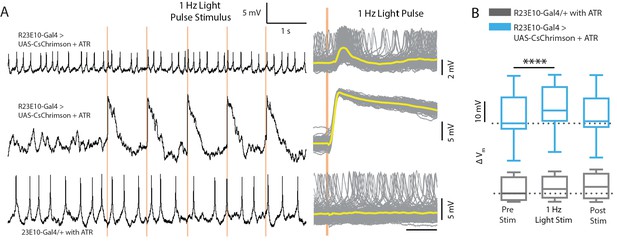
1Hz stimulation.
(A) Example traces (left) of a CsChrimson-expressing spiking cell (top), a CsChrimson-expressing non-spiking cell (middle), and a non-CsChrimson-expressing cell (bottom) when exposed to 1 Hz light pulse with 5 ms exposure per pulse (orange shading). Superimposed traces from all 120 trials for the corresponding cells shown on the left (right). Solid yellow lines indicate mean values. (B) Boxplots show median membrane potential for CsChrimson-expressing cells (cyan, n = 13) and for non-CsChrimson-expressing cells (gray, n = 4) before, during, and after 1 Hz light stimulation (****p<0.0001, Friedman test with Dunn’s multiple comparisons to pre-stimulus condition).
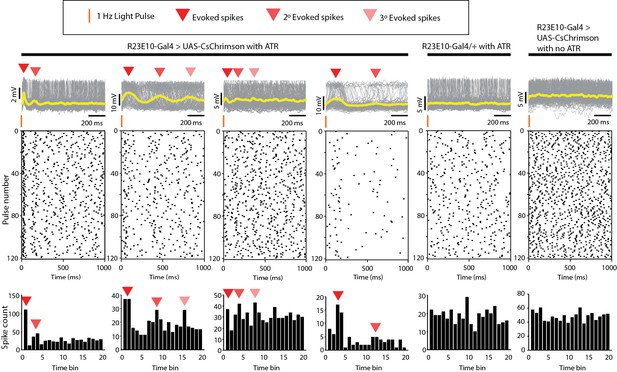
Whole-cell patch physiology in wildtype and INX6 knockdown flies.
Top panels show combined traces (yellow lines indicate mean values) for all 120 pulses for all CsChrimson-expressing R23E10 neurons that showed the presence of evoked spikes (red arrows) in response to the light stimulus pulses at 1 Hz with 5 ms exposure time each pulse, and the absence of any evoked spikes for the wildtype controls (right, 500 ms exposure for R23E10-Gal4 > UAS CsChrimson with no ATR), with spiking events presented as raster plots (middle), and corresponding peristimulus time histograms (bottom).
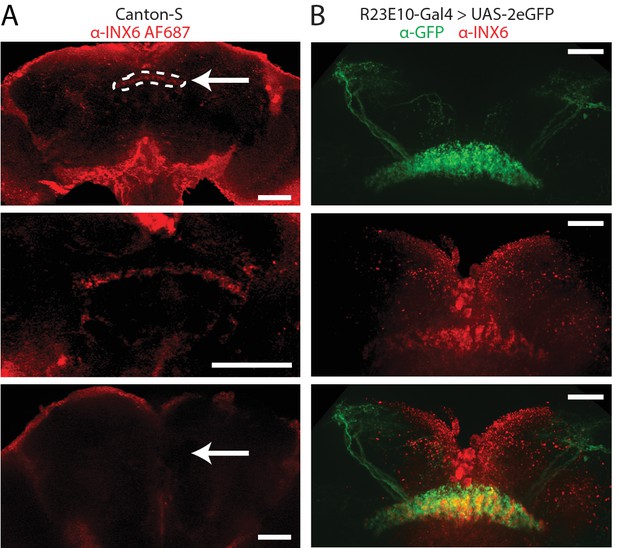
INX6 dFB localization.
(A) INX6 antibody staining (red) in wildtype Canton-S flies at 20x (top) and 60x (middle). White arrow indicates the location of the dFB and white dashes outline it. Staining using the secondary antibody alone (bottom, 20x) shows no detectable reactivity in the dFB. Scale bars = 50 μm. (B) INX6 antibody staining (red, middle) with GFP antibody staining in flies expressing GFP in R23E10 neurons (UAS-GFP/+;R23E10-Gal4/+, green, top). Overlap between these regions (bottom) indicates the presence of INX6 in these neurons (47.5% co-localization, see 'Materials and methods'). Scale bars = 25 μm. See also Figure 4—figure supplements 1 and 2.
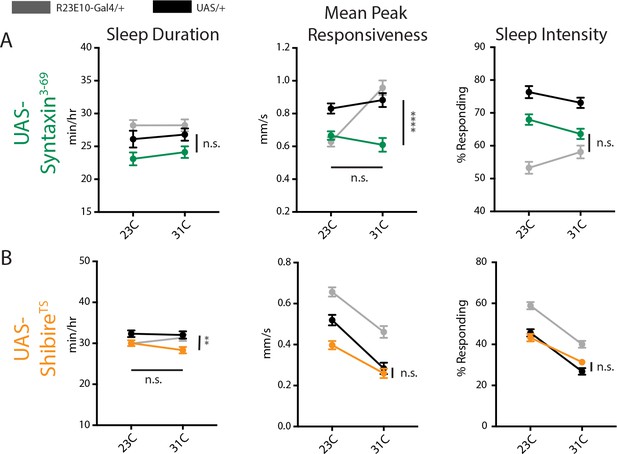
Acute synaptic manipulations.
(A–B) Mean day sleep duration (min/hr), mean peak responsiveness (mm/s) and sleep intensity (% responding) at 23C and 31C for flies expressing UAS-Syntaxin3-69 (green, n = 71) or UAS-ShibireTS (orange, n = 67) in R23E10-Gal4 neurons. R23E10-Gal4/+ (n = 48 in Syntaxin3-69 experiments, n = 68 in ShibireTS experiments) and UAS/+ (UAS-Syntaxin3-69/+n = 71, UAS- ShibireTS/+n = 51) controls are shown in gray and black, respectively. Error bars indicate SEM and asterisks indicate significance (**p<0.01, ****p<0.0001 two-way ANOVA, n.s. = not significant, adjusted for multiple comparisons (Dunnett)). Vertical comparisons are between genetic controls whereas horizontal comparisons are between temperature shifts (activation). For clarity, all genetic comparisons are between Gal4 >UAS flies and their closest genetic control at 31C activation. In cases where we saw significant differences from controls, there was no significant change between restrictive and permissive temperature conditions ([A], middle panel, mean peak responsiveness and [B], left panel, sleep duration).
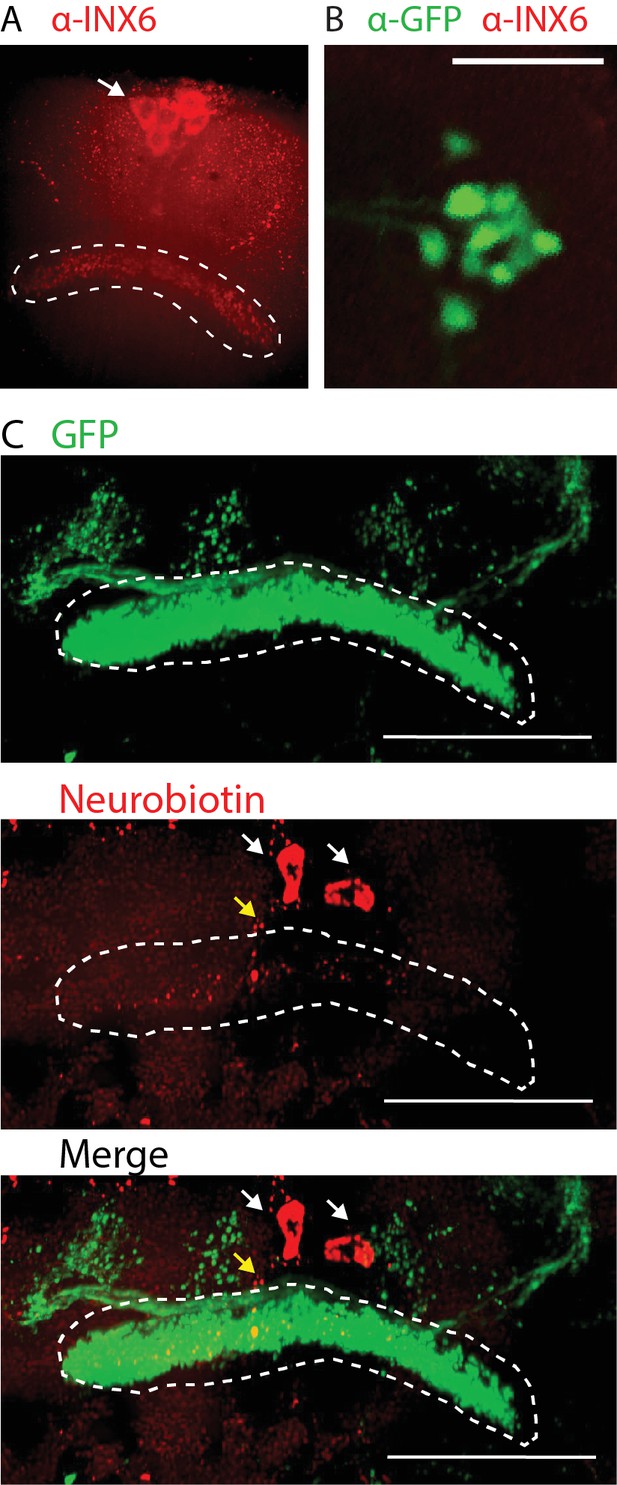
INX6 in dFB neurons.
(A) INX6 antibody labeling shows reactivity in the dFB (outlined) and in PI cells (arrow). (B) R23E10-Gal4 cell bodies (green) showed no overlap with INX6 staining. Scale bar: 25 μm. (C) Images taken from Video 1. dFB is highlighted by the white dashed line. From left to right: GFP expression in R23E10-Gal4; neurobiotin labeling overlaps with the expression of R23E10-Gal4 and also labels a separate set of neurons (white arrows) and corresponding axons (yellow arrow); merged image of 23E10-Gal4 and neurobiotin labeling. Scale bar: 25 µm.
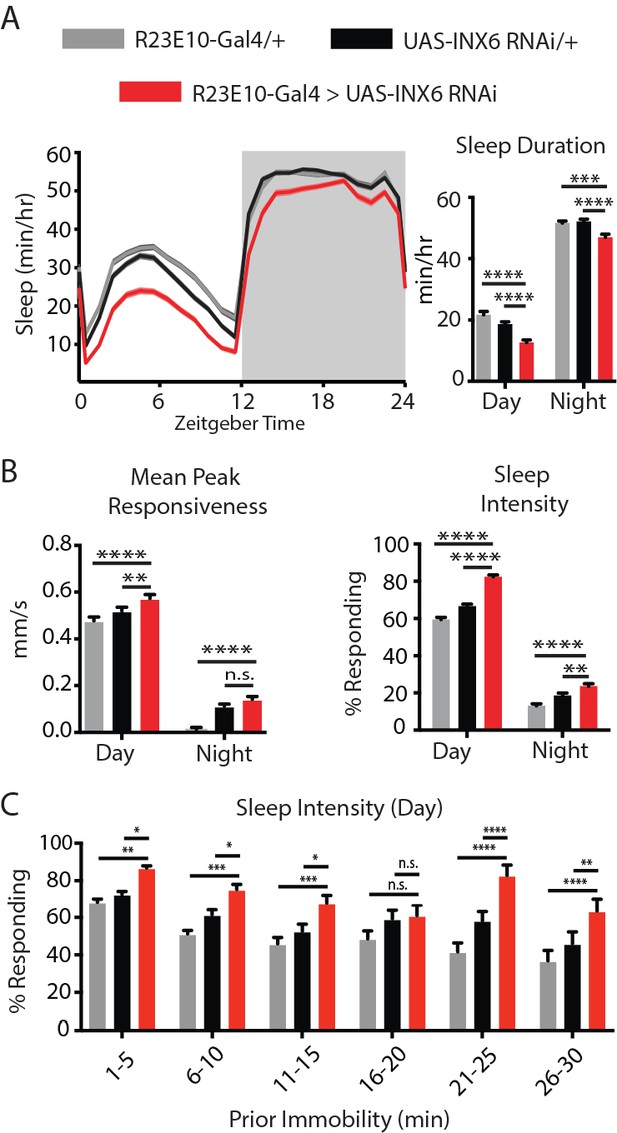
INX6 effects on behavior.
(A) Mean sleep duration (min/hr) and (B) mean peak responsiveness (mm/s) and sleep intensity (% responding) for R23E10-Gal4/+;UAS-INX6-RNAi/+ (red, n = 85) compared to R23E10-Gal4/+ (gray, n = 83) and UAS-INX6-RNAi/+ (black, n = 84) controls. (C) Sleep intensity for inactivity bins of 5 min for the first 30 min of inactivity. See also Figure 2—figure supplement 1. Shading and error bars indicate SEM, asterisks indicate significance (*p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, two-way ANOVA, adjusted for multiple comparison (Dunnett). See also Figure 5—figure supplements 1 and 2.
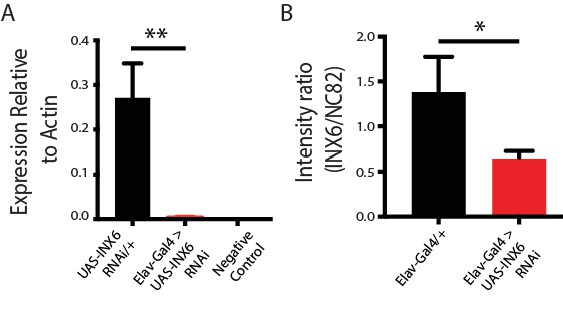
INX6 RNAi effectiveness.
(A) qRT-PCR shows relative amount of inx6 mRNA normalized to the housekeeping gene Actin in pan-neuronal knockdown using elav-Gal4. **p<0.01, t-test. (B) Average intensity ratio (± SEM) in the dFB (INX6 vs NC82 background) in Elav-Gal4/+;UAS-INX6-RNAi/+flies (n = 5) compared to genetic controls (n = 4). *p<0.05, t-test.

Behavioral effects of acute downregulation of INX6 in adult flies.
(A) Mean sleep duration (min/hr), (B) peak responsiveness (mm/s) (C) and sleep intensity (% responding) for tubpGAL80TS/+;R23E10-GAL4/UAS-INX6-RNAi (red, n = 34) compared to negative tubpGAL80TS/+;UAS-INX6 RNAi/+ (grey, n = 34) and positive UAS-INX6-RNAi/R23E10-Gal4 (black, n = 34) controls, following heating to 31°C for 24 hr. Data are average over two days at 25°C. Error bars indicate SEM, asterisks indicate significance *p<0.05 **p<0.01, two-way ANOVA, adjusted for multiple comparisons (Dunnett).
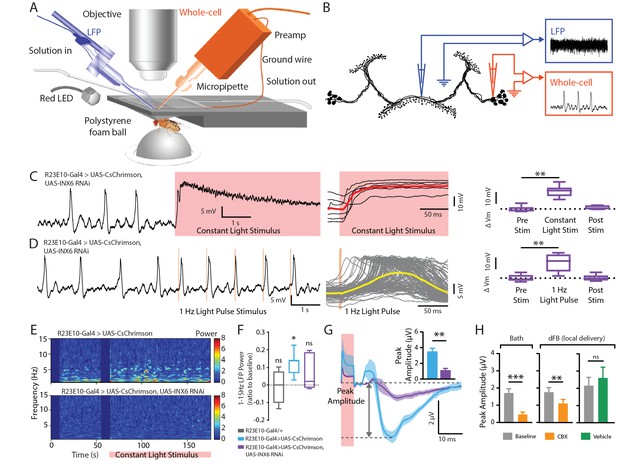
Electrophysiological effects of INX6 knockdown in activated R23E10 neurons.
(A) In vivo adult Drosophila electrophysiology recording setup with a whole-cell patch clamp (orange) targeting R23E10 cell bodies and a local field potential (blue) electrode targeting the dFB. (B) Local field-potential (LFP) recordings were obtained within the presynaptic arborizations of the R23E10 neurons in the fan-shaped body, while whole-cell recordings were obtained from the cell bodies of R23E10 neurons. (C) Example trace (left) of a CsChrimson-expressing (UAS-CsChrimson/+; UAS-INX6 RNAi/+; R23E10-Gal4/+ with ATR) cell with INX6 knockdown when exposed to constant red light (red shading). Superimposed traces from multiple cell recordings (middle). The solid red line indicates mean values. Boxplots show median membrane potential for CsChrimson-expressing cells with INX6 knockdown before, during and after constant light stimulation (n = 6, **p<0.01, Friedman test with Dunn’s multiple comparisons to the pre-stimulus condition). (D) Example trace (left) of a CsChrimson-expressing cell with INX6 knockdown when exposed to 1 Hz light pulse with 5 ms exposure per pulse (orange shading). Superimposed traces from all 120 trials (middle) for the representative cell shown on the left. Solid yellow line indicates mean value. Boxplots show median membrane potential for CsChrimson-expressing cells with INX6 knockdown before, during, and after 1 Hz light stimulation (n = 6, **p<0.01, Friedman test with Dunn’s multiple comparisons to pre-stimulus condition). (E) Local field potential recordings from the dorsal fan-shaped body represented as power within the 1–15 Hz frequency range, in response to a constant light stimulus (pink bar) with one representative fly shown from each strain. (F) Boxplots show median 1–15 Hz local field potential power (±SEM) for the duration of the constant light stimulus relative to pre-stimulation power (normalized to zero) in UAS-CsChrimson/+;R23E10-Gal4/+ with ATR (blue, n = 7), UAS-CsChrimson/+; UAS-INX6 RNAi/+; R23E10-Gal4/+ with ATR (purple, n = 6), and R23E10-Gal4/+ with ATR (gray, n = 4). Only UAS-CsChrimson/+;R23E10-Gal4/+ showed a significant increase in 1–15 Hz activity when exposed to constant red light (*p<0.05, ns = not significant, by Wilcoxon signed rank test). (G) Peak amplitude of the average LFP response (±SEM) to 1 Hz light pulse stimulus is significantly reduced for UAS-CsChrimson/+; UAS-INX6 RNAi/+; R23E10-Gal4/+ (purple, n = 6), compared to UAS-CsChrimson/+;R23E10-Gal4/+ (blue, n = 7) (**p<0.01, by Mann-Whitney test). (H) Peak LFP amplitude (±SEM) in response to 1 Hz stimulus in the presence of bath-applied (n = 6) or locally injected (n = 4) carbenoxolone (CBX, orange) compared to baseline (gray) or vehicle (green, n = 5). ***p<0.001,**p<0.01, by paired t-test. See also Figure 6—figure supplement 1.

Combined traces (yellow lines indicate mean values) for all 120 pulses for each of the recorded CsChrimson-expressing R23E10 neurons with INX6 knockdown.
All neurons responded to 5 ms light stimulus with burst firing in the absence of stereotypical subsequent firing in the 1,000 ms that follows. See also Figure 3—figure supplement 2.
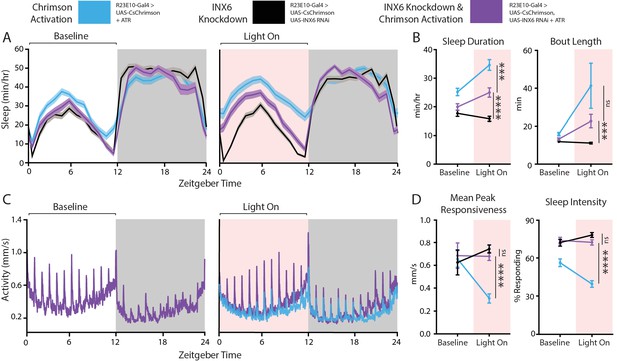
Behavioral effects of INX6 knockdown in activated R23E10 neurons.
(A–D) Three different behavioral conditions comparing flies with R23E10 neurons that can be activated (UAS-CsChrimson/+; R23E10-Gal4/+with ATR, blue, n = 50) with flies where R23E10 neurons cannot be activated but have INX6 knocked down (UAS CsChrimson/+; UAS-INX6 RNAi/R23E10-Gal4 no ATR, black, n = 51) and flies with R23E10 neurons that can be activated and have INX6 knocked down (UAS-CsChrimson/+; UAS-INX6 RNAi/R23E10-Gal4 with ATR, purple, n = 51) for effects on sleep and responsiveness following red light activation. (A) Mean sleep duration (min/hr, shading indicates SEM) during the 24 hr before red light activation, then during the next 24 hr during which red light is delivered for 12 hr during the day (pink shading). (B) Comparison between the 12-hr day period without red light (baseline) and the period of red-light activation in terms of sleep duration and bout length. (C) Mean activity (mm/s) for UAS-CSChrimson/+; UAS-INX6 RNAi/R23E10-Gal4 with ATR for the same time periods as (A). UAS-CsChrimson/+; R23E10-Gal4/+ with ATR is overlaid (blue) during the 'Light On' period to show differences in responses to hourly stimuli. Other traces (black) not shown for clarity; see (D) for summarized control data. (D) Comparison between the 12-hr day period without red light (baseline) and the period of red-light activation in terms of peak responsiveness and sleep intensity. Error bars indicate SEM and asterisks indicate significance (***p<0.001, ****p<0.0001, t-tests).

Acute inhibition of the dFB.
(A) Example average activity trace (speed ± SEM) of ATR-fed R23E10/GtACR-3M flies responding to stimuli 15 minutes apart (gray dashed lines); 5min GtACR activation (green shading), with 1min prior to the stimulus, is alternated with trials without green light. (B) Same acute experiment as in A, for non-ATR fed controls. (C) Average responsiveness ( ± SEM) in same flies as in A&B, for a 12-hour experiment. (D) Average sleep duration ( ± SEM) for same flies as in A&B, for a 12-hour experiment. N=16 flies for all..
Videos
3D reconstruction of dye coupling experiment.
https://doi.org/10.7554/eLife.37105.014Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (D. melanogaster) | R23E10-Gal4 | Bloomington | RRID:BDSC_49032 | |
| Genetic reagent (D. melanogaster) | C5-Gal4 | doi: 10.1002/ ssscne.22284 | Paul Shaw Lab | |
| Genetic reagent (D. melanogaster) | 104y-Gal4 | Paul Shaw Lab | ||
| Genetic reagent (D. melanogaster) | UAS-CsChrimson | doi: 10.1038/ nmeth.2836 | provided by Vivek Jarayaman Lab | |
| Genetic reagent (D. melanogaster) | UAS-2eGFP | Bloomington | RRID:BDSC_32186 | |
| Genetic reagent (D. melanogaster) | UAS-NaChBac | Bloomington | RRID:BDSC_9469 | |
| Genetic reagent (D. melanogaster) | UAS-syntaxin3-69 | Fly Base | FBal0092503 | |
| Genetic reagent (D. melanogaster) | UAS-shibireTS | Paul Shaw Lab | Gene ID: 45928 | |
| Genetic reagent (D. melanogaster) | tubpGAL80ts | Bloomington | RRID:BDSC_7108 | |
| Genetic reagent (D. melanogaster) | UAS-INX6 RNAi | VDRC | v8638 | Provided by the Chia-Lin Wu Lab |
| Antibody | Rabbit anti -INX6 | Provided by the Chia-Lin Wu Lab | 1:1,000 | |
| Antibody | Goat anti-mouse AlexaFluor488 | Invitrogen | Catalog # A-10680 | 1:200 |
| Antibody | Goat anti-rabbit AlexaFluor568 | Invitrogen | Catalog # A-11011 | 1:200 |
| Antibody | Goat anti-rabbit AlexaFluor647 | Invitrogen | Catalog # A-21244 | 1:200 |
| Antibody | Mouse anti-NC82 | DSHB | AB_2314866 | 1:10 |
| Antibody | Goat anti-rabbit AlexaFluor488 | Invitrogen | Catalog # A-11008 | 1:200 |
| Chemical compound, drug | Neurobiotin | Vector Labs | Cat. No: SP-1120 | |
| Software, algorithm | DART | bfklab | http://www.bfklab.com/ | |
| Software, algorithm | MATLAB code | This paper | 142faca | https://github.com/melvynyap/gap-junction-sleep-control (copy archived at https://github.com/elifesciences-publications/gap-junction-sleep-control) |
| Chemical compound, drug | All-trans retinal | SIGMA-Aldrich | SID 24899355 | |
| Chemical compound, drug | Vectashield | Vector Labs | Cat. No: H-1000 | |
| Chemical compound, drug | Streptavidin | Invitrogen | Catalog number: S32357 | 1:200 |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.37105.021





