Structural plasticity of actin-spectrin membrane skeleton and functional role of actin and spectrin in axon degeneration
Figures
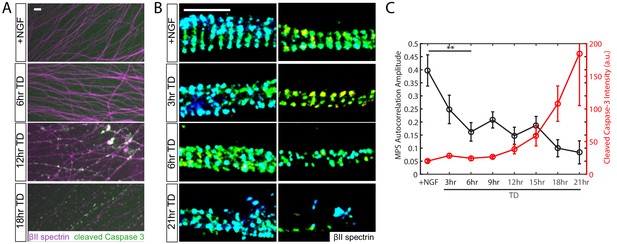
MPS disassembly upon TD precedes observable Caspase-3 activation and axon fragmentation.
Dissociated and re-aggregated wildtype (WT) DRG neurons were cultured for 7 days in the presence of 50 ng/ml NGF and subsequently deprived of NGF (trophic deprivation, TD) for the indicated times. (A) Wide-field images of WT axons fixed after a TD time course and stained with antibodies against βII-spectrin (purple) and cleaved (activated) Caspases-3 (green). Scale bar: 20 µm. (B) Representative 3D STORM images of βII-spectrin in axonal regions during a TD time course. Scale bar: 1 µm. (C) Quantification of the degree of periodicity of βII-spectrin calculated from STORM images, and cleaved Caspase-3 intensity measured by conventional wide-field microscopy. Autocorrelation analysis is applied to each STORM image of βII-spectrin (Figure 1—figure supplement 1) and the autocorrelation amplitude, as defined in Figure 1—figure supplement 1C, provides a quantification of the degree of periodicity. Axons with highly periodic MPS produce larger autocorrelation amplitude values as compared to axons with lower degree of periodicity. The reported value is the average autocorrelation amplitude derived from many axon regions selected at random, and only a few representative examples are shown in (B). Statistics: data are represented as mean ± SEM. Axon number: 19–41 per condition. **p≤0.01. The p value is derived from a two-sided Kolmogorov-Smirnov test. The individual values of MPS autocorrelation amplitudes and cleaved caspase three intensity are listed in Figure 1—source data 1.
-
Figure 1—source data 1
This spreadsheet contains the values of MPS autocorrelation amplitudes and cleaved Caspase-3 intensity used to generate Figure 1C.
- https://doi.org/10.7554/eLife.38730.006
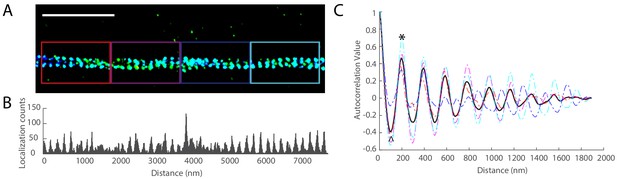
Autocorrelation analysis of the MPS.
(A) The 3D-STORM image of βII-spectrin in an axon region. Scale bar 2 µm. (B) The 1D single-molecule localization distribution of βII-spectrin in the region shown in (A). The bin size is 10 nm. (C) The autocorrelation functions of localization distribution in segmented regions (1900 nm in length for each) of the axon in (A). The color represents the corresponding regions in (A). The black curve represents the average autocorrelation function of the whole axon. The autocorrelation amplitude of an axon is defined as the difference in autocorrelation values between the local maximum around 190 nm (as marked by ‘*') and the local minimum around 95 nm (as marked by ‘^') in the average autocorrelation curve of the axon.
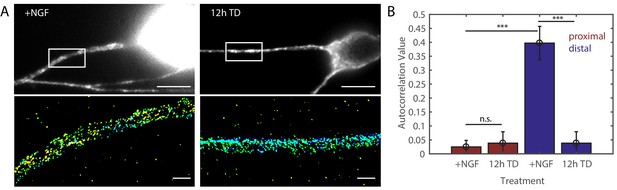
βII-spectrin shows substantially less periodicity in axon regions proximal to the cell body in DRG neurons.
Wildtype (WT) DRG neurons were fully dissociated and cultured homogeneously at lower density (to avoid axon bundling) for 7 days in the presence of 50 ng/ml NGF (+NGF) and subsequently deprived of NGF for 12 hr (12 hr TD). (A) Top: wide-field images of proximal axons fixed after the indicated treatment and stained with antibodies against βII-spectrin. Scale bar: 10 µm. Bottom: representative 3D STORM images of βII-spectrin in axonal regions indicated in the top panel. Scale bar: 1 µm. (B) Quantification of the degree of periodicity of βII-spectrin calculated from STORM images. Statistics: Data are represented as mean ± SEM. Axon number: 6–8 per condition for proximal axons. The data for distal axon regions are reproduced from Figure 1 for comparison. ***p≤0.001. p-Values are derived from a two-sided Kolmogorov-Smirnov test. The individual values of MPS autocorrelation amplitudes in proximal axons are listed in Figure 1—figure supplement 2—source data 1.
-
Figure 1—figure supplement 2—source data 1
This spreadsheet contains the values of MPS autocorrelation amplitudes in proximal axons used to generate Figure 1—figure supplement 2B.
- https://doi.org/10.7554/eLife.38730.005
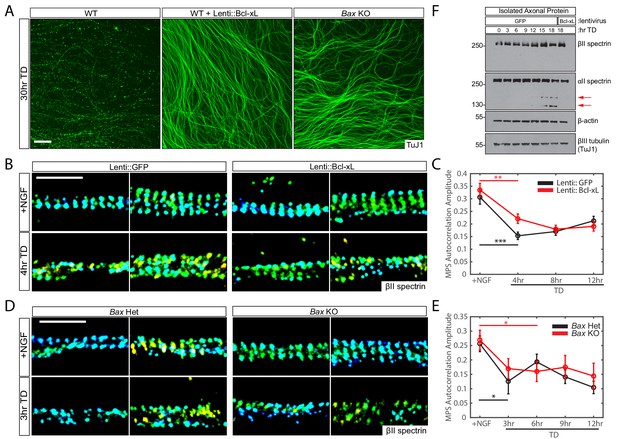
The disassembly of MPS upon TD is independent of apoptotic pathway activation.
(A) Representative images of 7-day in vitro (DIV) DRG cultures from indicated genotypes and treatments (described as below) following 30 hr TD. Axons are visualized with an antibody to βIII Tubulin (TuJ1). Scale bar: 100 µm. (B and C) Dissociated and re-aggregated WT DRG cultures were transduced with lentivirus expressing GFP (B-left panels, C-black curve) or Bcl-xL (B-right panels, C-red curve) one day after plating and deprived of NGF (TD) for varying durations beginning at 8 DIV. Representative 3D STORM images of βII-spectrin in axonal regions in the presence of NGF (top) or at 4 hr of TD (bottom) are shown in (B). Scale bar: 1 µm. βII-spectrin autocorrelation amplitude values during the TD time course are quantified in (C). Statistics: Data are represented as mean ± SEM. Axon number: 66–104 per condition. **p≤0.01; ***p≤0.001. p-Values are derived from a two-sided Kolmogorov-Smirnov test. The individual values of MPS autocorrelation amplitudes are listed in Figure 2—source data 1. (D and E) Dissociated and re-aggregated DRG cultures from Bax heterozygous (Het, D-left panels, E-black curve) or homozygous knockout (KO, D-right panels, E-red curve) embryos were deprived of NGF (TD) for varying durations starting at 7 DIV. Representative 3D STORM images of βII-spectrin in axonal regions in the presence of NGF (top) or at 3 hr of TD (bottom) are shown in (D). Scale bar: 1 µm. Quantification of autocorrelation amplitude values for βII-spectrin during the TD time course is shown in (E). Statistics: Data are represented as mean ± SEM. Axon number: 27–53 per condition. *p≤0.05. p-Values are derived from a two-sided Kolmogorov-Smirnov test. The individual values of MPS autocorrelation amplitudes are listed in Figure 2—source data 1. (F) Protein lysates of isolated axons from 8 DIV DRG cultures expressing the indicated lentiviral constructs (GFP or Bcl-xL) were generated at the indicated time points of TD. Immunoblotting was performed using the indicated antibodies. Red arrows indicate the appearance of degeneration-associated cleavage products of αII spectrin.
-
Figure 2—source data 1
This spreadsheet contains the values of MPS autocorrelation amplitudes used to generate Figure 2C,E.
- https://doi.org/10.7554/eLife.38730.012
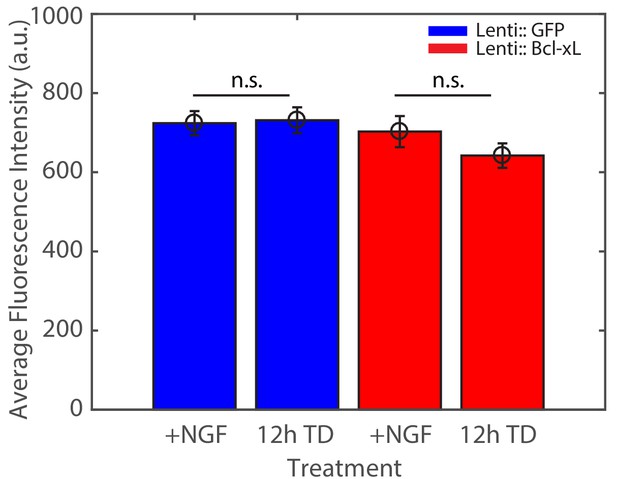
TD does not induce global reduction in axonal βII-spectrin abundance during the first 12 hr of TD.
The axonal abundance of βII-spectrin, measured by the average fluorescence intensity of βII-spectrin immunostaining in axons, remains unchanged during the first 12 hr of TD in both WT cultures and cultures overexpressing Bcl-xL to block axon degeneration. Statistics: Data are represented as mean ± SEM. Axon number: 66–104 per condition. n.s. p>0.05. p-Values are derived from a two-sided Kolmogorov-Smirnov test. The individual values of fluorescence intensities are listed in Figure 2—figure supplement 1—source data 1.
-
Figure 2—figure supplement 1—source data 1
This spreadsheet contains the values of fluorescence intensity used to generate Figure 2—figure supplement 1.
- https://doi.org/10.7554/eLife.38730.009
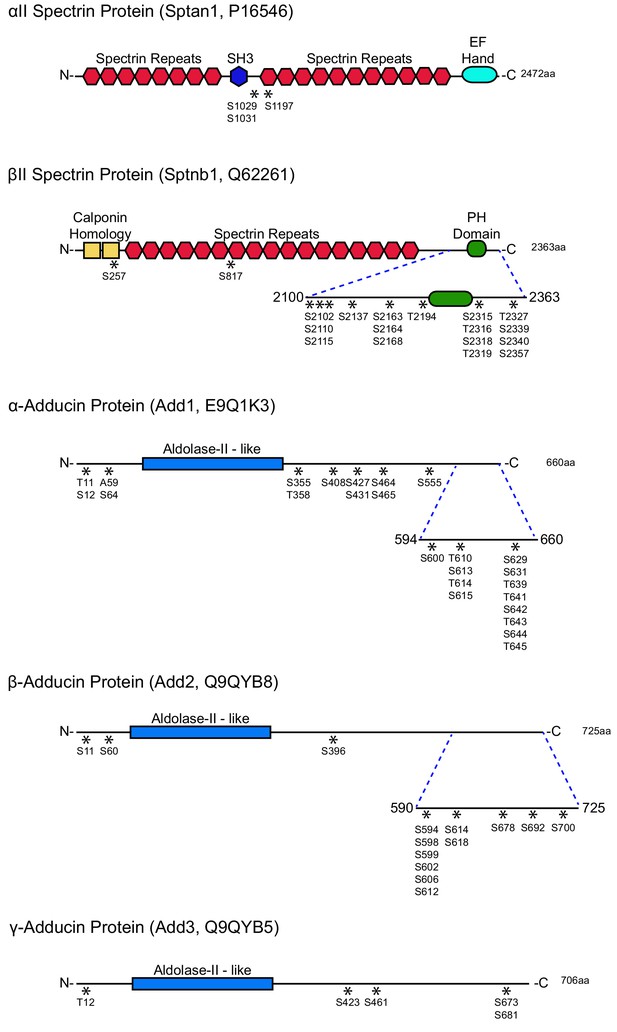
MPS components αII-spectrin, βII-spectrin, and adducin are widely phosphorylated during TD.
As a part of a large-scale, unbiased phosphoproteomic screen in axons undergoing TD, numerous de novo phosphorylations were detected on αII-spectrin, βII-spectrin, α-adducin, β−adducin and γ-adducin proteins, as indicated by the ‘*” symbols. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE (Vizcaíno et al., 2016) partner repository with the dataset identifier PXD009854. Detailed quantifications of phosphorylation sites are listed in Figure 2—figure supplement 2—source data 1.
-
Figure 2—figure supplement 2—source data 1
This spreadsheet contains the information of the phosphorylation sites shown in Figure 2—figure supplement 2.
- https://doi.org/10.7554/eLife.38730.011
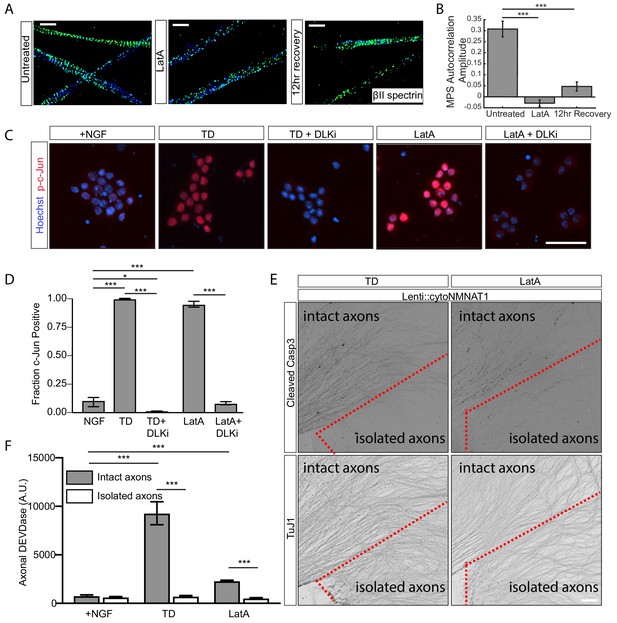
Depolymerization of actin filaments disrupts MPS and induces pro-degenerative signals.
20 µM of latrunculin A (LatA) or vehicle was applied to relevant DRG cultures (7 DIV) for 3 hr in the presence of NGF. (A, B) Representative 3D STORM images of βII-spectrin in axonal regions in untreated (left), LatA-treated (middle), 12-hr recovery (cultured for an extra 12 hr after LatA removal, right) cultures are shown in (A) and quantified in (B). Scale bar: 1 µm. Statistics: Data are represented as mean ± SEM. Axon number: 27–36 per condition. ***p≤0.001. p-Values are derived from a two-sided Kolmogorov-Smirnov test. The individual values of MPS autocorrelation amplitudes are listed in Figure 3—source data 1. (C) 7 DIV DRG cultures were subjected to 6 hr TD or 6 hr treatment with 20 µM LatA, either alone or in the presence of 1 μM DLK inhibitor (DLKi) GNE-3511. Cultures were stained with the DNA dye Hoechst 33342 (blue) and an antibody to phosphorylated c-Jun (Serine 73) (p-c-Jun) (red). Cell bodies are visualized. Scale bar: 50 µm. (D) Quantification of the fraction of phosphorylated c-Jun positive cell bodies in (C). Data are represented as mean ± SEM. N = 3 independent experiments. *p≤0.05, ***p≤0.001, two-way ANOVA with Bonferroni post-test. The individual values of the fraction of phosphorylated c-Jun positive cell bodies are listed in Figure 3—source data 2. (E) Cultures of WT DRGs were established that expressed lentiviral cytoplasmic NMNAT1. A subset of axons were physically severed from their cell bodies (isolated axons, locate at the lower half of each field of view and are encircled with a dotted red line) while the rest of axons (intact axons, upper left of each field of view) remain connected to cell bodies, followed by either 12 hr TD (left) or 12 hr application of 20 µM LatA. Cultures were stained for cleaved Caspase-3 (top) to indicated Caspase-3 activity and βIII tubulin (TuJ1, bottom) to indicate axons. Scale bar: 100 µm. (F) In parallel cultures with (E), at 8 DIV, axons were either severed away from their cell bodies (‘isolated axons’) or left intact (‘intact axons’) and subjected to 16 hr of TD or 16 hr treatment with 20 μM LatA in the presence of NGF. At the end of the assay (16 hr time point), cell bodies were removed from the ‘intact axons,’ leaving all cultures with axons but no cell bodies. At the point, axonal DEVDase activity was measured. Data are represented as mean ± SEM. N = 3 independent experiments. ***p≤0.001, two-way ANOVA with Bonferroni post-test. The individual values of axonal DEVDase activities are listed in Figure 3—source data 3.
-
Figure 3—source data 1
This spreadsheet contains the values of MPS autocorrelation amplitudes used to generate Figure 3B.
- https://doi.org/10.7554/eLife.38730.015
-
Figure 3—source data 2
This spreadsheet contains the values of fraction of p-c-Jun(+) cells used to generate Figure 3D.
- https://doi.org/10.7554/eLife.38730.016
-
Figure 3—source data 3
This spreadsheet contains the values of axonal DEVDase used to generate Figure 3F.
- https://doi.org/10.7554/eLife.38730.017
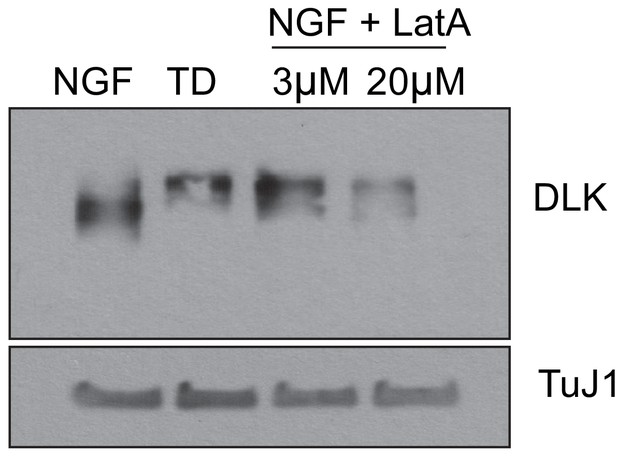
Latrunculin A treatment induces activation of DLK mimicking TD.
WT DRGs were cultured in the presence on NGF. At 7 DIV, cultures were subjected to 12 hr of treatment as indicated. At the 12 hr time point, cell bodies were physically removed from the cultures and axons were directly lysed in the culture dish as previously described (Simon et al., 2016). Activation of DLK occurs as a slight increase in apparent molecular weight on a western blot, indicative of activating phosphorylation, as previously described (Huntwork-Rodriguez et al., 2013). Representative western blot from three independent biological replicates is shown.
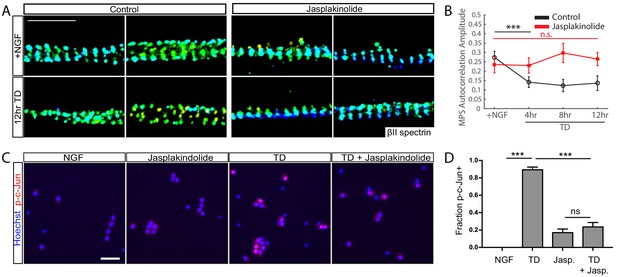
Stabilizing actin filaments preserves MPS and blocks pro-degenerative retrograde signals.
WT DRG cultures (7 DIV) were pretreated with 10 µM Jasplakinolide (Jasp) or vehicle for 30 min, followed by a TD time course in the continued presence of Jasp. (A) Representative 3D STORM images of βII-spectrin in axonal regions in cultures without (left) or with (right) Jasp treatment in the presence of NGF (top) or at 12 hr of TD (bottom). Scale bar: 1 µm. (B) Autocorrelation amplitude values for βII-spectrin in cultures without (black) or with (red) application of Jasp. Statistics: Data are represented as mean ± SEM. Axon number: 39–61 per condition. ***p≤0.001; n.s. p>0.05. p-Values are derived from a two-sided Kolmogorov-Smirnov test. The individual values of MPS autocorrelation amplitudes are listed in Figure 4—source data 1. (C) Immunoreactivity for phosphorylated c-Jun (p-c-Jun) in 7 DIV DRG cultures in the presence of NGF or following an 8 hr TD, without or with 1 µM Jasp pre-incubation for 30 min prior to the assay, as indicated. Scale bar: 50 µm. (D) Quantification of the fraction of phosphorylated c-Jun positive cell bodies. Statistics: Data are represented as mean ± SEM. N = 3 independent experiments,~400 cell neurons per assay. ***p≤0.001. The individual values of the fraction of phosphorylated c-Jun positive cell bodies are listed in Figure 4—source data 2.
-
Figure 4—source data 1
This spreadsheet contains the values of MPS autocorrelation amplitudes used to generate Figure 4B.
- https://doi.org/10.7554/eLife.38730.022
-
Figure 4—source data 2
This spreadsheet contains the values of fraction of p-c-Jun(+) cells used to generate Figure 4D.
- https://doi.org/10.7554/eLife.38730.023
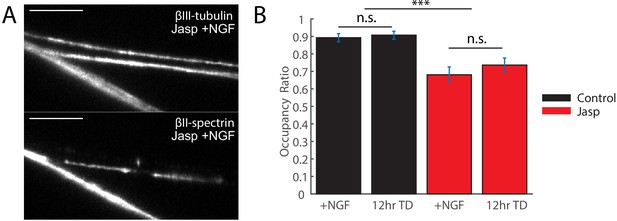
Jasplakinolide treatment stabilizes MPS upon TD but removes MPS from a fraction of DRG axonal segments.
10 µM Jasplakinolide (Jasp) or vehicle (control) was applied to 7 DIV WT DRG cultures 30 min before and during TD or in media containing NGF for the same duration. (A) Conventional images of βIII-tubulin (TuJ1, top) and βII-spectrin (bottom) of axonal regions in a Jasp-treated sample. βII-spectrin is absent in some regions of axons marked by βIII-tubulin. Scale bar: 10 µm. (B) Quantification of occupancy ratio in Jasp-treated (red) and control (black) samples in the presence of NGF or at 12 hr TD. Occupancy ratio of an axon is defined as the ratio of the length along an axon occupied by βII-spectrin. In axons undergoing TD, even though the MPS (a sub-micrometer structure) is disrupted as shown previously and spectrin is released from the lattice structure into a free form, the axonal spectrin amount remains unchanged and thus the occupational ratio remains unchanged. Axons are identified by βIII-tubulin staining. Statistics: Data are represented as mean ± SEM. Axon number: 22–29 per condition. ***p≤0.001; n.s. p>0.05. The individual values of occupancy ratio are listed in Figure 4—figure supplement 1—source data 1.
-
Figure 4—figure supplement 1—source data 1
This spreadsheet contains the values of occupancy ratio used to generate Figure 4—figure supplement 1.
- https://doi.org/10.7554/eLife.38730.020
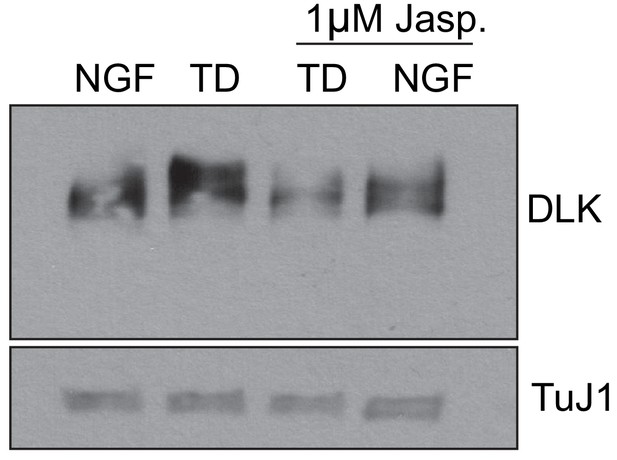
Jasplakinolide treatment occludes the TD-induced activation of axonal DLK.
DRGs were cultured in the presence on NGF. At 7 DIV, cultures were subjected to 12 hr of treatment as indicated. At the 12 hr time point cell bodies were physically removed from the cultures and axons were directly lysed in the culture dish as previously described (Simon et al., 2016). Activation of DLK (i.e. the slight increase in apparent molecular weight) was not observed in the Jasp treated neurons. Representative Western blot from three independent biological replicates is shown.
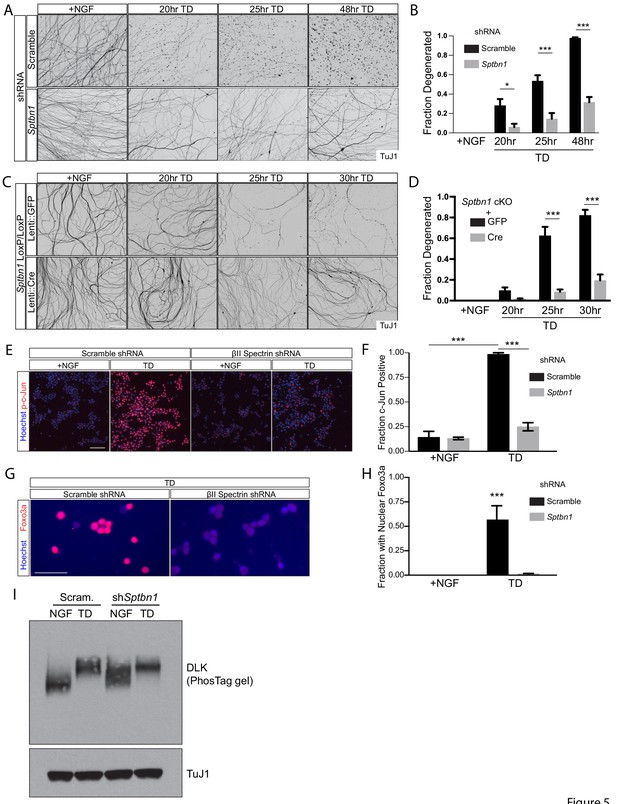
Depletion of the MPS component βII spectrin inhibits retrograde signaling and protects axons from degeneration.
(A, B) Dissociated and re-aggregated WT DRG cultures were infected with AV-shRNA against βII-spectrin (Sptbn1) or scrambled control at 1 DIV and deprived of NGF (TD) for the indicated time points starting at 8 DIV. Axons are visualized with βIII tubulin (TuJ1) immunostaining in (A) and degenerated axons are quantified over time in (B). Scale bar: 100 µm. Statistics: n = 4 independent experiments. *p≤0.05, ***p≤0.001, two-way ANOVA with Bonferroni post-test. The individual values of ratio of axons degenerated are listed in Figure 5—source data 1. (C, D) Dissociated and re-aggregated DRG cultures from the indicated genotype were transduced with lentivirus expressing GFP as a control or lentivirus expressing Cre recombinase to delete the βII-spectrin (Sptbn1) gene from 1 DIV. The cultures were deprived of NGF (TD) for the indicated time points starting at 8 DIV. Axon degeneration was visualized in (C) and quantified over time in (D). Scale bar: 100 µm. Statistics: Data are represented as mean ± SEM. n = 3 independent experiments. ***p≤0.001, two-way ANOVA with Bonferroni post-test. The individual values of ratio of axons degenerated are listed in Figure 5—source data 2. (E) DRG cultures expressing AV-based scrambled shRNA or shRNA against βII-spectrin were subjected to 6 hr TD and stained with an antibody to phosphorylated c-Jun (p-c-Jun). Scale bar: 50 µm. (F) Quantification of the fraction of cells immunoreactive for p-c-Jun from (E). Data are represented as mean ± SEM. n = 3 independent experiments. ***p≤0.001, two-way ANOVA with Bonferroni post-test. The individual values of the fraction of phosphorylated c-Jun-positive cell bodies are listed in Figure 5—source data 3. (G) As in (E) but for cultures stained with antibody to Foxo3a. Nuclear import of Foxo3a is visualized by co-localization with the DNA dye Hoechst 33342. (H) Quantification of the fraction of cells from (G) where Foxo3a immunoreactivity appears to co-localize with Hoechst 33342 staining. Data are represented as mean ± SEM. n = 3 independent experiments. ***p≤0.001, two-way ANOVA with Bonferroni post-test. The individual values of the fraction of cells with nuclear Foxo3a are listed in Figure 5—source data 4. (I) Cultures were infected at 1 DIV with AVs expressing scrambled shRNA or shRNA against βII spectrin (Sptbn1). At 8 DIV, cultures were subjected to TD (12 hr), followed by removal of cell bodies and axon-specific lysis. Samples were subjected to immunoblotting as indicated. The TD-associated shift in DLK molecular weight was visualized using a phos-Tag gel (see Materials and methods). Representative western blot from three independent biological replicates.
-
Figure 5—source data 1
This spreadsheet contains the values of fraction of axons degenerated used to generate Figure 5B.
- https://doi.org/10.7554/eLife.38730.032
-
Figure 5—source data 2
This spreadsheet contains the values of fraction of axons degenerated used to generate Figure 5D.
- https://doi.org/10.7554/eLife.38730.033
-
Figure 5—source data 3
This spreadsheet contains the values of fraction of p-c-Jun(+) cells used to generate Figure 5F.
- https://doi.org/10.7554/eLife.38730.034
-
Figure 5—source data 4
This spreadsheet contains the values of fraction of cells with nuclear Foxo3a used to generate Figure 5H.
- https://doi.org/10.7554/eLife.38730.035
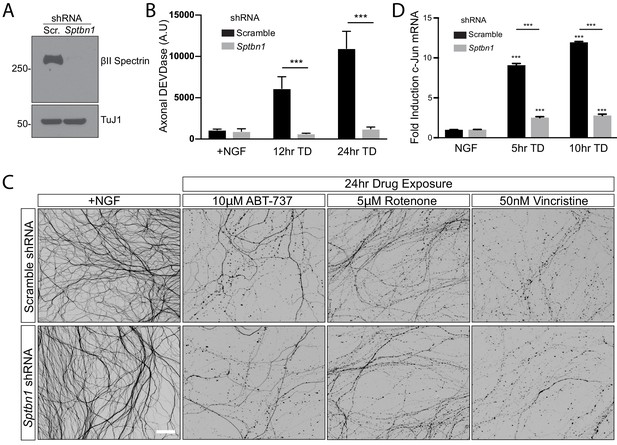
Loss of βII spectrin occludes increase in Caspase-3/7-like enzymatic activities during TD but offers no appreciable protection against apoptosis induced by various drugs.
(A) Knockdown of βII-spectrin was confirmed by immunoblot at 7 DIV in cultures expressing AV-based scrambled shRNA (Scr.) or shRNA against βII-spectrin (Sptbn1). (B) For a time course of TD, cell bodies were physically removed and the remaining axons were lysed in a buffer containing Caspase-3/7 Glo reagent. Cleavage of the preferred Caspase-3 substrate DEVD is reported. Statistics: Data are presented as mean ± SEM. ***p≤0.001, two-way ANOVA with Bonferroni post-test. N = 3 independent experiments. The individual values of axonal DEVDase activities are listed in Figure 5—figure supplement 1—source data 1. (C) 8 DIV WT DRG cultures infected with control shRNA or shRNA against βII-spectrin were subjected to 24 hr exposure to the Bcl-2/Bcl-xL/Bcl-w antagonist ABT-737, the mitochondrial toxin rotenone, or the tubulin depolymerizing chemotherapeutic agent vincristine. No overt axon protection was seen in any of these conditions. Scale bar: 100 µm. (D) 8DIV cultures expressing either scrambled shRNA or βII spectrin (Sptbn1) shRNAs were subjected to TD for the indicated times followed by lysis to harvest total RNA. Induction of c-Jun mRNA was quantified based on real time PCR measurements, compared to the control gene GAPDH. Data are presented as mean ± SEM. ***p≤0.001, two-way ANOVA with Bonferroni post-test. N = 3 independent experiments. The individual values of fold induction c-Jun mRNA are listed in Figure 5—figure supplement 1—source data 2.
-
Figure 5—figure supplement 1—source data 1
This spreadsheet contains the values of axonal DEVDase used to generate Figure 5—figure supplement 1B.
- https://doi.org/10.7554/eLife.38730.026
-
Figure 5—figure supplement 1—source data 2
This spreadsheet contains the values of fold induction c-Jun mRNA used to generate Figure 5—figure supplement 1D.
- https://doi.org/10.7554/eLife.38730.027
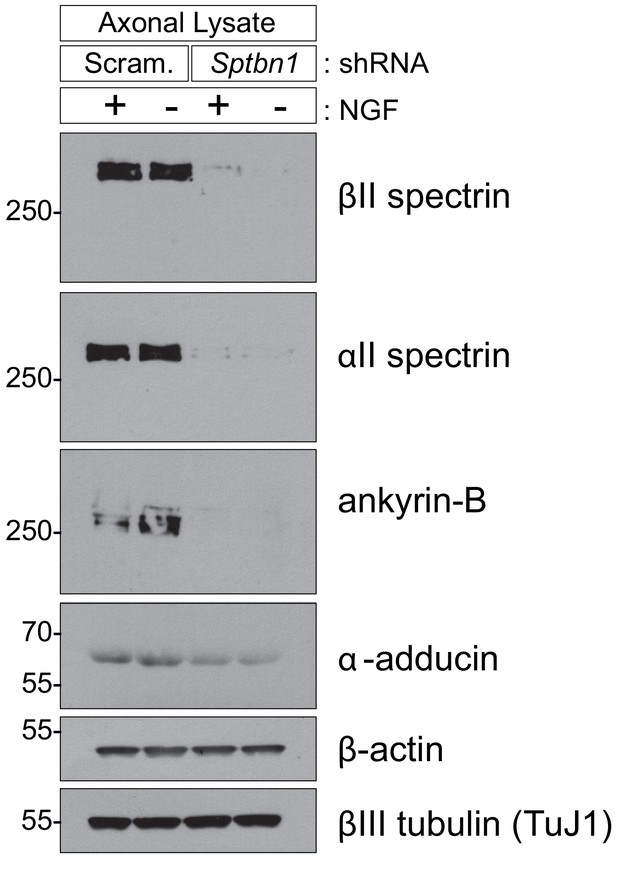
βII spectrin depletion destabilizes some other MPS components.
Axon-specific protein lysates from 8DIV cultures expressing either scrambled (Scram.) or βII spectrin (Sptbn1) shRNAs were subjected to immunoblotting using the indicated antibodies. The axonal abundance of βII spectrin, αII spectrin and ankyrin-B gets greatly depleted upon βII spectrin knockdown and α-adducin gets mildly depleted, whereas the abundance of β-actin and βIII tubulin remains constant. Representative western blot from two independent biological replicates is shown.
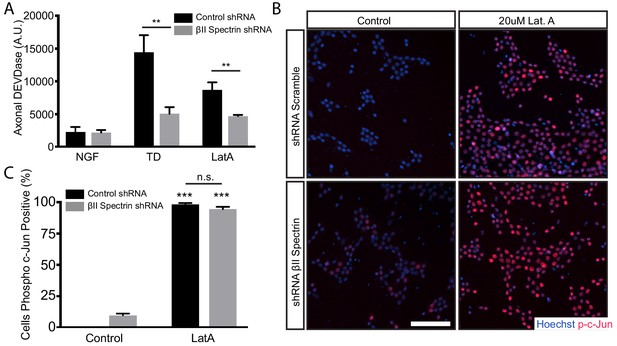
Loss of βII spectrin prevents the LatA-induced appearance of axonal DEVDase activity but not LatA-induced rise in phospho-c-Jun.
(A) Cultures were infected at 1DIV with AVs expressing scrambled shRNA or shRNA against βII spectrin (Sptbn1). At 8DIV cultures were subjected to 16 hr of TD or 16 hr treatment with 20 μM LatA in the presence of NGF. At the end of the assay (16 hr time point), cell bodies were removed and axonal DEVDase activity was measured. Data are represented as mean ± SEM. N = 3 independent experiments. **p≤0.01, n.s. p>0.05, two-way ANOVA with Bonferroni post-test. The individual values of axonal DEVDase activities are listed in Figure 5—figure supplement 3—source data 1. (B) 8 DIV DRG cultures infected with AVs expressing either control (scrambled) or shRNA against βΙΙ Spectrin on 1 DIV were subjected to no treatment (control) or 12 hr treatment with 20 µM LatA. Cultures were stained with the DNA dye Hoechst 33342 (blue) and an antibody to phosphorylated c-Jun (Serine73) (p-c-Jun) (red). Cell bodies are visualized. Scale bar: 100 µm. (C) Quantification of the fraction of phosphorylated c-Jun-positive cell bodies in (B). Data are represented as mean ± SEM. ***p≤0.001; n.s. p>0.05. N = 2 independent experiments. The individual values of the fraction of phosphorylated c-Jun-positive cell bodies are listed in Figure 5—figure supplement 3—source data 2.
-
Figure 5—figure supplement 3—source data 1
This spreadsheet contains the values of axonal DEVDase used to generate Figure 5—figure supplement 3A.
- https://doi.org/10.7554/eLife.38730.030
-
Figure 5—figure supplement 3—source data 2
This spreadsheet contains the values of fraction of p-c-Jun(+) cells used to generate Figure 5—figure supplement 3C.
- https://doi.org/10.7554/eLife.38730.031
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (M. musculus) | Sptbn1-flox | Galiano et al., 2012 | RRID:MGI:5431637 | Dr. Matt Rasband (Baylor College of Medicine) |
| Genetic reagent (M. musculus) | Bax KO | Knudson et al., 1995 | RRID:MGI:1857429 | Dr. Stanley Korsmeyer (via Jackson Labortory) |
| Antibody | mouse monoclonal anti-βII spectrin, clone 42 | Santa Cruz | Cat. # sc-136074; RRID:AB_2194501 | IF (1:100), WB (1:500) |
| Antibody | rabbit anti-Tubulin β 3 | BioLegend | Cat. # 845502; RRID:AB_2566589 | IF (1:2000) |
| Antibody | mouse monoclonal anti-Tubulin β 3 | BioLegend | Cat. # MMS-435P; RRID:AB_2313773 | IF (1:2000), WB (1:1000) |
| Antibody | rabbit anti-cleaved Caspase-3 | Cell Signaling | Cat. # 9661; RRID:AB_2341188 | IF (1:100) |
| Antibody | rabbit monoclonal anti-cleaved Caspase-3 | Cell Signaling | Cat. # 9664; RRID: AB_2070042 | IF (1:100) |
| Antibody | Rabbit monoclonal anti-phosphorylated c-Jun S73 | Cell Signaling | Cat. # 3270; RRID: AB_2129575 | IF (1:500) |
| Antibody | rabbit anti-Foxo3a | Cell Signaling | Cat. # 12829; RRID: AB_2636990 | IF (1:200) |
| Antibody | rabbit polyclonal anti-DLK | Genetex | Cat. # GTX124127 | WB (1:500) |
| Antibody | mouse anti-αII spectrin | BioLegend | Cat. # 803201; RRID: AB_2564660 | WB (1:250) |
| Antibody | rabbit anti-α-adducin | Abcam | Cat. # ab51130; RRID: AB_867519 | WB (1:100) |
| Antibody | mouse anti-Ankyrin B | Neuromab | Cat. # 75–145; RRID: AB_10673095 | WB (1:100) |
| Antibody | rabbit monoclonal anti-β actin | Revmab | Cat. # 31-1013-00; RRID:AB_2716368 | WB (1:3000) |
| Chemical compound, drug | Hoechst 33342 | ThermoFisher Scientific | Cat. # H3570 | IF (1:10,000) |
| Chemical compound, drug | Jasplakinolide | Millipore Sigma | Cat. # 420107 M | Drugs were pre-dissolved in culture medium (sonicate shortly if necessary) for MPS imaging and in DMSO for other assays. |
| Chemical compound, drug | Jasplakinolide | Abcam | Cat. # 141409 | |
| Chemical compound, drug | Latrunculin A | Millipore Sigma | Cat. # L5163 | |
| Chemical compound, drug | Latrunculin A | Abcam | Cat. # 144290 | |
| Chemical compound, drug | GNE-3511 | Millipore Sigma | Cat. # 533168 | |
| Chemical compound, drug | ABT-737 | Selleck Chem | Cat. # S1002 | |
| Commercial assay, kit | Caspase-Glo 3/7 reagent | Promega | Cat. # G8090 | |
| Transfected construct (adenovirus) | βII-spectrin silencing, Gift from Baylor College of Medicine (Matthew Rasband) | Galiano et al., 2012 | ||
| Transfected construct (adenovirus) | Scrambled shRNA with GFP | Vector Biolabs | Cat. # 1122 | |
| Transfected construct (lentivirus) | Lentivirus expressing mouse Bcl-xL cDNA | Simon et al., 2016 | ||
| Transfected construct (lentivirus) | Lentivirus expressing GFP DNA | Simon et al., 2016 | ||
| Transfected construct (lentivirus) | Lentivirus expressing Cre recombinase | Simon et al., 2016 | ||
| Transfected construct (lentivirus) | Lentivirus expressing cytoplasmic NMNAT1 cDNA | Simon et al., 2016 |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.38730.036





