Potentiation of P2RX7 as a host-directed strategy for control of mycobacterial infection
Figures
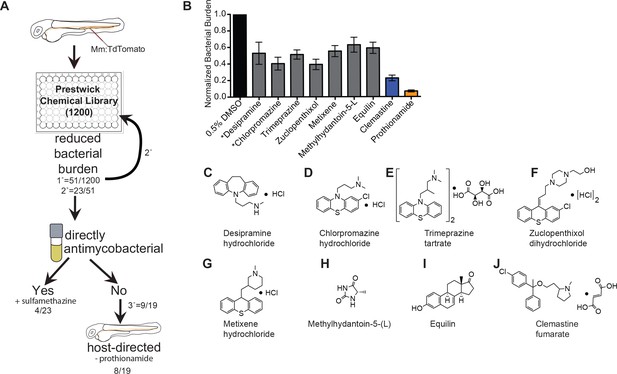
An in vivo zebrafish chemical screen identifies six novel host-directed therapies.
(A) Schematic of chemical screen. 1200 compounds in the Prestwick Library were screened at a final concentration of 5 µM in 0.5% DMSO for substantial reductions in bacterial burden. Three larvae were placed in each well and imaged at 5 days post-infection (dpi). 1° denotes primary screen, 2° secondary, and 3° tertiary screens. Numerator represents number of hits after that screening step over the total number of compounds tested in that step. Mm = Mycobacterium marinum (B) Hits from chemical screen determined to reduce bacterial burden compared to DMSO control. All drug concentrations are 5 µM in 0.5% DMSO. Bacterial burden by fluorescence is normalized to DMSO control for each experiment. Data shown is representative of three experiments with n > 15 animals for each experiment. Error bars are s.d. *represents drugs with previously identified host-directed mechanism (C–J), Chemical structures of drugs identified as reducing bacterial burden in a host-directed manner.
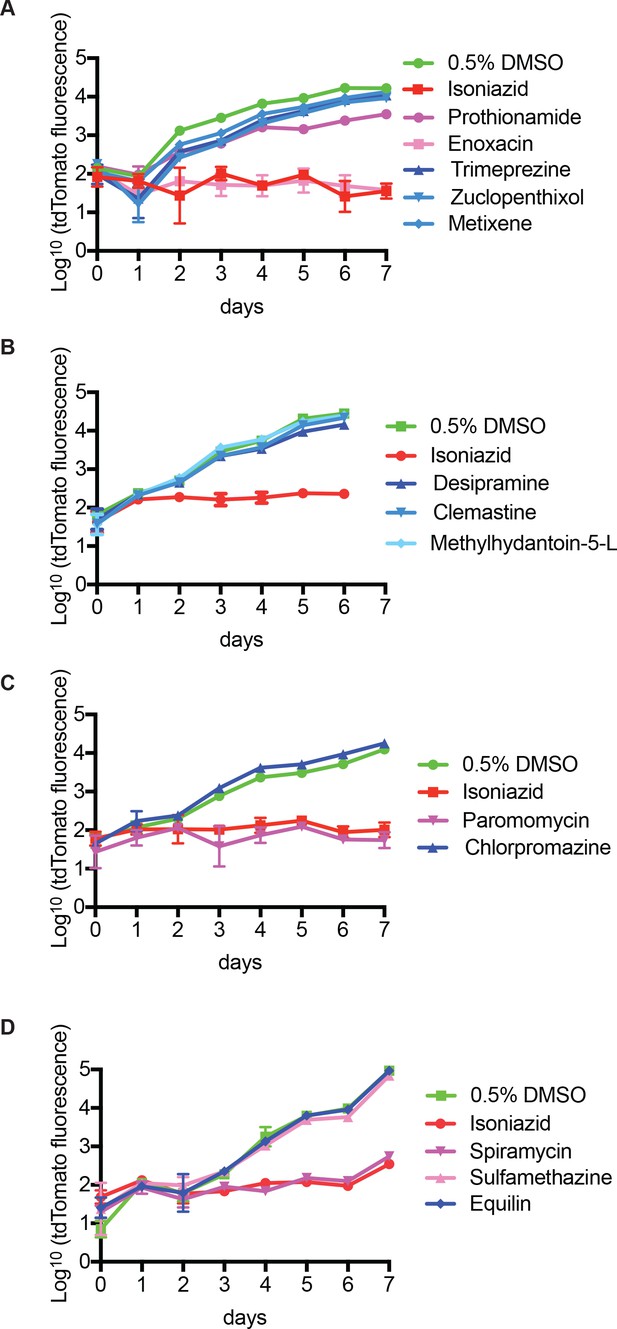
An in vivo zebrafish chemical screen identifies four directly antimycobacterial compounds.
(A–D) Mm:tdTomato bacterial broth culture grown in the presence of compounds identified from the chemical screen (results of 2° screen in Figure 1) at 5 µM in 0.5% DMSO (shades of blue), 0.5% DMSO as a vehicle control (green) or 200 µg/mL isoniazid (red). Previously known antimicrobial agents are in purple and were excluded from further analysis, regardless of their effects on bacterial growth in broth culture.
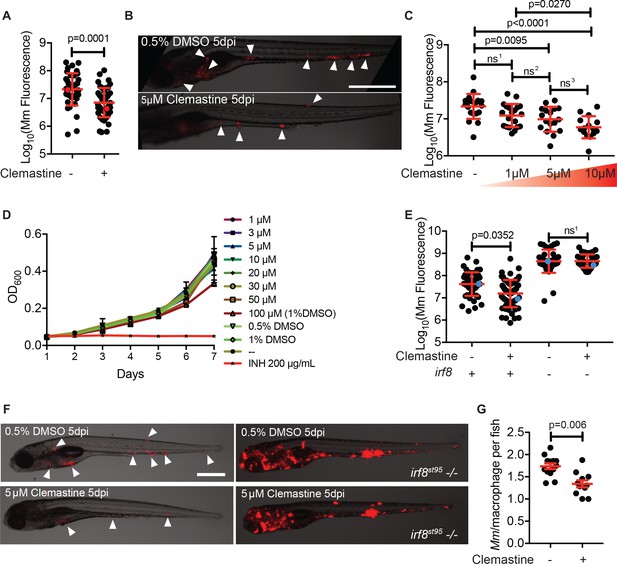
Clemastine activity is host-dependent, dose-responsive, and requires macrophages.
(A) Bacterial burden per animal assessed by Mm:tdTomato fluorescence 5 days post infection (dpi) after treatment with 0.5% DMSO vehicle or 5 µM clemastine. Red dots denote the corresponding image shown in Figure 2B. Representative of five experiments. (B) Representative images from experiment in Figure 2A (red dots). Mm:tdTomato infection treated with 0.5% DMSO vehicle or 5 µM clemastine in 0.5% DMSO. White arrows denote regions of bacterial foci. Scale bar is 500 µm. (C) Quantification of Mm:tdTomato fluorescence at 5 dpi in zebrafish larvae treated with increasing concentrations of clemastine. (D) Mm:tdTomato bacterial broth culture grown in the presence of increasing concentrations of clemastine, compared to 0.5% DMSO, 1% DMSO, no vehicle (-), and 200 µg/mL isoniazid. (E) Bacterial burden of wildtype and heterozygous siblings or irf8st95 mutants, which lack macrophages, treated with 0.5% DMSO or 5 µM clemastine. Blue dots indicate animals in Figure 2F. Data are pooled from two biological replicates. (F) Representative infections from irf8st95 mutants and wildtype/heterozygous siblings from each treatment group in Figure 2E at 5 dpi. Wildtype animals (left) were equally brightened (no change in gamma settings) to show contrast of bacteria and brightfield. The irf8st95-/- example animals (right) were not brightened. (G) Number of bacteria per macrophage during treatment with 0.5% DMSO or 5 µM clemastine, 1 dpi. Each dot represents the mean number of intracellular Mm:mCerulean bacteria inside macrophages of one Tg (mfap4:tdTomato)xt11 animal at 24 hpi infected with ~ 50 CFU. Representative of three independent experiments. (A) Two-tailed, unpaired t-test. (C) Ordinary one-way ANOVA with Tukey’s multiple comparison test, ns1 = 0.0864, ns2 = 0.7799, ns3 = 0.2415. Post-test for linear trend, p<0.0001. (E) Kruskal-Wallis ANOVA for unequal variances with Dunn’s multiple comparisons test, ns1 > 0.9999 (A,C,E) Error bars are s.d. (G) Two-tailed unpaired t-test; error bars are s.e.m. p-Values from statistical tests on untransformed data are provided in Supplementary file 2.
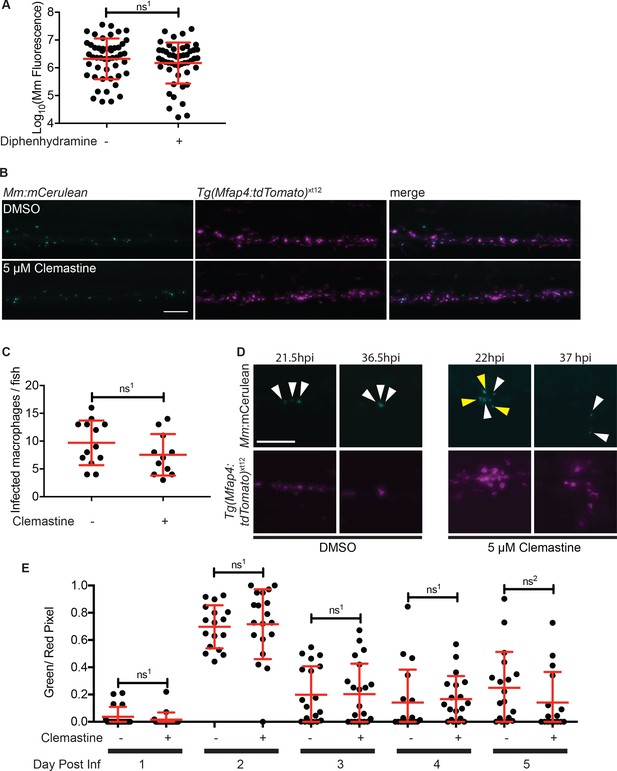
Macrophage function during clemastine treatment.
(A) Bacterial burden per animal assessed by Mm:tdTomato fluorescence 5 days post-infection after treatment with 0.5% DMSO or 5 µM diphenhydramine. (B) Example images from quantification in Figure 2G. Bacteria are Mm:mCerulean and macrophages Tg(mfap4:tdTomato)xt12 are false colored in magenta, maximum intensity projection of 5 z stacks of 10 µm. Scale bar is 100 µm. (C) Number of infected macrophages per fish. Each dot represents the total number of infected macrophages in each animal from experiment in Figure 2G. (D) Stills from Video 1. Scale bar is 100 µm. Maximum intensity projection of 4 z-stacks of 15 µm. White arrows mark bacteria that are present in both initial and final timeframes, and yellow arrows mark individual bacteria that disappear during the timelapse. (E) Ratio of green to red fluorescent bacteria from Mm:aprA’::GFP,smyc::mCherry infection (~ 50 CFU) in wildtype zebrafish larvae over the course of a 5-day infection during treatment with 0.5% DMSO or 5 µM clemastine. (A) Unpaired t-test, ns1 = 0.3147, error bars are s.d. (C) Unpaired t-test, ns1 = 0.1907, error bars are s.d. (E) Unpaired t-tests between treatments for each day, ns1 > 0.9999, ns2 = 0.5505, error bars are s.d. p-Values from all transformed and untransformed data are provided in Supplementary file 3.
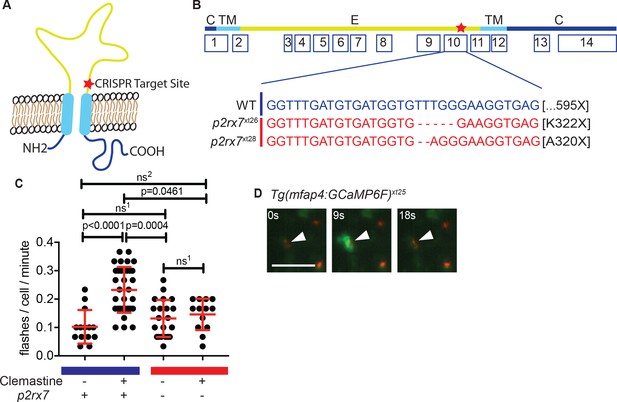
Clemastine increases frequency of macrophage calcium transients in a p2rx7-dependent manner.
(A) Schematic of P2rx7 receptor, based on human structure, with CRISPR target site in exon 10 denoted with a star. (B) CRISPR/Cas9-mediated lesions in p2rx7 include a five base pair deletion (p2rx7xt26) and a two base pair deletion (p2rx7xt28) leading to a premature stop codon in exon 10. Red bar denotes p2rx7 mutants and blue bar denotes WT animals. (C) Quantification of calcium flashes observed with light-sheet microscopy in Tg(mfap4:GCaMP6F)xt25 and p2rx7xt26;Tg(mfap4:GCaMP6F)xt25 infected with ~ 50 CFU Mm:tdTomato. Each dot represents the number of flashes observed in a single infected macrophage in an animal during a 30-min time-course during treatment with 0.5% DMSO or 5 µM clemastine, 4–5 hpi, n > 3 fish for each group. All cells counted were visibly infected with Mm:tdTomato and scoring performed blind to genotype or treatment. (D) Representative calcium transient. Panels are stills from a light-sheet video in an untreated Tg(mfap4:GCaMP6F)xt25 animal infected with Mm:tdTomato. Scale bar is 25 µm. (C) Kruskal-Wallis ANOVA for unequal variances, Dunn’s multiple comparison test. ns1 > 0.9999, ns2 = 0.7007 All error bars are s.d.; p values from statistical tests on untransformed data are provided in Supplementary file 2.
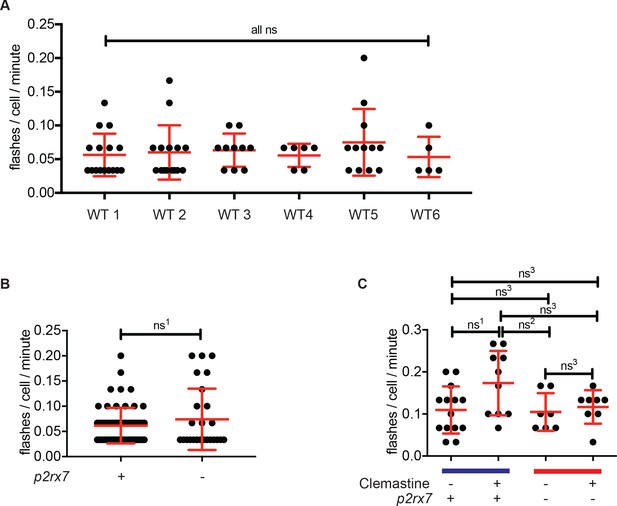
Light-sheet video quantification reveals calcium dynamics in macrophages.
(A) Enumerated calcium transients per cell per minute in six independent, uninfected Tg(mfap4:GCaMP6F)xt25 larvae. (B) Enumerated calcium transients per cell per minute in Tg(mfap4:GCaMP6F)xt25 and p2rx7xt26;Tg(mfap4:GCaMP6F)xt25 untreated, uninfected larvae. Pooled flash counts from >3 animals per group (C) Enumerated calcium transients from uninfected cells from animals infected with Mm:tdTomato (~50 CFU), treated with 5 µM clemastine or 0.5% DMSO in Tg(mfap4:GCaMP6F)xt25 and p2rx7xt26;Tg(mfap4:GCaMP6F)xt25 larvae, pooled from >3 animals per group. The blue line represents wildtype animals and the red line represents p2rx7 mutants. (A) Ordinary one-way ANOVA with Tukey’s multiple comparison test. All error bars are s.d., p values for all comparisons are in Supplementary file 3. (B) Unpaired t-test with Welch’s correction for unequal standard deviations, ns1 = 0.3206. (C) Kruskal-Wallis test for unequal variances, with Dunn’s multiple comparison test, ns1 = 0.2407, ns2 = 0.3357, ns3 > 0.9999. P values from all transformed and untransformed data are provided in Supplementary file 3.
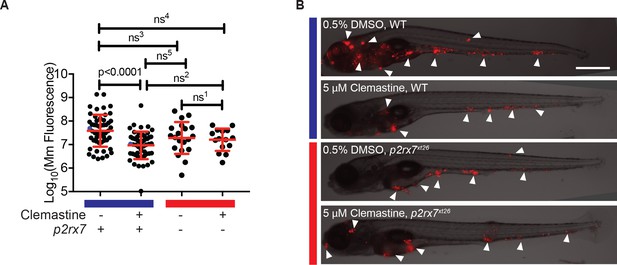
The effect of clemastine on bacterial burden is p2rx7 dependent.
(A) Quantification of Mm:tdTomato fluorescence at 5 days post-infection (dpi) in wildtype and p2rx7xt26 larvae treated with 0.5% DMSO or 5 µM clemastine, infected with ~ 100–150 CFU. Blue bar denotes wildtype larvae and red denotes p2rx7xt26 mutants. Red and blue dots denote the corresponding image shown in (B). Representative of 12 independent experiments, with > 15 fish per group, presented in Figure 4—figure supplement 1A–B. (B) Representative fish from each treatment group in Figure 4A, 5 dpi. Blue bar labels wildtype *AB fish; red bar labels p2rx7xt26. Scale bar is 500 µm. White arrows mark regions of bacterial foci. (A) Ordinary one-way ANOVA, Tukey’s multiple comparison test, ns1 = 0.9862, ns2 = 0.5662, ns3 = 0.2451, ns4 = 0.1652, ns5 = 0.2375. All error bars are s.d.; p values from statistical tests on untransformed data are provided in Supplementary file 2.
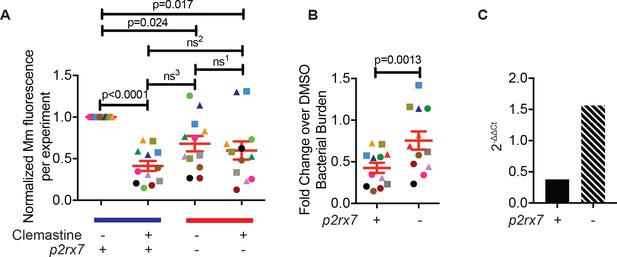
Clemastine requires functional P2rx7 to reduce bacterial burden in vivo.
(A) Combined experiments testing clemastine’s efficacy in wildtype and p2rx7 larvae. Each dot is the average bacterial burden for each treatment group, normalized to the experiment’s wildtype DMSO-treated group. Bacterial burdens per experiment are color-coded (e.g. all light blue squares are from the same experiment). Circles represent experiments performed with the p2rx7xt26 allele, squares represent experiments performed with the p2rx7xt28 allele, and triangles represent experiments performed with transheterozygous p2rx7xt28,xt26 larvae. The blue line represents wildtype animals and the red line represents p2rx7 mutants. Experiment in Figure 4A–B is represented by a gray square. (B) Fold change bacterial burden per experiment assessed by Mm:tdTomato fluorescence 5 dpi after treatment with 0.5% DMSO, 5 µM clemastine in wildtype and p2rx7 mutants. Each genotype is normalized to the DMSO-treated control within the genotype. Each point is color-matched to Figure 4—figure supplement 1A, representing the fold change reduction in bacterial burden within each genotype. (C) 16S rRNA qPCR, represented as the fold change over DMSO normalized within genotype from the experiment represented with green circles in Figure 4—figure supplement 1A–B. (A) Row-matching one-way ANOVA with Geissor-Greenhouse Correction, Tukey’s multiple comparison test. Error bars are s.e.m, ns1 = 0.7854, ns2 = 0.2855, ns3 = 0.0769 (B) Paired t-test, error bars are s.e.m. (C) No statistics performed, represents one biological replicate performed in triplicate.
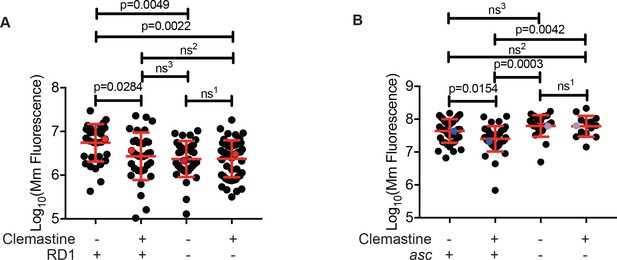
Clemastine efficacy requires cytosolic access and inflammasome signaling.
(A) Quantification of bacterial burden of Mm:Wasabi and MmΔRD1:Wasabi in wildtype larvae treated with 0.5% DMSO or 5 µM clemastine, five dpi. Representative of three independent experiments. Each dot represents an individual animal’s bacterial burden by fluorescence. Red dots denote the animals represented in Figure 5—figure supplement 1A. (B) Quantification of bacterial burden of Mm:tdTomato in pycard (asc) mutants and wildtype/heterozygous siblings after 0.5% DMSO or 5 µM clemastine treatment, 5dpi. Each dot represents an individual animal’s bacterial burden by fluorescence. Representative of three independent experiments. Blue (WT/het) and purple (asc mutants) dots denote representative larvae in Figure 5—figure supplement 2B. Fold change over DMSO for each genotype is presented in Figure 5—figure supplement 2C. (A) Ordinary one-way ANOVA with Tukey’s multiple comparison test. All error bars are s.d. ns1 > 0.9999, ns2 = 0.9452, ns3 = 0.9430. (B) One-way ANOVA with Tukey’s multiple comparison test. All error bars are s.d. ns1 = 0.9998, ns2 = 0.5798, ns3 = 0.3723. p Values from statistical tests on untransformed data are provided in Supplementary file 2.
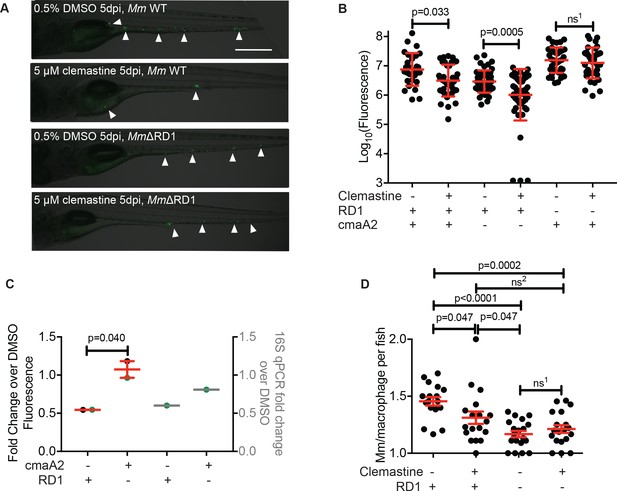
Clemastine does not enhance microbicidal macrophage activities in MmΔRD1 infections.
(A) Representative animals from the red dots in Figure 5A. Animals were infected with Mm:Wasabi (~50 CFU) and Mm:ΔRD1:Wasabi (~ 50 CFU) and treated with 0.5% DMSO or 5 μM clemastine, 5 dpi. Scale bar is 500 μm. (B) Bacterial burden per animal assessed by fluorescence 5 days post-infection after treatment with 0.5% DMSO or 5 µM clemastine. Infections were performed with Mm:tdTomato (RD1+ and cmaA2+), Tn01901:mCerulean (cmaA2-) or Mm:ΔRD1:Wasabi (RD1-). Each dot represents a single animal bacterial burden by fluorescence, represented as fold chance over DMSO in Figure 5—figure supplement 1C. Representative of two independent experiments. (C) Fold change in bacterial burden per experiment assessed by fluorescence of bacteria (left y-axis) or 16S rRNA qPCR (right y-axis, gray error bars). The experiment in Figure 5—figure supplement 1B is represented with green dots. Each bacterial strain is normalized to the DMSO-treated control within each bacterial strain. (D) Number of Mm:Wasabi or Mm:ΔRD1:Wasabi bacteria per macrophage during treatment with 0.5% DMSO or 5 μM clemastine, 1 dpi. Each dot represents the average number of Mm:Wasabi bacteria inside the macrophages of Tg(mfap4:tdTomato)xt12 of a zebrafish larva. Representative of three experiments. (B) Ordinary one-way ANOVA with Sidak’s multiple comparison test within bacterial strains, ns1 = 0.8757. Error bars are s.d. (C) Paired t-test on left y-axis. Error bars are s.e.m. No statistics were performed on the right y-axis, represents one biological replicate performed in triplicate. (D) Ordinary one-way ANOVA with Holm-Sidak’s multiple comparison test, ns1 = 0.4243, ns2 =0.1495. All error bars are s.e.m., p values from all transformed and untransformed data are provided in Supplementary file 3.
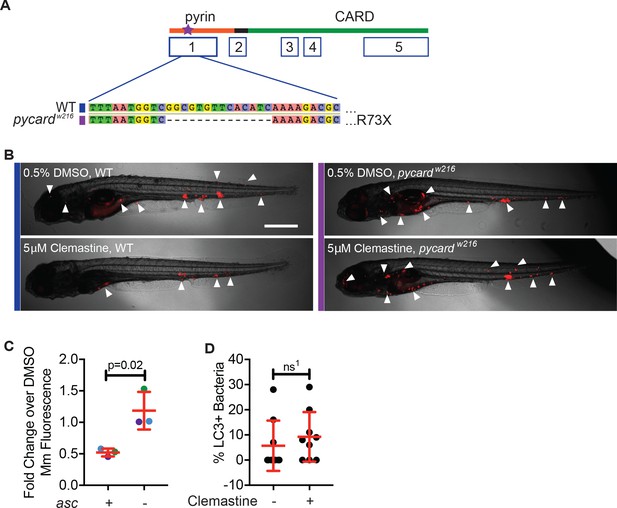
Clemastine requires inflammasome components to reduce bacterial burden.
(A) TALEN-mediated lesions in pycard, the zebrafish orthologue of asc, to generate a 14 base pair deletion in exon 1, known as ascw216. (B) Representative infected animals 5 dpi from purple and blue dots in Figure 5B. Blue bar represents wildtype animals and purple bar represents ascw216 infected with Mm:tdTomato. Scale bar is 500 μm. (C) Fold change in bacterial burden per experiment assessed by fluorescence of bacteria. The experiment in Figure 5B is represented with blue dots. Each genotype is normalized to the DMSO-treated control within that genotype. (D) Percent of bacteria decorated with GFP:LC-3 puncta, 3 dpi. Each dot represents percent of bacteria with GFP:Lc-3 puncta from a single animal. Representative of two experiments. (C) Unpaired t-test, error bars are s.e.m. (D) Unpaired t-test, error bars are s.d., ns1 = 0.4585. p Values from all transformed and untransformed data are provided in Supplementary file 3.
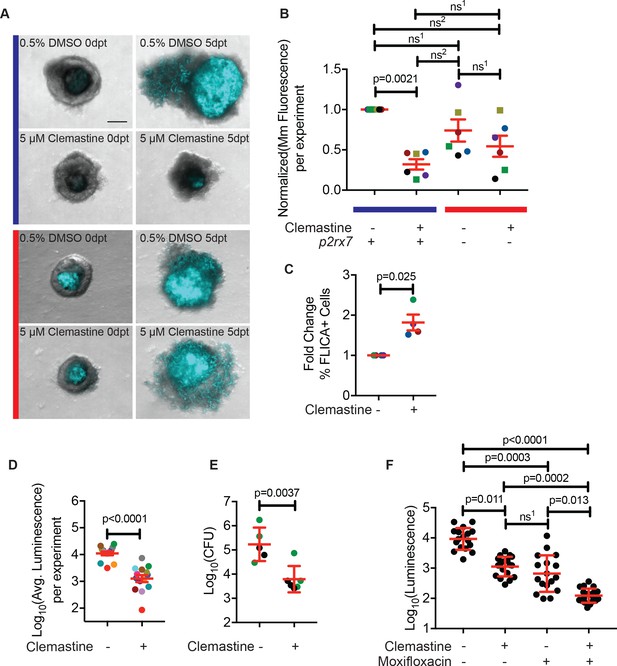
Clemastine reduces bacterial growth in granuloma explants.
(A) Representative granuloma explants treated with 5 µM clemastine or 0.5% DMSO from wildtype or p2rx7xt26 adult animals (2 weeks post-infection) at zero days post treatment (dpt) and 5 dpt. Animals were infected with ~300 CFU Mm:mCerulean. Blue line denotes granulomas from wildtype adult fish and red line denotes granulomas from p2rx7xt26 adult fish. Images are maximum intensity projections of spinning disk confocal images, 15 steps of 10 µm. DIC and Mm:mCerulean channels are merged. Quantification in Figure 6—figure supplement 1A, averages from this experiment are denoted in the green squares of Figure 6B. (B) Quantification of 6 independent experiments in which Mm:mCerluean fluorescence at 5dpt is normalized to the DMSO treatment of wildtype granuloma explants. Each dot is the average bacterial burden at 5 dpt for each treatment group, normalized to the experiment’s wildtype explants treated with DMSO. Bacterial burdens per experiment are color-coded (e.g. red dots are all from the same experiment). Squares represent granulomas from p2rx7xt28 adult infections and circles represent granulomas fromp2rx7xt26 adult infections. The blue line represents wildtype animals and the red line represents p2rx7 mutants. Fold change of bacterial burden over DMSO-treated granulomas for each genotype is in Figure 6—figure supplement 1B using the same colors for each experiment. (C) Quantification of FLICA-positive cells from FACS analysis after treatment with DMSO or 5 µM clemastine 2 dpt. Each dot represents the treatment averages from an independent experiment, normalized to the DMSO treatment for each experiment with at least three biological replicates per experiment. Gating strategy provided in Figure 6—figure supplement 2A and individual experimental averages in Figure 6—figure supplement 2B. (D) Quantification by luminescence of Mm:Lux granulomas 7 dpt after treatment with 0.5% DMSO or 5 µM clemastine, with each dot representing a single experiment’s average luminescence for each treatment. Luminescence values are color-coded between experiments. Granuloma explants used for CFU plating are colored with green dots and represented in Figure 6E as green dots. Fold change of bacterial burden over DMSO-treated granulomas in each treatment is in Figure 6—figure supplement 3A. (E) Quantification of bacterial load by colony-forming units (CFU) 8 dpt with DMSO or 5 µM clemastine. Each dot represents a single granuloma. Fold change of bacterial burden over DMSO-treated granulomas in each treatment is in Figure 6—figure supplement 3A. (F) Quantification by luminescence of Mm:Lux granulomas seven dpt after treatment with 0.5% DMSO, 5 µM clemastine, 2 µg/mL moxifloxacin, or combination clemastine and moxifloxacin. Data are pooled from two experiments. Fold change of bacterial burden over DMSO-treated granulomas in each treatment is in Figure 6—figure supplement 3C. (B) Friedman Test (paired) ANOVA with Dunn’s multiple comparisons test ns1 > 0.9999, ns2 = 0.1521. All error bars are s.e.m. (C) Unpaired t-test, error bars are s.e.m. (D) Paired t-test, error bars are s.e.m. (E) Unpaired t-test, error bars are s.d. (F) Kruskal-Wallis ANOVA with Dunn’s multiple comparisons test, ns1 > 0.9999. All error bars are s.d. p-Values from statistical tests on untransformed data are provided in Supplementary file 2.
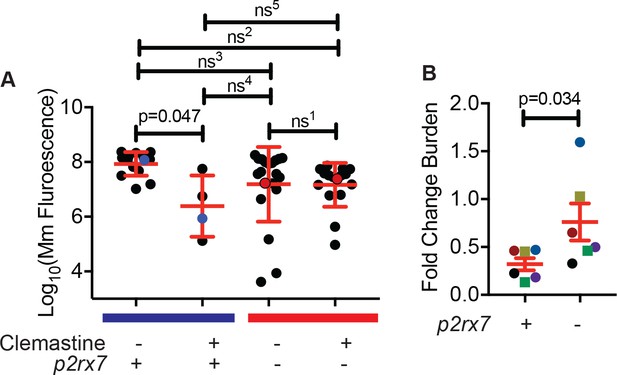
Clemastine reduces bacterial growth in granuloma explants in a P2rx7 dependent manner.
(A) Quantification of Mm:mCerluean fluorescence of each granuloma explant at 5 dpt. This experiment contains the granulomas in Figure 6A, denoted with blue (WT) and red (p2rx7 mutants) dots. (B) Fold change over DMSO for all granuloma explant experiments (Figure 6B), with same color coding. Each point represents fluorescence averages after 5 days of treatment with 5 µM clemastine over the average fluorescence of 0.5% DMSO treatment within each genotype (WT or p2rx7 mutant) for an experiment. (A) Ordinary one-way ANOVA with Tukey’s multiple comparisons test, ns1 > 0.9999, ns2 = 0.1790, ns3 = 0.1635, ns4 = 0.4829, ns5 = 0.5220. All error bars are s.d. (B) Paired t-test, error bars are s.e.m. p-Values from all transformed and untransformed data are provided in Supplementary file 3.
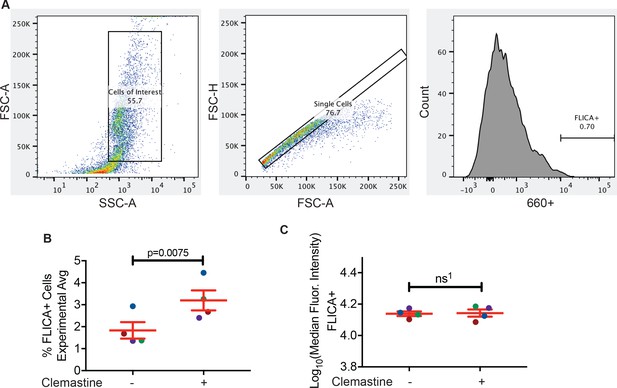
Clemastine enhances activation of caspase-1 in granuloma explants.
(A) Gating strategy for flow-cytometry based FLICA assay. (B) Quantification of FLICA-positive cells from FACS analysis after treatment of granuloma explants with DMSO or 5 µM clemastine 2 dpt. Each dot represents the average from an independent experiment, with at least three biological replicates per experiment. Fold change over DMSO presented in Figure 6C, with matched color-coded experiments (i.e. the dark blue dots are from the same experiment). (C) Quantification of the median fluorescence intensity (MFI) of FLICA-positive cells for each experiment. Each dot represents the averages from an independent experiment, with at least three biological replicates per experiment. Color-coded per experiment, matching Figure 6—figure supplement 2B and Figure 6C. (B) Paired t-test, error bars are s.e.m. (C) Unpaired t-test, error bars are s.e.m., ns1 = 0.8875. p-Values from all transformed and untransformed data are provided in Supplementary file 3.
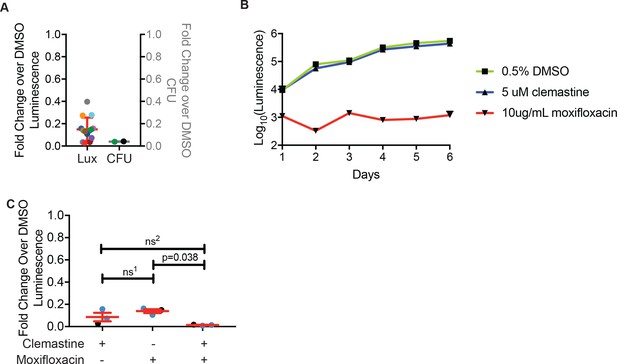
Clemastine reduces bacterial growth in granuloma explants, as measured by luminescence and CFU-based assays.
(A) Fold change over DMSO from granuloma explants in Figure 6D, with same color coding. On the left-y axis, each dot represents the average of luminescence values after 7 days of treatment with 5 µM clemastine over the average luminescence of 0.5% DMSO treatment. The right y-axis represents the fold change in CFU: average CFU from 5 µM clemastine treatment over the average CFU from 0.5% DMSO treatment. Green dots represent the green dots on the left y-axis. (B) Growth curve of Mm:Lux broth culture grown in the presence of 0.5% DMSO, 5 µM clemastine, or 10 µg/mL moxifloxacin for 6 days. (C) Fold change over DMSO during treatment with 5 µM clemastine, 2 µg/mL moxifloxacin, or both. Each dot represents an experimental luminescence average of each treatment divided by the average DMSO-treatment by luminescence. Blue dots represent the data shown in Figure 6F. (A) No statistics performed. See Figure 6D for same data presented as average luminescence per experiment. (C) Repeated measures (paired) ANOVA with Tukey’s multiple comparisons test, ns1 = 0.6479, ns2 = 0.3420.
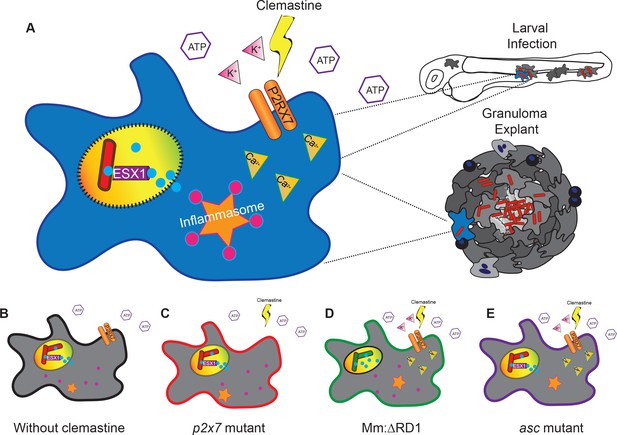
A model describing clemastine as a host-directed drug against mycobacterial infection in both nascent and established infections.
Clemastine potentiates the purinergic channel, P2rx7, to increase calcium transients within infected macrophages. Clemastine enhances microbicidal activities within macrophages to kill intracellular bacteria (A, B) in a manner that requires p2rx7 (C), intact mycobacterial ESX-1 (D), and a functional inflammasome (E).
Videos
Clemastine treatment results in clearing of bacteria by host macrophages.
Split screen video of the caudal hematopoietic tissue of a Tg(mfap4:tdTomato-CAAX)xt6 (false colored magenta) zebrafish larva infected with ~50 CFU of Mm:mCerulean. Left side of the screen is an animal treated with 0.5% DMSO. Right side is an animal treated with 5 µM clemastine. In vivo timelapse is 17–34 hr post infection, with frames every 10 min. Maximum intensity projection of 4 z stacks of 15 μm, 30 frames per second. Stills of single channels are in Figure 2—figure supplement 1D.
Wildtype and p2rx7 mutants exhibit similar calcium transients in uninfected larvae.
Split screen video of Tg(mfap4:GCaMP6F)xt25 (left) and p2rx7xt26;Tg(mfap4:GCaMP6F)xt25 (right) uninfected larvae, 2 days post fertilization (dpf). 30 min light-sheet microscopy timelapse, acquiring every 8.8 s. Maximum intensity projection of 80 steps of 1 μm, 30 frames per second. Flashes are marked with either a circle (WT) or a square frame (mutant). Yellow frames represent cells that only flash once. Other colors (white, green, blue, red, green, cyan) represent cells that flash more than once, with the same cell marked in the same color throughout the timelapse. Only cells that are present during the whole video are marked. Whole cell flashes, not subcellular flickers, are marked.
Clemastine enhances calcium transients in wildtype infected zebrafish larvae.
Split screen video of Tg(mfap4:GCaMP6F)xt25larval zebrafish 2 dpf, infected with ~ 50 CFU. Mm:TdTomato, 4 hr post infection, treated with 0.5% DMSO (left) or 5 µM clemastine (right). 30 min light-sheet microscopy timelapse, acquiring every 8.8 s. Maximum intensity projection of 80 steps of 1 μm, 30 frames per second. Flashes are marked with either a circle or a square frame. Yellow frames represent cells that only flash once. Other colors (white, green, blue, red, green, cyan) represent cells that flash more than once, with the same cell marked in the same color throughout the timelapse. Only cells that are present during the whole video are marked. Whole cell flashes, not subcellular flickers, are marked.
Clemastine does not enhance calcium transients in p2rx7 mutant infected zebrafish larvae.
Split screen video of p2rx7xt26;Tg(mfap4:GCaMP6F)xt25 larval zebrafish two dpf, infected with ~ 50 CFU Mm:TdTomato, 4 hr post infection, treated with 0.5% DMSO (left) or 5 µM clemastine (right). 30 min light-sheet microscopy timelapse, acquiring every 8.8 s. Maximum intensity projection of 80 steps of 1 μm, 30 frames per second. Flashes are marked with either a circle or a square frame. Yellow frames represent cells that only flash once. Other colors (white, green, blue, red, green, cyan) represent cells that flash more than once, with the same cell marked in the same color throughout the timelapse. Only cells that are present during the whole video are marked. Whole cell flashes, not subcellular flickers, are marked.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Mycobacterium marinum + Photorhabdus luminescens) | Lux | Addgene, (Andreu et al., 2010). | ID_Addgene: pMV306hsp | |
| Strain, strain background (Danio rerio) | *AB | https://zfin.org/ZDB-GENO-960809-7 | ||
| Genetic reagent (Danio rerio) | mfap4: GCaMP6Fxt25 | this paper | ||
| Genetic reagent (Danio rerio) | p2rx7xt26 | this paper | ||
| Genetic reagent (Danio rerio) | p2rx7xt28 | this paper | ||
| Genetic reagent (Danio rerio) | ascw216 | this paper | ||
| Genetic reagent (Danio rerio) | mfap4:tdTomato | Walton et al., 2015, https://doi.org/10.1371/journal.pone.0138949 | ||
| Genetic reagent (Mycobacterium marinum) | cmaA2 | this paper | ||
| Genetic reagent (Mycobacterium marinum) | RD1 | Volkman et al., 2004, https://doi.org/10.1371/journal.pbio.0020367 | ||
| Commercial assay or kit | FLICA | ImmunoChemistry Technologies | ID_Immuno Chemistry Technologies:9122 | |
| Chemical compound, drug | Clemastine fumarate | Sigma | ID_Sigma:SML0445 | |
| Software, algorithm other, Chemical Screening Platform | ImageJ | Rueden et al., 2017 | ||
| Other, Chemical Screening Platform | Prestwick Chemical Library | http://www.prestwickchemical.com/libraries-screening-lib-pcl.html |
Additional files
-
Supplementary file 1
Summary of identified compounds.
Compounds with blue background are host directed and compounds in red are known anti-infectives. Asterisk (*) represents compounds with previously identified host-directed anti-mycobacterial activities.
- https://doi.org/10.7554/eLife.39123.022
-
Supplementary file 2
Summary of p values and statistical tests for Figures 1–6.
Statistics performed on transformed data are in yellow and untransformed is in gray. The table is organized by figure number, in chronological order. Statistical tests are listed and transformation equation provided, where applicable. When a paired t-test is performed on paired data, the results of the unpaired t-test are also given. When both paired and unpaired are potentially appropriate (in the case of paired data with ineffective pairing), both test results are given. Statistical test results in italics denote tests not presented within the figures.
- https://doi.org/10.7554/eLife.39123.023
-
Supplementary file 3
Summary of p values and statistical tests for the figure supplements to Figures 1-6.
Statistics performed on transformed data are in orange and untransformed is in light blue. The table is organized by figure number, in chronological order. Statistical tests are listed and transformation equation provided, where applicable. When a paired t-test is performed on paired data, the results of the unpaired t-test are also given. When both paired and unpaired are potentially appropriate (in the case of paired data with ineffective pairing), both test results are given. Statistical test results in italics denote tests not presented within the figure.
- https://doi.org/10.7554/eLife.39123.024
-
Transparent reporting form
- https://doi.org/10.7554/eLife.39123.025





