Environmentally-induced epigenetic conversion of a piRNA cluster
Figures
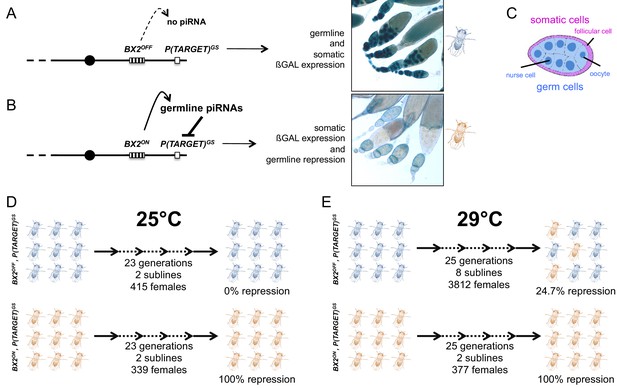
Functional assay of the BX2 epigenetic state.
Females carrying either one of the BX2 epialleles and P(TARGET)GS were analyzed. (A) When BX2 is OFF for production of piRNAs (BX2OFF), no repression of P(TARGET)GS occurs, allowing expression of ß-Galactosidase in both germline and in somatic lineages in ovaries (ß-Galactosidase staining). BX2OFF is illustrated by a blue fly. (B) When BX2 is ON for production of piRNAs (BX2ON), repression of P(TARGET)GS occurs only in the germline lineage. BX2ON is illustrated by a light brown fly. (C) Drawing of an intermediate egg chamber showing germ cells (nurse cells and oocyte in blue) surrounded by somatic follicular cells (in pink), adapted from Figure 1A from Frydman and Spradling (2001). (D) At 25°C, BX2OFF and BX2ON are stable over generations. (E) At 29°C, BX2OFF can be converted into BX2ON, while BX2ON is stable over generations.
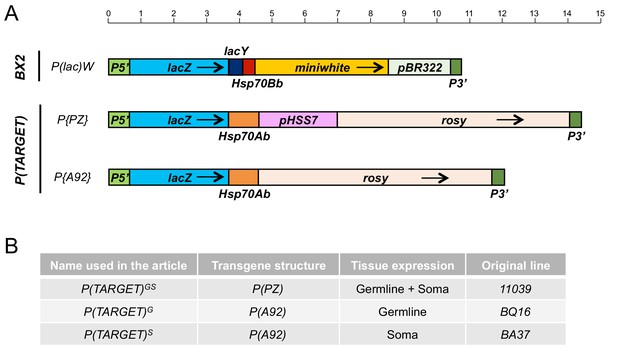
Schematic maps, drawn at scale, of the different transgenes used in this study.
BX2 is a cluster of seven P(lac)W insertion in tandem. P(TARGET) corresponds to euchromatic transgenes used as the reporter. The common transcribed sequence between BX2 and P(TARGET) transgenes is 3547 bp long, from the transcription start at position 87 in P5’ to the end of lacZ sequence. Hsp70 sequences corresponds to transcription termination signal and are different between P(lac)W and P(TARGET) transgenes. A very few small RNAs matched these Hsp70 sequences, and their number is not affected by temperature. Black arrows indicate transcription orientation. Upper scale is in kb.
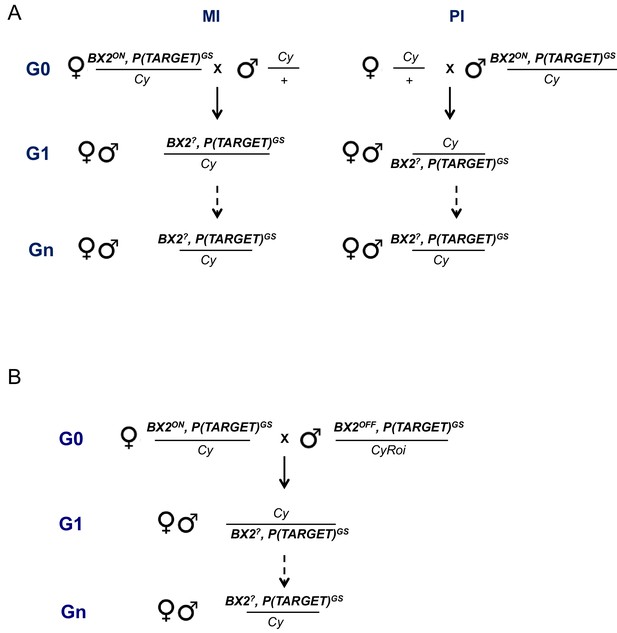
Maternal (A) and paramutagenic (B) effect of BX2ON lines.
(A) Reciprocal crosses were performed at 25°C between BX2ON, P(TARGET)GS flies (either BX2Θ or BX2*) and flies carrying a balancer of the second chromosome (Cy). Lines were established with G1 individuals of maternal or paternal inheritance (named MI or PI, respectively) that carry the BX2, P(TARGET)GS chromosome, associated with white+ phenotype conferred by BX2 transgenes, over Cy and maintained at 25°C. Silencing capacities of these lines were tested over generations by ovarian ß-Galactosidase staining (Supplementary file 3). (B) The capacity of maternally inherited BX2ON piRNAs to activate a BX2OFF cluster was tested as shown on the mating scheme. BX2ON, P(TARGET)GS females (either BX2Θ or BX2*) were crossed at 25°C with BX2OFF, P(TARGET)GS males and lines were established with G1 individuals which maternally inherited piRNAs and paternally inherited the BX2 cluster. Lines were distinguished thanks to two distinct balancers, Cy and CyRoi that are devoid of transgenes and carrying different dominant markers. These lines were maintained at 25°C and their silencing capacities were tested over generations by intra-strain ovarian ß-Galactosidase staining (Supplementary file 4).
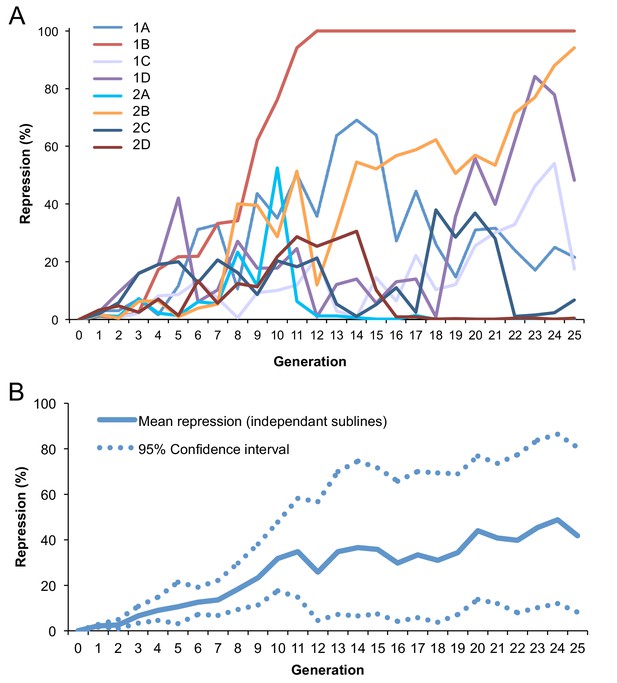
Dynamic of conversion across generations at 29°C.
(A) Fraction of repression across generations for eight independent BX2, P(TARGET)GS lines incubated at 29°C. The fraction of repressed egg chambers was determined at each generation (Supplementary file 6). BX2 conversion occurred in each line tested with various dynamics: one of them appears to be homogeneous and stable from the 12th generation (1B) while others still fluctuate. 2A subline was lost at the 18th generation. (B) Mean repression and 95% confidence interval of repression frequency for the eight independent lines. Frequencies were modified (arcsine square root) prior to calculate means and confidence intervals. Data were then back transformed (sine-squared) for graphical representation.
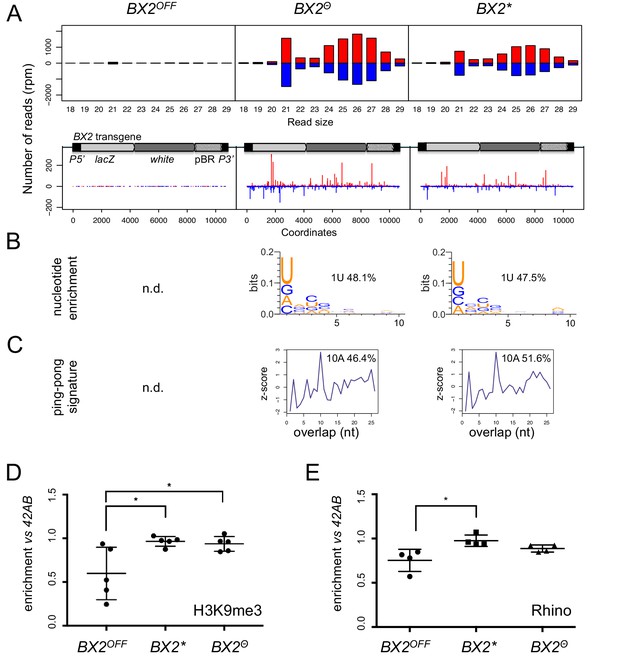
BX2Θ and BX2* produce piRNAs and are enriched in H3K9me3.
(A) Size distribution of ovarian small RNAs matching BX2 transgene sequences reveals that both BX2Θ and BX2* but not BX2OFF produce 21 nt siRNAs and 23–29-nt piRNAs (upper panels, pBR = backbone plasmid pBR322). Positive and negative values correspond to sense (red) and antisense (blue) reads, respectively. Unique 23–29 nt mappers are shown on the BX2 transgene sequences (lower panels). (B) Percentage of 23–29 nt small RNAs from BX2Θ and BX2* matching transgene sequence with a U at the first position are shown. n.d.: not determined due to low number of reads. (C) Relative frequency (z-score) of overlapping sense-antisense 23–29 nt RNA pairs reveals an enrichment of 10 nt overlapping corresponding to the ping-pong signature. (D) H3K9me3 and (E) Rhino binding on the BX2 transgene in ovaries of BX2OFF, BX2Θ and BX2* strains revealed by chromatin immunoprecipitation (ChIP) followed by quantitative PCR (qPCR) on specific white sequences. In both ‘ON’ strains, BX2Θ and BX2*, H3K9me3 and Rhino levels over the transgene are very similar and higher than in the BX2OFF strain (unpaired t-test was used to calculate significance of the differences (p<0.05, n = 5).
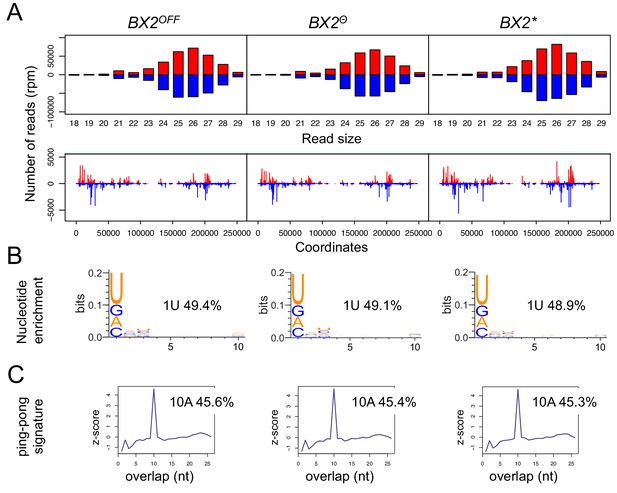
42AB piRNA production is not altered in different BX2 epigenetic contexts.
(A) The size distribution and alignments of unique reads that match the 42AB piRNA cluster show that all genotypes produce similar profile of small RNAs corresponding to 42AB sequence (upper panels). Unique 23–29 nt mappers are shown on the 42AB sequence (lower panels). Positive and negative values correspond to sense (red) and antisense (blue) reads, respectively. (B) Percentage of 1U-containing small RNAs from each genotype that match 42AB sequence. (C) Relative frequency (z-score) of overlapping sense-antisense 23–29 nt RNA pairs reveals an enrichment of 10-nucleotides overlapping small RNAs, which corresponds to the ping-pong signature.
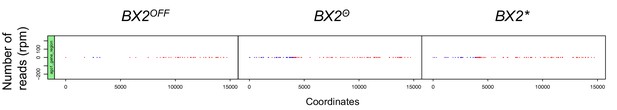
The AGO1 gene region does not produce piRNA by itself.
Alignments of unique 23–29 nt reads that match the AGO1 gene region (from nucleotide 13943387 to 13958089 on the 2R chromosome) show that all genotypes produce no significant levels of piRNAs. Compare this result to that of piRNAs matching BX2 sequences (Figure 2, same y-axis scale). Positive and negative values correspond to sense (red) and antisense (blue) reads, respectively. This observation demonstrates that the AGO1 gene region is not a natural piRNA producing locus.
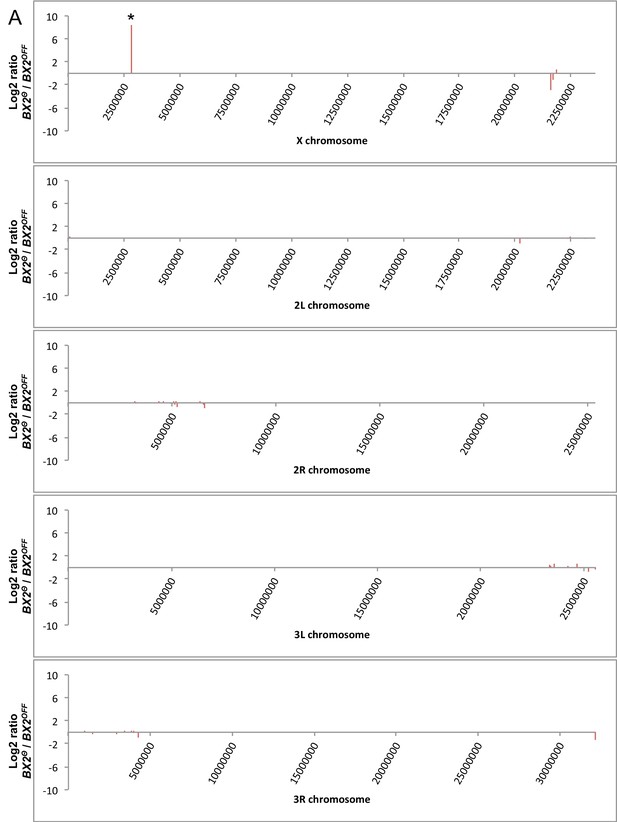
Mapping the genome for regions stably producing new piRNAs.
(A) Unique piRNAs matching Drosophila chromosomes were kept only if they correspond to specific positions in BX2Θ compared to BX2OFF. The number of reads per specific positions was then resampled per windows of 50 kilobases. Background noise was reduced by considering only regions that match more than five reads per kilobase on average on both libraries. Log2 ratio of the number of specific reads from BX2Θ as compared to those from BX2OFF was plotted along chromosomes (50 kb window). Only one region on the X chromosome presents a high increase (log2 ratio >8.5, marked by an asterisk). (B) These small RNAs match exons of the white gene and correspond to piRNAs produced by P(lacW) transgenes corresponding to the BX2Θ locus. No other regions than BX2 stably produce new piRNAs.
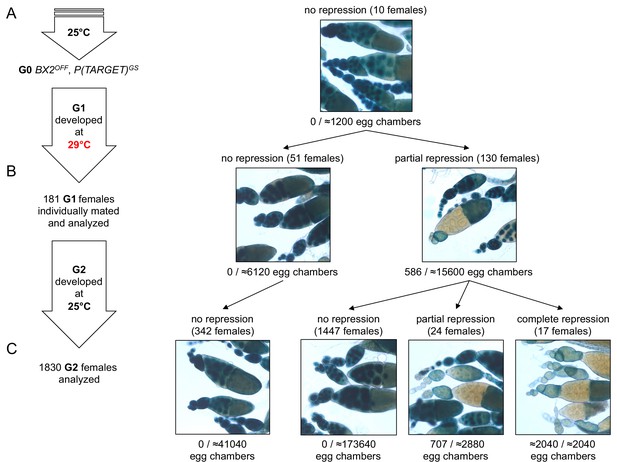
BX2 conversion at 29°C occurs in one generation at a low rate.
(A) G0 females carrying the P(TARGET)GS reporter and BX2OFF laid eggs at 25°C during three days. The BX2OFF state of these females was confirmed after the three days at 25°C by ß-Galactosidase staining (number of egg chambers ≥ 1200). (B) Their eggs were allowed to develop at 29°C until emergence of the next generation. G1 females (n = 181) were individually mated with two siblings and left to lay for three days at 25°C. G1 females were then individually stained for ß-Galactosidase expression. Strikingly, 130 females (71.8%) show ß-Galactosidase repression in some egg chambers (586 among ≈21700 - estimation of the total egg chamber number among 181 females). The BX2OFF into BX2ON conversion frequency is ≈2.7%. (C) Analysis of each G1 female progeny developed at 25°C by ß-Galactosidase staining. The progeny of the 51 G1 females that did not present repression maintained BX2OFF state (n flies = 342). Most of the progeny of the 130 G1 females presenting conversion show no repression (97.2%, n flies = 1488) while 41 females present partial (n = 24) or complete (n = 17) repression of the germline expression of ß-Galactosidase.
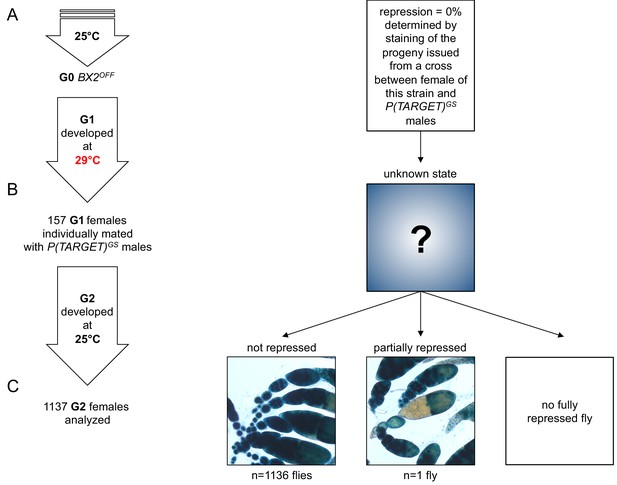
No BX2 conversion at 29°C without the P(TARGET)GS reporter transgene.
This experiment is similar to the one in Figure 3, except that P(TARGET)GS is absent in these flies incubated at 29°C. (A) The BX2OFF state of G0 females was confirmed by staining the progeny of a cross between females from this strain and P(TARGET)GS males. G0 females maintained at 25°C lay eggs at 25°C during three days. (B) Eggs were then transferred at 29°C until emergence of the next generation. 157 G1 females were individually mated with two siblings and left to lay for three days at 25°C. It was not possible to know the epigenetic state of BX2 in these females because of the absence of a reporter transgene. (C) Analysis of the progeny of G1 females reveals that only one female among 1137 presents a partial repression (only one ovariole). Complete repression was never observed.
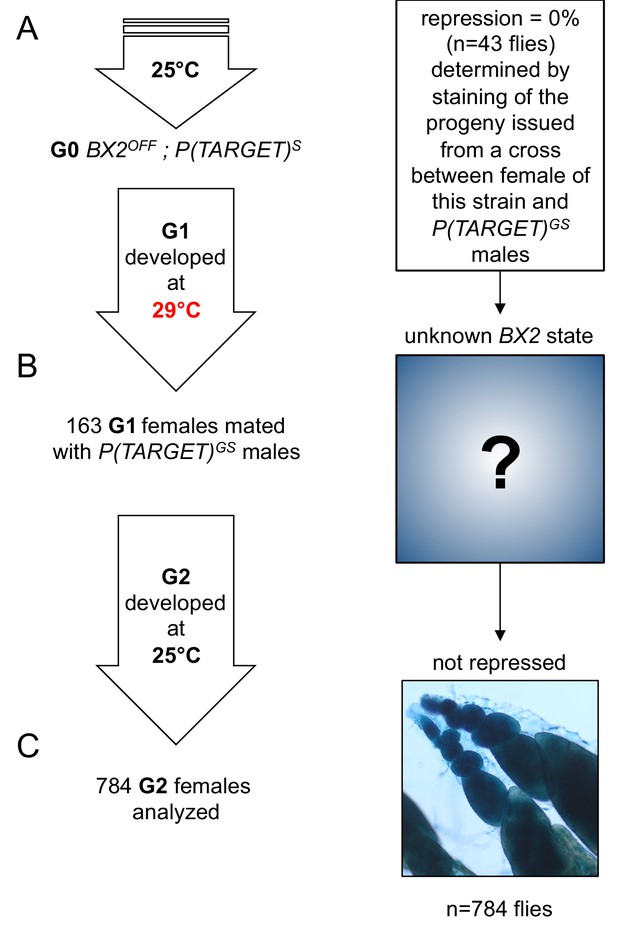
No BX2 conversion at 29°C with a transgene that is not expressed in the germline.
This experiment is similar to the one in Figure 3, except that P(TARGET)GS has been replaced by P(TARGET)S expressed only in the somatic cells surrounding the germ cells. (A) The BX2OFF state of G0 females was confirmed by staining the progeny of a cross between females from this strain and P(TARGET)GS males. G0 females maintained at 25°C lay eggs at 25°C during three days. (B) Eggs were then transferred at 29°C until emergence of the next generation. 163 G1 females were mated en masse with males bearing P(TARGET)GS and left to lay for three days at 25°C. It was not possible to know the epigenetic state of BX2 in these females because of the absence of a germinally-expressed reporter transgene. (C) Analysis of the progeny of G1 females reveals that no repression was observed among 784 analyzed flies (≈94080 estimated egg chambers).
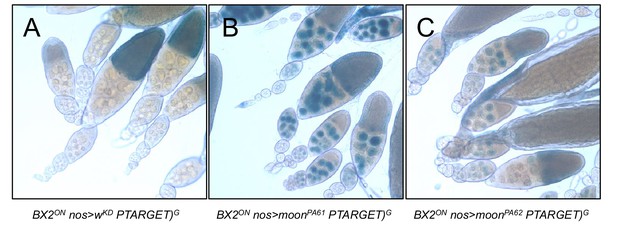
BX2ON silencing depends on moonshiner.
ß-Galactosidase staining of ovaries from females harboring BX2ON, P(TARGET)G, the nosGAL4 driver and a RNAi against the white gene as a control (A) or a RNAi against moonshiner (moonPA61 and moonPA62, B and C, respectively). Both of the moon knockdown lines affect the silencing induces by BX2ON showing that moonshiner is required for the BX2ON state.
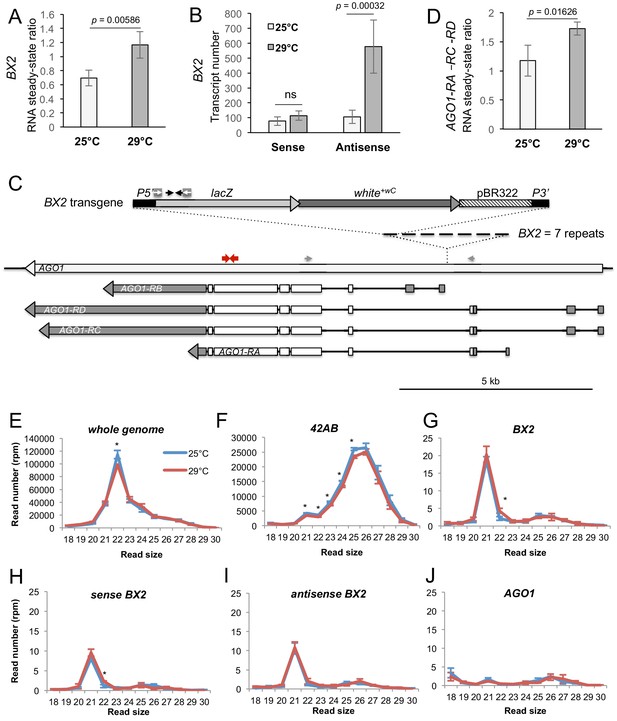
BX2OFF antisense RNA increase at 29°C.
(A) RT-qPCR experiments revealed that the steady-state level of ovarian lacZ RNAs from BX2 is more abundant at 29°C (n = 5) compared to 25°C (n = 6). (B) Sense-specific RT-qPCR experiments revealed that only antisense transcripts from BX2, corresponding to antisense lacZ transcripts, are increased (25°C n = 6, 29°C n = 4). Significant p-values are given (bilateral Student's t-test). ns: not significant. (C) Map of the BX2 locus containing seven P(lacW) transgenes inserted into the AGO1 gene. P(lacW) and AGO1 are drawn to scale. The lacZ gene contained in P(lacW) and AGO1 are transcriptionally in opposite directions. Black arrows show lacZ primers used for (A) and (B) experiments. White arrows show primers used for sense-specific reverse transcription in (B) experiment. Grey arrows show AGO1 primers used for (D) experiment: these primers are specific for AGO1 transcripts (RA, RC and RD) that originate from promoters located upstream the BX2 insertion point and, thus, are potentially convergent to BX2. Red arrows show primers used to measure steady-state of all AGO1 isoforms (see Figure 4—figure supplement 1D). (D) RT-qPCR experiments performed on flies devoid of P(lacW) transgenes (w1118 context) revealed that the steady-state level of ovarian AGO1-RA, -RC and -RD RNA isoforms is more abundant at 29°C (n = 5) compared to 25°C (n = 6). (E–I) To compare small RNAs at 25 versus 29°C, total RNAs were extracted from BX2OFF ovaries dissected from adults incubated at 25°C or 29°C. Three samples were tested for each temperature. Small RNAs from 18 to 30 nt were deep sequenced. For each library, normalization has been performed for 1 million reads matching the Drosophila genome (rpm, Supplementary file 7). Size distributions of unique reads that match reference sequences are given. (E) Small RNAs matching the Drosophila genome present similar profiles in both temperatures except for 22 nt RNAs that are more represented at 25°C. (F) The 21 to 25 nt reads matching the 42AB piRNA cluster that range from 21 to 25 nt are slightly more abundant at 25°C. (G) Strikingly, almost only 21 nt RNAs match BX2 sequence. They are equally distributed among sense (H) and antisense (I) sequences at both temperatures. (J) No small RNAs corresponding to the AGO1 gene can be detected whatever the temperature. *=p < 0.05, bilateral Student's t-test.
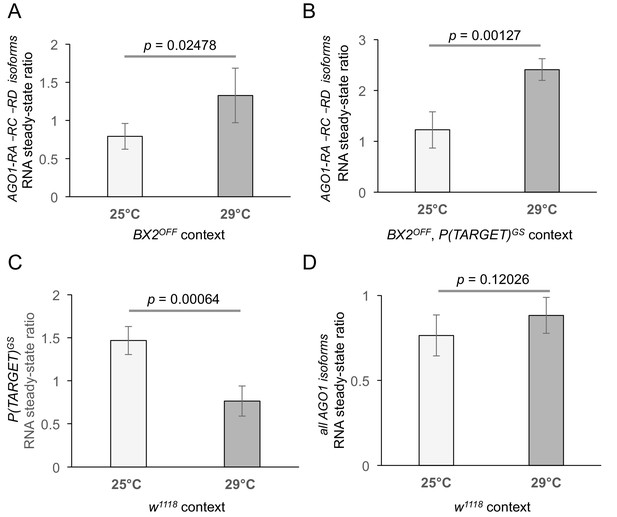
Complementary RNA steady-state measurements by quantitative RT-PCR.
(A, B) Measurement of AGO1 RNA at 25°C and 29°C in different genetic contexts. (A) BX2OFF flies at 25°C (n = 6) and 29°C (n = 4). As in the w1118 background experiment (shown in Figure 4D), the amount of AGO1 transcripts is increased at 29°C. (B) Same as (A) except that RNA was extracted from flies carrying BX2OFF and P(TARGET)GS. Again, the amount of AGO1 transcripts is increased at 29°C (n = 4) compared to 25°C (n = 4). (C) The amount of P(TARGET)GS transcripts is decreased at 29°C compared to 25°C. RT-qPCR experiments performed on total RNA extracted from ovaries of flies containing only P(TARGET)GS transgene in w1118 context revealed that the steady-state level of lacZ RNA from P(TARGET)GS is less abundant at 29°C (n = 6) compared to 25°C (n = 3). (D) Total steady-state AGO1 RNA was measured in w1118 context at 25°C (n = 6) and 29°C (n = 5) using a set of primers located downstream the BX2 insertion point (represented as red arrows on Figure 4C). A slight but non significant difference is observed. p-value are given (bilateral Student's t-test).
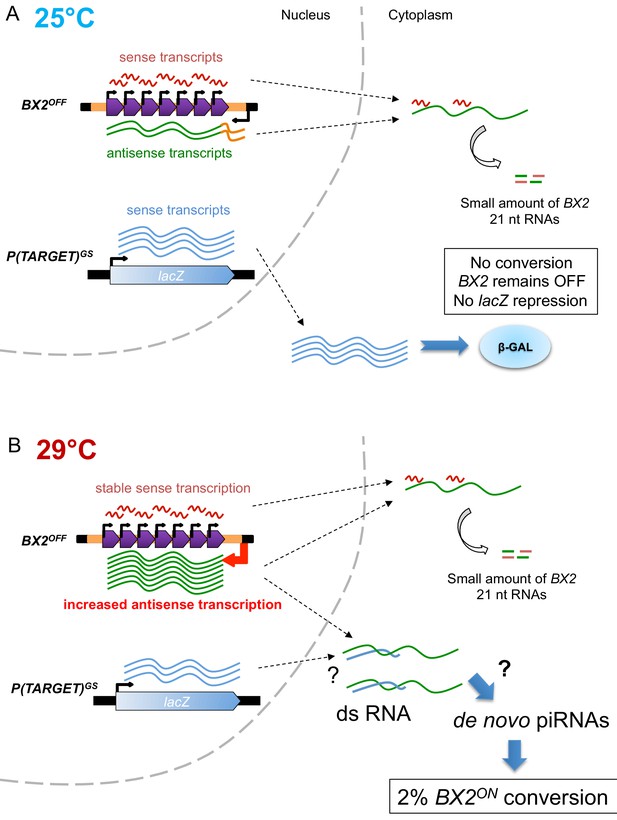
Model of BX2 activation at 29°C.
(A) At 25°C, a low bidirectional transcription of the BX2 cluster leads to a small production of 21 nt RNAs. BX2OFF is stable at 25°C, no conversion event is observed and the lacZ reporter gene from P(TARGET)GS remains active throughout generations. (B) At 29°C, a specific increase in antisense transcription occurs, presumably due to a higher activity of the promoter of the AGO1 gene (orange box). This excess of BX2 antisense RNA could interact with sense P(TARGET)GS transcripts to produce double stranded RNA. Through a yet unknown mechanism, such dsRNA could lead to the formation of de novo BX2 piRNAs. These piRNAs could in turn trigger the conversion of BX2OFF into an active piRNA cluster, a phenomenon observed based on the repression of the lacZ reporter gene of P(TARGET)GS. The BX2 conversion is a rare event (≈2% per generation) but once achieved, BX2ON remains active throughout generations due to the maternal inheritance of homologous piRNAs and the paramutation of the paternal BX2OFF allele.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Drosophila melanogaster) | AGO1 | NA | FLYB: FBgn0262739 | |
| Gene (D. melanogaster) | RpL32 | NA | FLYB: FBgn0002626 | |
| Gene (D. melanogaster) | eEF5 | NA | FLYB: FBgn0285952 | |
| Gene (D. melanogaster) | Moonshiner | NA | FLYB: FBgn0030373 | |
| Strain, strain background (D. melanogaster) | w1118 | Laboratory Stock | FLYB: FBal0018186 | |
| Strain, strain background (D. melanogaster) | BX2 | Dorer and Henikoff, 1994 PMID:8020105 | FLYB: FBti0016766 | |
| Strain, strain background (D. melanogaster) | P(TARGET)GS | Bloomington Drosophila Stock Center | FLYB: FBst0011039 | also called P-1039 |
| Strain, strain background (D. melanogaster) | P(TARGET)G | Bloomington Drosophila Stock Center | FLYB: FBti0003435 | also called BQ16 |
| Strain, strain background (D. melanogaster) | P(TARGET)S | Bloomington Drosophila Stock Center | FLYB: FBti0003418 | also called BA37 |
| Strain, strain background (D. melanogaster) | nosGAL4 | Bloomington Drosophila Stock Center | FLYB: FBti0131635, RRID:BDSC_32180 | |
| Genetic reagent (D. melanogaster) | P(lacW) | PMID: 2558049 | FLYB: FBtp0000204 | |
| Genetic reagent (D. melanogaster) | P(PZ) | Mlodzik and Hiromi, 1992 doi: 10.1016/B978-0-12-185267-2.50030–1 | FLYB: FBtp0000210 | |
| Genetic reagent (D. melanogaster) | P(A92) | PMID: 2827169 | FLYB: FBtp0000154 | |
| Genetic reagent (D. melanogaster) | Moon shRNA PA61 | Andersen et al., 2017 doi:10.1038/nature23482 | Dr. Julius Brennecke (Institute of Molecular Biotechnology, Vienna) | |
| Genetic reagent (D. melanogaster) | Moon shRNA PA62 | Andersen et al., 2017 doi:10.1038/nature23482 | Dr. Julius Brennecke (Institute of Molecular Biotechnology, Vienna) | |
| Antibody | Mouse IgG polyclonal antibody | Merck (ex-Millipore) | Cat# 12-371B, RRID:AB_2617156 | |
| Antibody | Rabbit polyclonal antibody against H3K9me3 | Merck (ex-Millipore) | Cat# 07–442 | |
| Antibody | Rabbit polyclonal antibody against Rhino | PMID: 19732946 | Dr. William Theurkauf (University of Massachusetts Medical School, Worcester) | |
| Sequence-based reagent | RT-qPCR primers | Sigma-Aldrich | ||
| Sequence-based reagent | RT-qPCR primers | Eurogentech | ||
| Commercial assay or kit | RNeasy kit | Qiagen | Cat# 74104 | |
| Commercial assay or kit | Illumina TruSeq Small RNA library preparation kits | Fasteris | http://www.fasteris.com | |
| Commercial assay or kit | Revertaid RT | Thermo Scientific | EP0442 | |
| Commercial assay or kit | Random Hexamers | Invitrogen | N8080127 | |
| Commercial assay or kit | DNaseI (Rnase free) | New Englands Biolabs | M0303S | |
| Commercial assay or kit | dNTPs solution Mix | New Englands Biolabs | N0447S | |
| Commercial assay or kit | Ribolock RNA inhibitor | Thermo Scientific | EO0381 | |
| Commercial assay or kit | Ssofast Evagreen Supermix | Biorad | Cat# 172–5204 | |
| Commercial assay or kit | qPCR kit | Roche | Cat# 04887352001 | |
| Chemical compound, drug | TRIzol | Invitrogen | Cat# 15596026 | |
| Chemical compound, drug | Chloroform | Carlo Erba Reagents | Cat# 438601 | |
| Chemical compound, drug | Chloroform | Sigma-Aldrich | C2432 | |
| Chemical compound, drug | Ethanol (EtOH) | Merck millipore | Cat# 100983 | |
| Chemical compound, drug | Ethanol (EtOH) | Honeywell | Cat# 32221 | |
| Chemical compound, drug | Glycerol | VWR AnalaR NORMAPUR | Cat# 24388.295 | |
| Chemical compound, drug | Glutaraldehyde | Sigma Aldrich | G-5882 | |
| Chemical compound, drug | Potassium hexacyanoferrate(III) | Sigma Aldrich | P3667 | |
| Chemical compound, drug | Potassium hexacyanoferrate(II) trihydrate | Sigma Aldrich | P3289 | |
| Chemical compound, drug | X-Gal | Dutscher | Cat# 895014 | |
| Chemical compound, drug | NaCl | VWR AnalaR NORMAPUR | Cat# 27810.295 | |
| Chemical compound, drug | NaCl | Sigma-Aldrich | Cat# 31432 | |
| Chemical compound, drug | Formaldehyde | Sigma-Aldrich | Cat# 252549 | |
| Chemical compound, drug | Schneider Medium | Gibco | Cat# 21720–024 | |
| Chemical compound, drug | Insulin | Sigma-Aldrich | I4011 | |
| Chemical compound, drug | PBS | Ambion | AM9625 | |
| Chemical compound, drug | Triton | Sigma-Aldrich | T8787 | |
| Chemical compound, drug | KCl | Ambion | AM9640G | |
| Chemical compound, drug | HEPES | Fisher Scientific | BP299 | |
| Chemical compound, drug | IPEGAL | Sigma-Aldrich | Cat# 18896 | |
| Chemical compound, drug | DTT | Fisher Scientific | R0861 | |
| Chemical compound, drug | Na Butyrate | Sigma-Aldrich | Cat# 07596 | |
| Chemical compound, drug | EDTA free protease inhibitor | Roche | Cat# 04693159001 | |
| Chemical compound, drug | N lauryl sarkosyl | Sigma-Aldrich | L-5125 | |
| Chemical compound, drug | BSA | Fisher Scientific | BP9703 | |
| Chemical compound, drug | SDS 20% | Euromedex | EU0660-B | |
| Chemical compound, drug | Tris HCl | Invitrogen | Cat# 15504–020 | |
| Chemical compound, drug | Dynabeads A | Invitrogen | 10002D | |
| Chemical compound, drug | Glycine | Sigma-Aldrich | G8898 | |
| Chemical compound, drug | Isopropanol | VWR | Cat# 20842.298 | |
| Software, algorithm | Galaxy Server | ARTBIO | https://mississippi.snv.jussieu.fr/ | |
| Software, algorithm | Weblogo | Crooks et al., 2004 doi:10.1101/gr.849004 |
Additional files
-
Supplementary file 1
.Silencing capacities of BX2ON and BX2OFF lines throughout generations at 25°C and at 29°C.
BX2OFF and BX2ON are recombined lines carrying the P(TARGET)GS and the BX2 locus transgenes on the same chromosome. Numbers show the fraction of females harboring complete germline repression of P(TARGET)GS at each generation. Complete stability of the initial epigenetic state was observed at 25°C for BX2OFF and BX2ON lines, 0% repression (n = 415) and 100% repression (n = 339), respectively. At 29°C, all BX2OFF lines showed emergence of silencing capacities, 24.7% (n = 3812). BX2ON lines maintained their silencing capacities over generations at 29°C, 100% (n = 377). nt: not tested.
- https://doi.org/10.7554/eLife.39842.017
-
Supplementary file 2
Stability of BX2Θ lines.
Five BX2, P(TARGET)GS lines showing full repression capacities after 23 generations kept at 29°C were transferred at 25°C and tested for their silencing capacities throughout generations. Numbers show females presenting full P(TARGET)GS repression and the total number of tested flies. In all cases, the BX2ON epiallele induced by high temperature remains completely stable during 50 additional generations at 25°C.
- https://doi.org/10.7554/eLife.39842.018
-
Supplementary file 3
Maternal effect of BX2ON lines.
Reciprocal crosses were performed at 25°C between BX2ON, P(TARGET)GS (BX2Θ or BX2*) individuals and flies carrying a balancer of the second chromosome (Cy). For both the maternal and paternal inheritances (named MI and PI, respectively), lines were established and maintained at 25°C by crossing G1 individuals carrying the BX2Θ, P(TARGET)GS chromosome over Cy (Figure 1—figure supplement 2A). Silencing capacities of these lines was tested over generations by intra-strain ovarian ß-Galactosidase staining. Numbers represent the fraction of females showing complete repression of P(TARGET)GS. In all cases, maternal transmission of the BX2Θ cluster results in progeny showing complete repression capacities which are stable over generations whereas paternal transmission of the BX2Θ cluster results in the definitive loss of BX2 silencing capacities similarly to the BX2* epigenetic state.
- https://doi.org/10.7554/eLife.39842.019
-
Supplementary file 4
Paramutagenic effect of BX2ON lines.
The capacity of the cytoplasm of BX2ON females to activate a BX2OFF cluster was tested as shown in the mating scheme. BX2ON females (either BX2Θ or BX2*) were crossed with BX2OFF males, incubated at 25°C. Lines were established with G1 individuals, which have maternally inherited piRNAs and paternally inheritance of the BX2 cluster (Figure 1—figure supplement 2B). These lines were maintained at 25°C and their silencing capacities were tested over generations by crossing females with P(TARGET)GS males. Numbers represent the fraction of females showing complete repression of P(TARGET)GS. All of the derived lines showed complete silencing capacities over generations, revealing that the cytoplasm of BX2ON females, either BX2Θ or BX2*, can fully activate a BX2OFF cluster.
- https://doi.org/10.7554/eLife.39842.020
-
Supplementary file 5
Annotation of small RNA libraries.
Small RNAs were prepared from ovaries of females of the indicated genotype. Values for the different categories of sequences are the total number of sequence reads that matched reference libraries. For comparisons, libraries were normalized (normalization factor) to 1 million miRNA (miRNA rpm) or to 1 million Dmel reads (Dmel rpm).
- https://doi.org/10.7554/eLife.39842.021
-
Supplementary file 6
Silencing capacities of BX2ON and BX2OFF lines across generations cultured at 25°C and at 29°C.
Same as Supplementary file 1 except that egg chambers were monitored for P(TARGET)GS repression instead of whole ovaries. Numbers show the fraction of repressed egg chamber per generation.
- https://doi.org/10.7554/eLife.39842.022
-
Supplementary file 7
Annotation of small RNA libraries from BX2OFF raised at 25°C or 29°C.
Small RNAs were prepared from ovaries of females of the indicated genotype. Values for the different categories of sequences are the total number of sequence reads that matched reference libraries. For comparisons, libraries were normalized (normalization factor) to 1 million miRNA (miRNA rpm) or to 1 million Dmel reads (Dmel rpm).
- https://doi.org/10.7554/eLife.39842.023
-
Supplementary file 8
P(TARGET)GS requirement in the BX2 conversion process.
Comparison of the conversion frequency in one generation between BX2, P(TARGET)GS (Figure 3) and BX2 (Figure 3—figure supplement 1) genotypes. The difference between the presence and absence of the P(TARGET)GS transgene is highly significant (p=8.5×10−6, homogeneity χ2 = 23.35 with 2 degrees of freedom).
- https://doi.org/10.7554/eLife.39842.024
-
Supplementary file 9
Silencing capacities of BX2OFF lines recombined in a P(TARGET)GS background throughout generations developed at 29°C.
BX2OFF was initially recombined with a line carrying the P(TARGET)GS transgene to obtain the BX2OFF, P(TARGET)GS lines. From these crosses, eight independent recombinants without the P(TARGET)GS transgene were recovered and were further cultured at 29°C. To test if some of them acquired silencing capacities, females were crossed with males harboring the P(TARGET)GS transgene and their progeny was stained for ß-Galactosidase expression. Numbers show the fraction of females harboring complete germline repression of P(TARGET)GS at each generation. A complete stability of the initial epigenetic OFF state was observed for all recombinant lines.
- https://doi.org/10.7554/eLife.39842.025
-
Supplementary file 10
Silencing capacities of BX2OFF; P(TARGET)G lines throughout generations at 25°C and at 29°C.
Numbers show the fraction of females harboring complete germline repression of P(TARGET)G at each generation. Complete stability of the initial epigenetic state was observed at 25°C for BX2OFF, 0% repression (n = 189). At 29°C, BX2OFF; P(TARGET)G lines showed emergence of silencing capacities, 19.79% (n = 6766).
- https://doi.org/10.7554/eLife.39842.026
-
Supplementary file 11
Heat shock and saline stresses do not induce conversion of BX2OFF.
BX2OFF, P(TARGET)GS flies were raised during one generation either on classical cornmeal medium (control) at 25°C or were heat shocked for 1 hr at 37°C at the 0–2 hr embryo stage or were cultured on medium supplemented with 150 mM NaCl. The female progeny were then stained for ß-Galactosidase expression and egg chambers were individually monitored in order to detect any possible conversion event. No repressed egg chambers were observed (0/total number of egg chambers). Compared to results obtained at 29°C (from data observed in G1 in Figure 3), differences are significant: for heat shock experiment, p=7.2×10−25, homogeneity χ2 = 106.03 with 2 degrees of freedom and for NaCl experiment, p=4.9×10−27, homogeneity χ2 = 115.93 with 2 degrees of freedom.
- https://doi.org/10.7554/eLife.39842.027
-
Transparent reporting form
- https://doi.org/10.7554/eLife.39842.028





