FGF2-FGFR1 signaling regulates release of Leukemia-Protective exosomes from bone marrow stromal cells
Figures
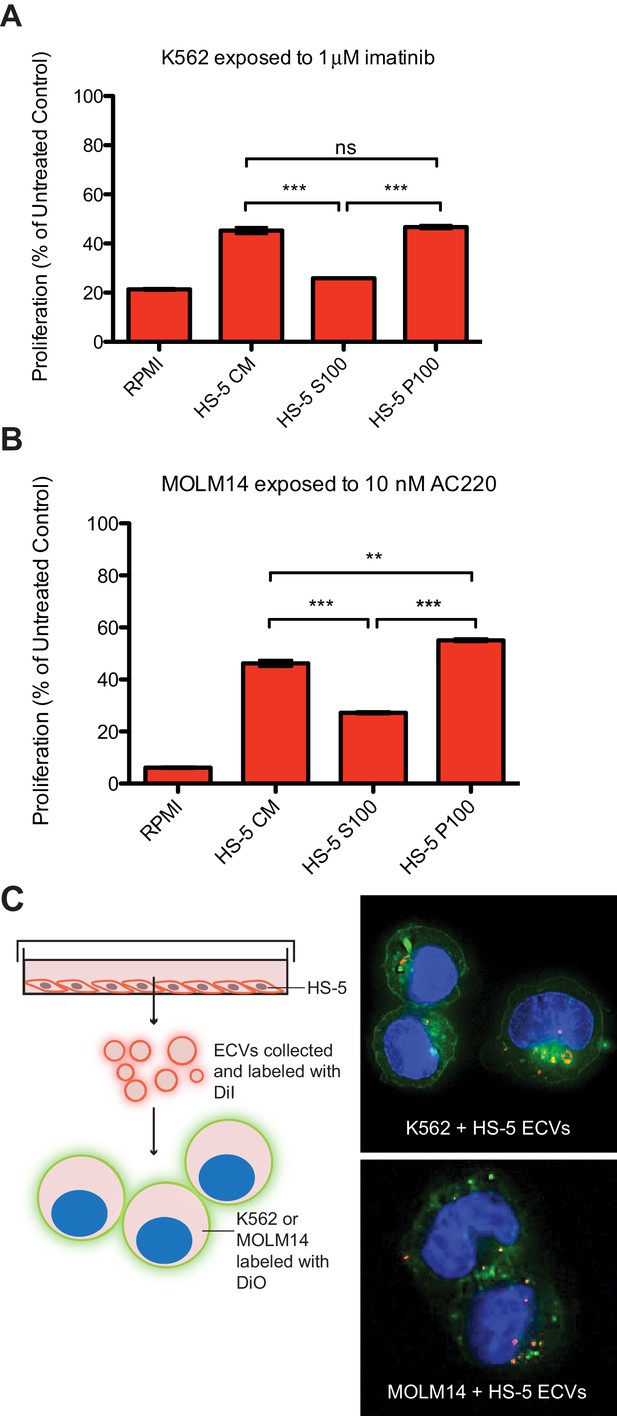
Extracellular vesicles (ECVs) secreted by HS-5 cells are internalized by MOLM14 and K562 cells and protect from treatment with AC220 or imatinib, respectively.
HS-5 conditioned media (CM) was collected and separated by ultracentrifugation at 100,000 g into a supernatant (S100) and pellet (P100) fraction containing ECVs. These fractions were incubated with (A) K562 cells ± 1 μM imatinib, or (B) MOLM14 cells ± 10 nM AC220, and viability measured by MTS assay after 48 hr. Values were normalized to respective untreated condition. All wells were plated in triplicate and error bars indicate standard deviation. RPMI is the media control. p values are indicated by *<0.05, **<0.005, and ***=0.0007. (C), MOLM14 and K562 cells were stained with DiO (green) tracer, washed, and immobilized on Poly-D-Lysine coated chamber slides. HS-5 P100 fraction was stained with DiI (red) tracer and added to the cells for a 24 hr incubation. Slides were stained with DAPI (blue) and imaged by confocal fluorescent microscopy. A movie of the z-stack images is included in Supplemental data.
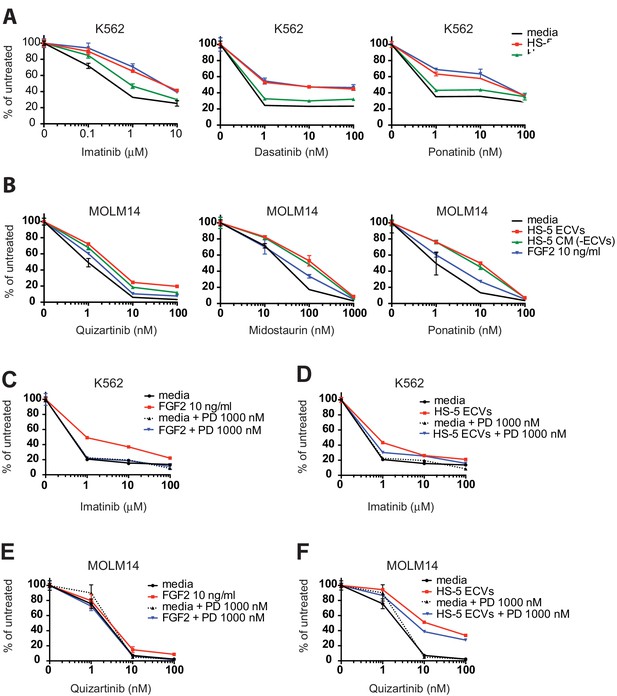
Comparison of protection from recombinant FGF2, HS-5 ECVs, and CM after ECV depletion (-ECV) in both K562 and MOLM14 cells.
HS-5 conditioned media (CM) was collected and separated by ultracentrifugation at 100,000 g into ECVs and CM without ECVs (-ECV). These fractions and RPMI media ± 10 ng/ml recombinant FGF2 were incubated with (A) K562 cells, or (B) MOLM14 cells with the concentrations of inhibitors as shown, and viability measured by MTS assay after 48 hr. Values were normalized to respective untreated condition. Ponatinib inhibits FGFRs around 100 nM and midostaurin inhibits FGFRs around 200 nM, and this activity blocks the protection of recombinant FGF2 completely (consistent with our previous work) and also blocks a portion of the protection of ECVs, which contain FGF2 as well as other proteins. K562 cells were incubated with media, (C) recombinant FGF2 10 ng/ml or (D) HS-5 ECVs and then treated with the indicated inhibitors ± 1000 nM PD173074 (PD). MOLM14 cells were incubated with media, (E) recombinant FGF2 10 ng/ml or (F) HS-5 ECVs and then treated with the indicated inhibitors ± 1000 nM PD173074 (PD). Viability was measured by MTS assay after 48 hr and normalized to respective untreated condition. In both cases, addition of PD has no effect with just media, but blocks protection by FGF2. PD also partially blocks protection by HS-5 ECVs, which contain FGF2. Wells were plated in triplicate and error bars indicate standard deviation.
HS-5 ECVs were stained with DiI (red) and K562 cells were stained with DiO (green) and incubated for 24 hr at 37°C as described in Materials and methods.
Cells were washed, placed on Poly-D-Lysine coated chamber slides, and DAPI-stained. Z-stack imaging was performed on an Olympus IX71 inverted microscope.
HS-5 ECVs were stained with DiI (red) and MOLM14 cells were stained with DiO (green) and incubated for 24 hr at 37°C as described in Materials and methods.
Cells were washed, placed on Poly-D-Lysine coated chamber slides, and DAPI-stained. Z-stack imaging was performed on an Olympus IX71 inverted microscope.
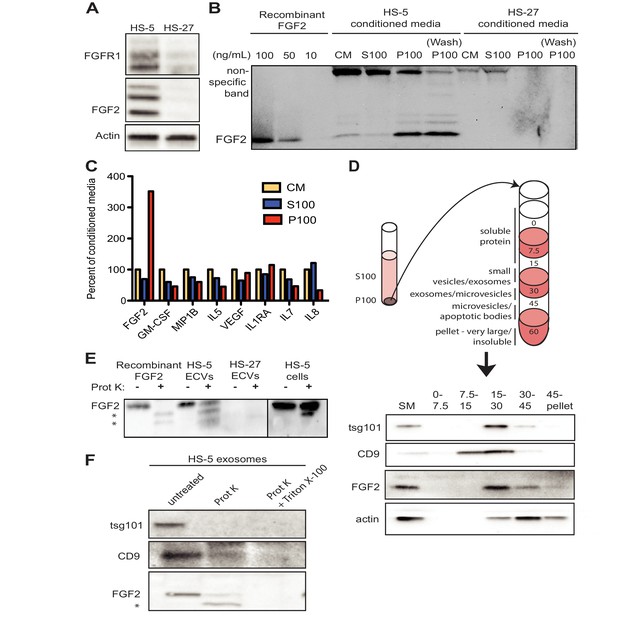
FGF2 is enriched in exosomes from HS-5 bone marrow stromal cells.
(A) Immunoblot of FGFR1, FGF2 and actin in HS-5 and HS-27 whole cell lysates. (B) HS-5 and HS-27 CM were ultracentrifuged at 100,000 g for 2 hr at four degrees C. CM, soluble protein (S100), and ECV (P100) fractions were collected and analyzed by immunoblot, using 10, 50, and 100 ng/ml recombinant FGF2 for comparison. The ultracentrifuge tube was also washed with detergent to remove adherent ECVs and material (detergent wash P100). (C) HS-5 CM, S100 and P100 fractions (concentrated ~10 fold compared to HS-5 CM) were solubilized in 0.1% NP-40 and analyzed by cytokine multiplex ELISA (Luminex). The S100 and P100 fractions were normalized to CM. (D) The HS-5 P100 fraction (starting material, or SM) was further fractionated on a sucrose step-gradient. Sucrose layer interfaces (0–7.5%, 7.5–15%, 15–30%, 30–45%, and 45%-pellet) were collected, lysed and analyzed by immunoblot with antibodies against the exosomal marker CD9, FGF2, and cytoplasmic marker actin. (E) HS-5 and HS-27 ECVs (P100), recombinant FGF2, and HS-5 cells were exposed to proteinase K and analyzed by immunoblot. (F) HS-5 exosomes were isolated by sucrose step-gradient (see panel D) and then exposed to proteinase K with or without detergent (0.1% Triton X-100, used to dissolve the lipid membrane). Samples were subjected to immunoblot analysis using antibodies against tsg101, CD9 and FGF2. The * indicates degraded FGF2 after partial proteinase K digestion.
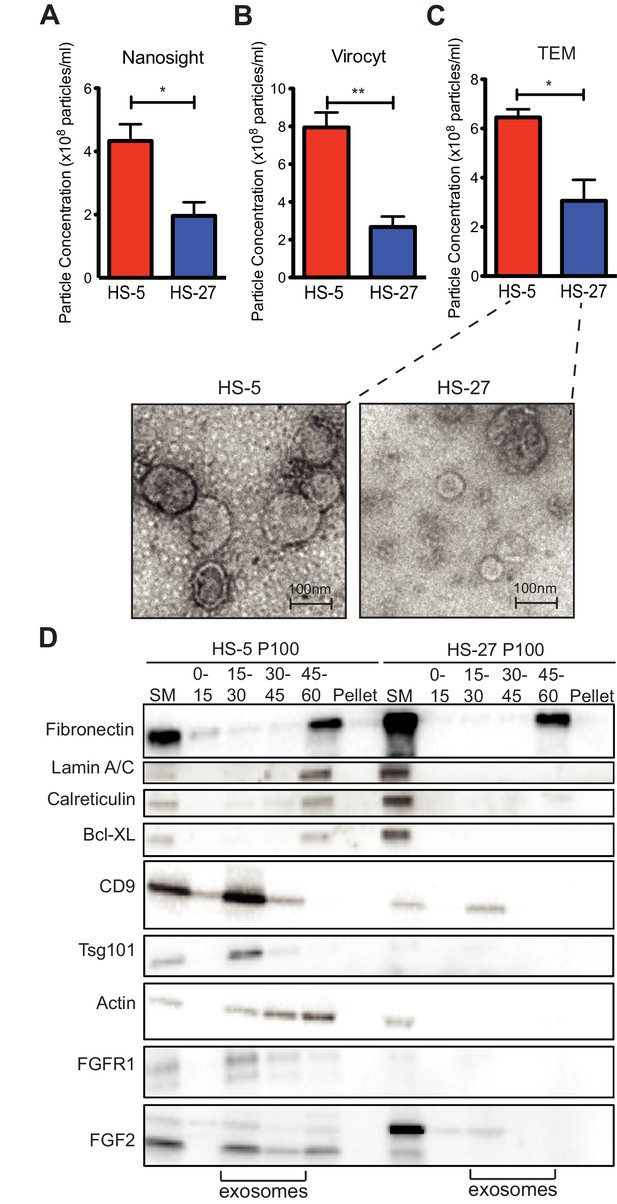
HS-5 cells secrete more exosomes than HS-27 cells.
Equal numbers of HS-5 and HS-27 cells were plated in RPMI with exosome-depleted FBS for 24 hr. The ECVs were pelleted by ultracentrifugation at 100,000 g for 2 hr at four degrees C and resuspended in PBS. ECVs were quantified by (A) Nanosight, a nanovesicle tracking analysis, (B) Virocyt Virus Counter, a proprietary flow cytometry using fluorescent dyes that stain both nucleic acid and protein, or (C) transmission electron microscopy. (D) HS-5 and HS-27 exosomes were collected by sucrose-step gradient and analyzed by transmission electron microscopy. Vesicles were quantified by counting in three 2 × 2 μm areas per sample. All experiments were done in triplicate, error bars represent standard deviation, p values are indicated by *<0.05, **<0.005. HS-5 and HS-27 ECVs (P100) were obtained by ultracentrifugation (starting material, or SM), and the exosome fraction was further purified by a sucrose step-gradient. Sucrose layer interfaces (0–7.5%, 7.5–15%, 15–30%, 30–45%, and 45%-pellet) were collected, lysed and analyzed by immunoblot. Blots were probed with antibodies against exosomal markers CD9 and tsg101; cell compartment markers: fibronectin, lamin A/C, BCL-XL; as well as FGFR1 and FGF2. The lanes with highest enrichment for CD9 and tsg-101, indicating exosomes, are marked below.
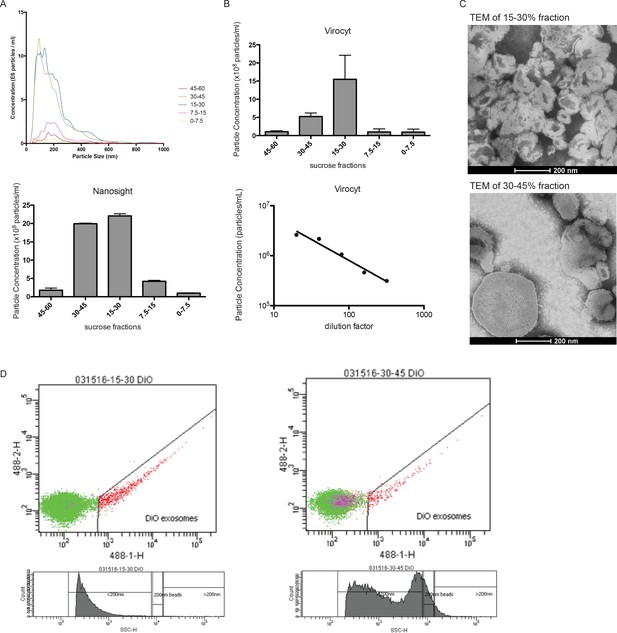
Methods for exosome quantification and further evaluation of microvesicle populations.
(A) CM fractionated by sucrose density gradient assessed for microvesicles by nanoparticle tracking analysis (Nanosight). (B) Fractions assessed by flow cytometry optimized for virus particles (Virocyt). (C) Transmission electron microscopy of sucrose fractions showing microvesicle size difference between 15–30% and 30–45% fractions. (D) Fractions dyed with fluorescent tracer and analyzed by flow cytometry. Size distribution of two different fractions shown relative to 200 nm beads.
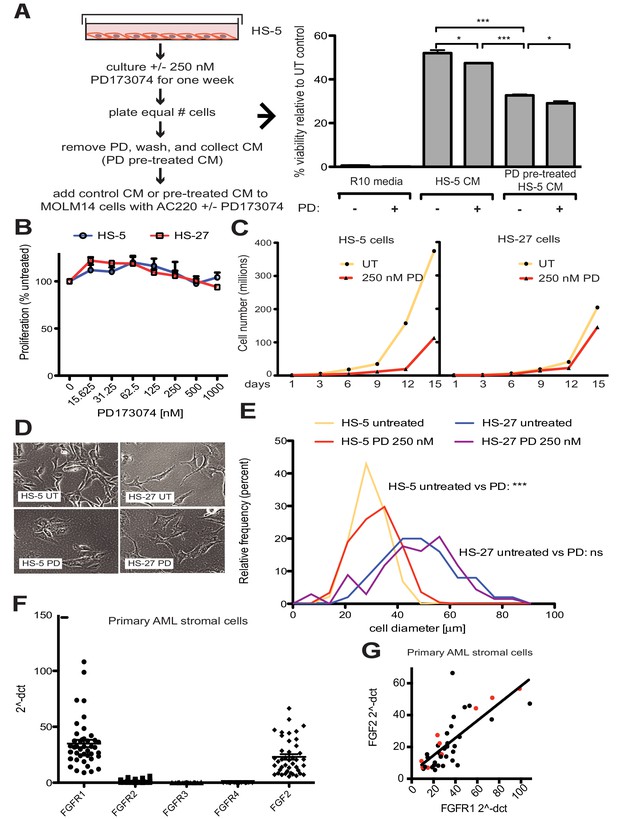
FGF2 is an autocrine growth factor in bone marrow stromal cells, and FGFR inhibition attenuates growth.
(A) HS-5 cells were cultured in media ± 250 nM PD173074 for one week and then equal numbers of cells were replated for comparison. After adhesion, the cells cultured in PD173074 were washed and fresh media added to collect CM. MOLM14 cells were resuspended in media, untreated HS-5 CM, and PD pre-treated HS-5 CM and treated with ± 10 nM AC220 and ± 250 nM PD173074. Viability was measured by MTS assay after 72 hr and values were normalized to the relevant UT control. Error bars represent standard deviation, p values are indicated by *<0.05, **<0.005, and ***=0.0007. (B) HS-5 and HS-27 cells were plated in triplicate on 96 well plates in a gradient of FGFR inhibitor PD173074. Proliferation was measured using MTS reagent after 72 hr. Error bars indicate standard deviation. (C) HS-5 and HS-27 cells were incubated media ± 250 nM PD173074 (PD). The number of viable cells was measured with Guava ViaCount every 3 days over a 15 day period. Fresh media and PD173074 was added every 3 days. (D) HS-5 and HS-27 cells were incubated in media ± 1 µM PD173074 for 1 week. Brightfield microscopy images were obtained using a 10X objective. (E) HS-5 cells were incubated in 4-well glass chamber slides in media ± 250 nM PD173074 (PD). Cells were stained with lipophilic tracer DiI for 24 hr, fixed, then nuclei stained with DAPI. Immunofluorescent images were analyzed with CellProfiler software to determine cell size (μm [Weisberg et al., 2009]) and number of cells for each size range was binned and graphically displayed. PD173074 had no effect on HS-27 growth, morphology or size, consistent with an on-target FGFR effect. (F) Ex vivo cultured primary bone marrow stromal cells from a series of leukemia patients (n = 42) were lysed for RNA extraction and cDNA synthesis. Taqman qPCR analysis was performed using FGFR1, FGFR2, FGFR3, FGFR4, and FGF2 Taqman primer assays and expression plotted (n = 42 for each except FGFR4 which is n = 41 due to failed PCR for one sample). (G) FGFR1 and FGF2 qPCR values (2^-ΔCT) were plotted against each other. There were 9 AML patients with FLT3 ITD (most newly diagnosed) and these patients are indicated with red dots. Linear regression produced a line fit with r2 = 0.5683 and slope significantly non-zero with p<0.0001.
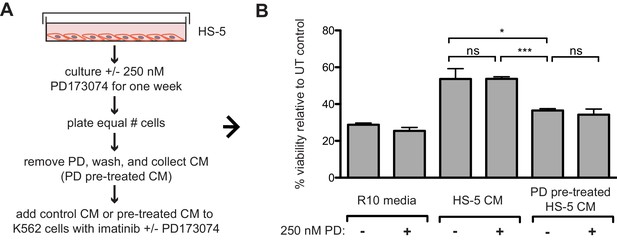
Pre-treatment of HS-5 stromal cells with FGFR inhibitor reduces protective properties of HS-5 CM when added to K562 cells exposed to imatinib.
(A) HS-5 cells were cultured in media ± 250 nM PD173074 for one week and then equal numbers of cells were replated for comparison. After adhesion, the cells cultured in PD173074 were washed and fresh media added to collect CM. K562 cells were resuspended in media, untreated HS-5 CM, and PD pre-treated HS-5 CM and treated with ± 1 uM imatinib and ± 250 nM PD173074. (B) Viability was measured by MTS assay after 72 hr and values were normalized to the relevant UT control. Error bars represent standard deviation, p values are indicated by *<0.05, **<0.005, and ***=0.0007.
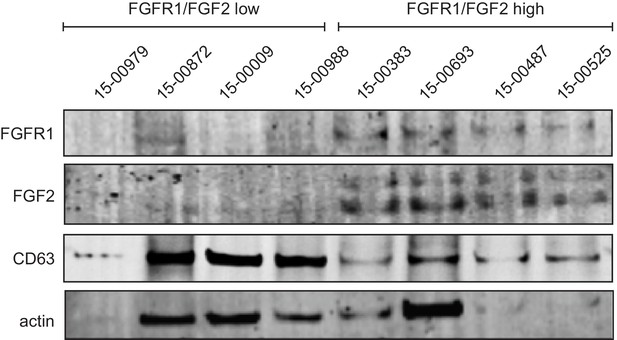
Cultures of primary human and mouse bone marrow stroma produce microvesicles containing FGF2.
Conditioned medium was collected from cultured human bone marrow stroma. Samples were ultracentrifuged and pellets were lysed with 78 μL of Cell Lysis Buffer (Cell Signaling Technologies Inc, Danvers, MA) containing a Complete Mini Protease Inhibitor Cocktail Tablet, Phosphatase Inhibitor Cocktail 2, and Phenylmethanesulfonyl Fluoride (PMSF) solution (Sigma-Aldrich Inc, St Louis, MO) and clarified by centrifugation at 14,000 g, 4°C for 15 min. All samples were loaded on NuPAGE 4–12% Bis-Tris gradient gels, ran in MES buffer (Thermo Fisher Scientific Inc, Waltham, MA), transferred on Immobilon-FL PVDF membranes (Millipore Inc, Billerica, MA), and blocked overnight at 4°C. Following overnight incubation, membranes were incubated with the following primary antibodies: anti-FGFR1, anti-FGF2, anti-CD63, anti-CD9, and anti-actin (Supplementary file 1) overnight at 4°C. The following day membranes were washed and probed with fluorescent IRDye 800CW goat anti-rabbit IgG and IRDye 680RD Goat anti-mouse IgG antibodies (Supplementary file 1). The membranes were imaged with the Odyssey Infrared Imaging System (LI-COR Biosciences).
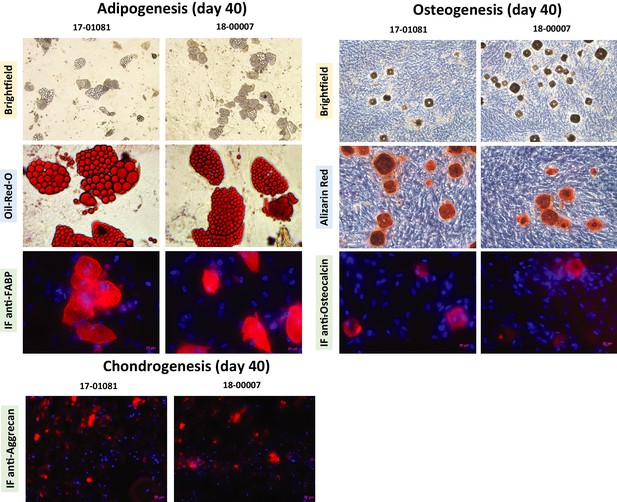
Cultured primary human bone marrow stroma exhibits trilineage differentiation.
Primary mesenchymal stromal cells at passage four were differentiated into adipocytes, osteocytes, and chondrocytes using the Human Mesenchymal Stem Cell Functional Identification Kit (R and D Systems Inc, Minneapolis, MN) according to the manufacturer’s instructions. Cells were cultured for 40 days with induction medium replaced every three days. On day 40 of induction, the cultures were washed with PBS and fixed for 20 min in 4% buffered paraformaldehyde at room temperature. The presence of adipogenic cells was confirmed with oil-red-o staining and immunofluorescence staining for fatty acid binding protein (FABP). Osteogenesis was assessed with alizarin red staining and immunofluorescence staining for osteocalcin. For chondrogenic pellets, upon fixing, pellets were cryosectioned at a thickness of 5 μm and stained for aggrecan using immunofluorescence.
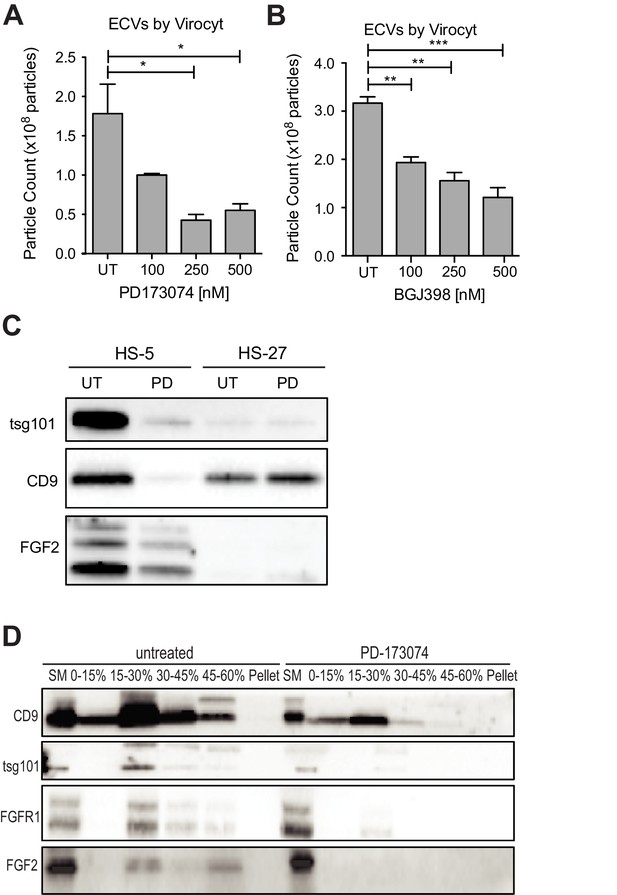
FGFR inhibition decreases exosome production in FGF2-expressing stroma.
HS-5 cells were exposed to a gradient of the FGFR inhibitors (A) PD173074 and (B) BGJ-398 for 48 hr prior to collecting CM. ECVs were pelleted by ultracentrifugation at 100,000 g and quantified by Virocyt Virus Counter. Error bars indicate standard deviation and p values are indicated by *<0.05. (C) HS-5 and HS-27 cells were incubated in media ± 1 µM PD173074 for 72 hr prior to collecting ECVs. ECVs were analyzed by immunoblot for FGF2. The exosome markers CD9 and tsg101 are also shown. (D) HS-5 cells were plated in media ± 1 µM PD173074 for 72 hr. P100 fractions were obtained by ultracentrifugation, and further fractionated on a sucrose step-gradient. The interfaces (0–7.5%, 7.5–15%, 15–30%, 30–45%, and 45%-pellet) were collected, lysed and processed by immunoblot with antibodies against the exosomal markers CD9 and tsg101 as well as FGFR1 and FGF2.
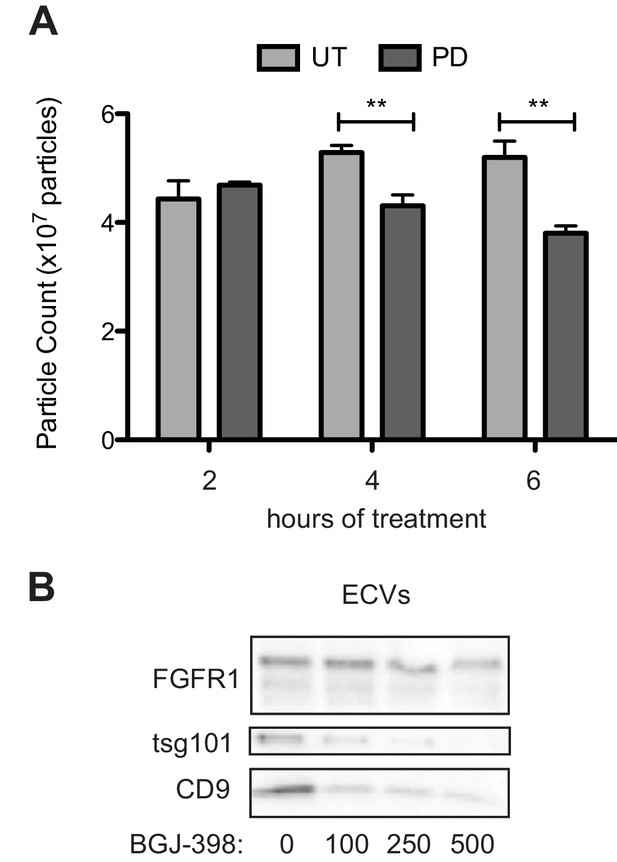
FGFR inhibition reduces HS-5 cell exosome secretion.
(A) HS-5 cells were exposed to 500 nM PD173074 (PD) for 2, 4 and 6 hr before collection of CM and ECVs as previously described. ECVs were quantified by Virocyt. Results obtained in triplicate. Error bars indicates standard deviation. **p<0.005 (B) HS-5 cells were exposed to a gradient of BGJ-398 for 72 hr before isolation of ECVs from CM. ECVs were lysed and run on immunoblot to demonstrate reduction in exosome markers (CD9 and tsg-101).
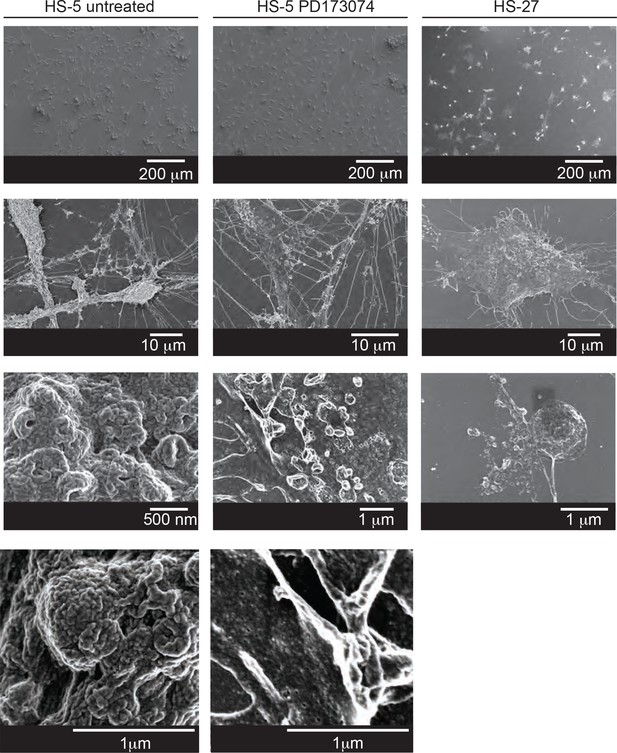
Scanning electron microscopy of HS-5 cells shows altered membrane dynamics after FGFR inhibition.
Cells grown on coverslips were fixed with 2% paraformaldehyde and 1% glutaraldehyde (Ted Pella Inc, Redding, CA, USA) in phosphate buffered saline for at least one hour. Following three rinses in phosphate buffered saline, the samples were immersed in 1% osmium tetroxide in phosphate buffered saline for one hour at room temperature. Following three buffer rinses, the samples were rinsed once in ddH2O before entering an ascending ethanol gradient, incubating for ten minutes in each mixture of ethanol:ddH2O (50%, 75%, to 95% ethanol). After two incubations in 100% ethanol the samples were critical point dried (Tousimis Samdri CPD, Rockville, MD, USA). Dried coverslips were mounted on aluminum stubs with carbon tape and silver paint and then sputter coated with 6–10 nm of gold-palladium (Hummer Sputter System, Anatech USA, Hayward, CA, USA). The samples were imaged on a FEI Helios Nanolab 660 (FEI Inc, Hillsboro, OR). Images were collected with the Elstar in-lens TLD detector or the Everhart-Thornley Detector at 1 kV accelerating voltage.
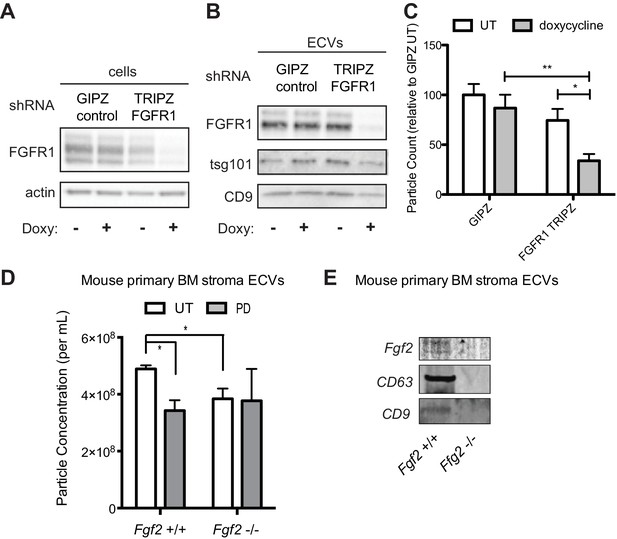
Genetic silencing of FGFR1 or deletion of FGF2 attenuates exosome secretion.
A doxycycline-inducible lentiviral shRNA targeting FGFR1 was used to create a stable HS-5 cell line. The cells were then treated with doxycycline to induce FGFR1 silencing and compared to a GIPZ lentiviral control. (A) Silencing of FGFR1 expression is shown by immunoblot of cell lysates. ECVs from doxycycline-treated cells were analyzed by (B) immunoblot or (C) Virocyt Virus Counter. *p<0.05. (D) Bone marrow was isolated from Fgf2 +/+ and -/- mice and cultured ex vivo to grow adherent marrow stroma. Equal numbers of cells were then plated, CM collected for 72 hr, and then ultracentrifuged to collect ECVs. The ECVs were quantified by Virocyt. *p<0.05. (E) Equal number of cultured marrow cells from Fgf2 +/+ and -/- mice were plated and then ECVs collected by ultracentrifugation and analyzed by immunoblot.
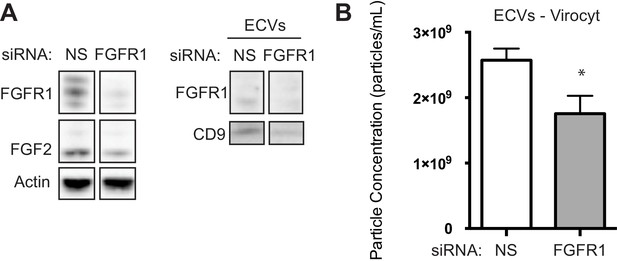
Genetic silencing of FGFR1 by siRNA reduces exosome secretion and protection capacity of HS-5 stromal cells.
FGFR1 siRNA pool was purchased from Thermo Fisher Scientific Dharmacon RNAi Technologies (Waltham, MA, USA). HS-5 cells were transfected with siRNAs using Lipofectamine 2000 reagent purchased from Thermo Fisher Scientific (Grand Island, NY, USA), according to manufacturer’s protocol. After 72 hr, cells were harvested, and cells and CM collected for analysis. siRNA effectively silences of FGFR1 in cells and leads to reduction in ECVs by (A) immunoblot and (B) Virocyt analysis.
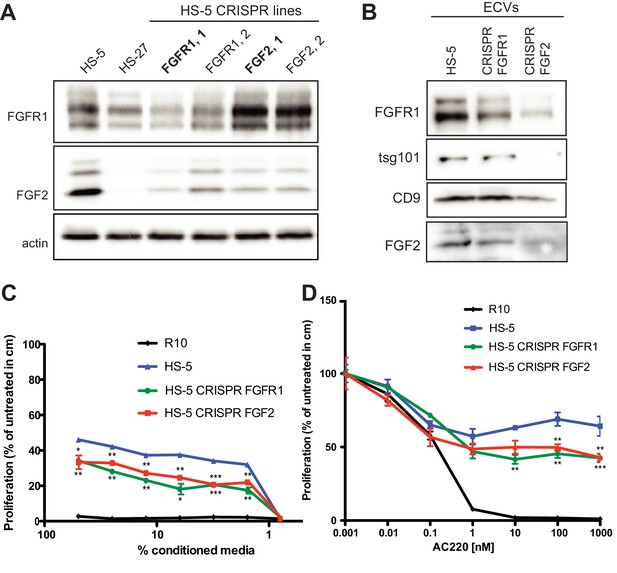
Genetic silencing of FGFR1 by CRISP/CAS9 reduces exosome secretion and protection capacity of HS-5 stromal cells.
(A) FGFR1 and FGF2 genes were knocked out in HS-5 cells by lentiviral CRISPR-Cas9 genome editing. Each gene was targeted with two single guide RNA sequences (labeled 1 or 2). However, once FGF2 and FGFR1 were genetically mutated, the HS-5 cells were unable to continue to grow, so we were only able to analyze the cell lines for a short time after CRISPR/CAS9 treatment, which initially results in a partial genetic silencing as demonstrated in panel A. Whole cell lysates were analyzed by immunoblot to demonstrate partial gene silencing. Constructs selected for subsequent experiments are indicated in bold. (B) ECVs from control HS-5 cells and CRISPR/Cas9 HS-5 cells were analyzed by immunoblot with antibodies against FGFR1, tsg101, CD9, FGF2, and actin. (C) CM was harvested from HS-5 cells, FGFR1 CRISPR/Cas9 HS-5 cells, and FGF2 CRISPR/Cas9 HS-5 cells after 72 hr. MOLM14 cells were plated in 96 well plates in 10 nM AC220 and media alone or with serial dilutions of CM. Proliferation was measured using MTS reagent after 48 hr. (D) CM was harvested from HS-5 cells, FGFR1 CRISPR/Cas9 HS-5 cells, and FGF2 CRISPR/Cas9 HS-5 cells after 72 hr. MOLM14 cells were plated in 96 well plates in media alone or CM and then graded concentrations of quizartinib (AC220). Proliferation was measured using MTS reagent after 48 hr. Error bars indicate standard deviation. All experiments were done in triplicate and p values are indicated by *<0.05, **<0.005, ***=0.0007.
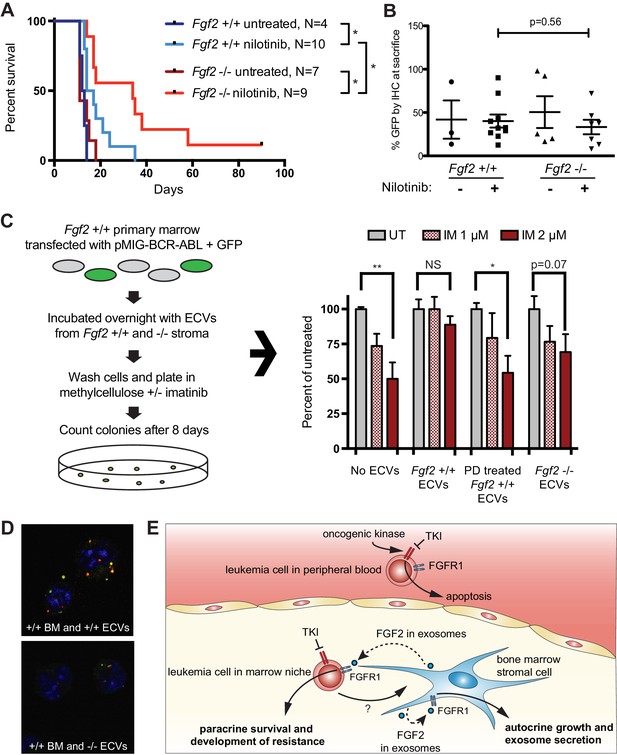
Fgf2 -/- mice survive significantly longer with TKI therapy in a murine BCR-ABL leukemia model.
Fgf2 +/+ bone marrow was removed from donor mice and spinoculated with pMIG BCR-ABL retrovirus containing an IRES-GFP marker. The transfected bone marrow was then transplanted into lethally irradiated Fgf2 +/+ or -/- recipients. Mice were treated with 75 mg/kg/day nilotinib by oral gavage starting on day 11 of transplant. (A) Survival curves of untreated and nilotinib-treated Fgf2 +/+ and -/- mice. (B) GFP in peripheral blood was evaluated weekly and at time of euthanasia to quantify disease burden. The average GFP (percent of nucleated cells) is shown and did not differ significantly between groups indicating that all animals developed similar disease burden. Error bars indicate standard deviation. (C) Bone marrow cells from Fgf2 +/+ mice were spinoculated with pMIG BCR-ABL retrovirus containing GFP-IRES. The cells were then incubated with ECVs obtained from Fgf2 +/+ and -/- primary stroma cultured alone or with 500 nM PD173074. The next day the incubated cells were washed three times to remove cytokines and exosomes and plated in cytokine-free methylcellulose ± imatinib. After 8 days, colonies were counted and normalized to untreated condition. Graph shown on right. Error bars indicate standard error of the mean. *p<0.05 and **p<0.005. (D) Lineage-negative bone marrow cells were isolated from Fgf2 +/+ mice and cells were stained with DiO (green) tracer, washed, and immobilized on Poly-D-lysine coated chamber slides. ECVs from bone marrow stroma of Fgf2 +/+ or -/- mice were stained with DiI (red) tracer and added to the cells for a 24 hr incubation. Slides were stained with DAPI (blue) and imaged by confocal fluorescent microscopy. Movie of the z-stack images are included as Figure 7—video 1 and 2. (E) Model of bone marrow stromal FGF2 autocrine signaling and paracrine protection of leukemia cells by FGF2-containing exosomes.
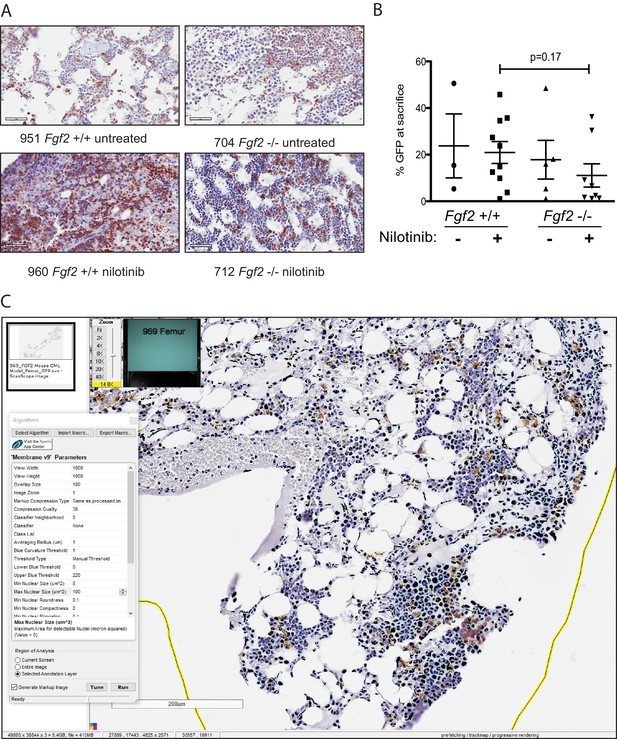
Fgf2 +/+ and -/- mice demonstrate engraftment of leukemia by immunohistochemistry (IHC) of GFP.
Fgf2 +/+ marrow was removed from donor mice and spinoculated with pMIG BCR-ABL retrovirus containing an IRES-GFP marker. The transfected marrow was then transplanted into lethally irradiated Fgf2 +/+ or -/- recipients. Mice were treated with 75 mg/kg/day nilotinib by oral gavage starting on day 11 of transplant. Lungs, spleens, and femurs were collected for analysis by hematoxylin and eosin staining and IHC for GFP as previously described (Traer et al. Blood 2012 and Traer et al. Cancer Research 2016). (A) IHC of GFP in marrows of representative mice to demonstrate engraftment of leukemia. (B) GFP in peripheral blood was evaluated weekly and at time of euthanasia to quantify disease burden. The average GFP (percent of nucleated cells) at time of euthanasia is shown and did not differ significantly between groups indicating that all animals developed similar disease burden prior to death. Error bars indicate standard deviation. (C) Illustration of patient tissue analysis using the Aperio ScanScope CS Slide Scanner. Bone marrow tissue selected for analysis showing results of analysis using the membrane-v9 algorithm.
ECVs collected from Fgf2 +/+ primary marrow stromal cell cultures were stained with DiI (red) and lineage-depleted hematopoietic cells from Fgf2 +/+ marrow were stained with DiO (green) and incubated for 24 hr at 37°C as described in Materials and methods.
Cells were washed, placed on Poly-D-Lysine coated chamber slides, and DAPI-stained. Z-stack imaging was performed on an Olympus IX71 inverted microscope.
ECVs collected from Fgf2 -/- primary marrow stromal cell cultures were stained with DiI (red) and lineage-depleted hematopoietic cells from Fgf2 +/+ marrow were stained with DiO (green) and incubated for 24 hr at 37°C as described in Materials and methods.
Cells were washed, placed on Poly-D-Lysine coated chamber slides, and DAPI-stained. Z-stack imaging was performed on an Olympus IX71 inverted microscope.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (homo sapiens) | FGF2 | NA | ||
| Gene (mus musculus) | Fgf2 | NA | ||
| Gene (homo sapiens) | FGFR1 | NA | ||
| Gene (mus musculus) | Fgfr1 | NA | ||
| Strain, strain background (mus musculus) | Fgf2tm1Doe/J Fgf2 +/+ and -/- mice | Jackson Laboratory | RRID:MGI:2679603 | |
| Genetic reagent (homo sapiens) | FGF2 | Thermo Fisher Scientific | shRNA in TRIPZ lentiviral vector | |
| Genetic reagent (homo sapiens) | FGFR1 | Thermo Fisher Scientific | shRNA in TRIPZ lentiviral vector | |
| Genetic reagent (homo sapiens) | AddGene | GeCKO lentiCRISPRv2 hSpCas9 and guide RNA | ||
| Genetic reagent (homo sapiens) | FGF2-1 | GenScript | CRISPR/Cas nine guide RNA design | |
| Genetic reagent (homo sapiens) | FGF2-2 | GenScript | CRISPR/Cas nine guide RNA design | |
| Genetic reagent (homo sapiens) | FGFR1-1 | GenScript | CRISPR/Cas nine guide RNA design | |
| Genetic reagent (homo sapiens) | FGFR1-2 | GenScript | CRISPR/Cas nine guide RNA design | |
| Genetic reagent (mus musculus) | pMIG with BCR-ABL and GFP | murine retrovirus | ||
| Cell line (homo sapiens) | MOLM14 | Dr. Yoshinobu Matsuo | RRID:CVCL_7916 | |
| Cell line (homo sapiens) | K562 | American Type Culture Collection | RRID:CVCL_0004 | |
| Cell line (homo sapiens) | HS-5 | Dr. Beverly Torok-Storb | RRID:CVCL_3720 | |
| Cell line (homo sapiens) | HS-27 | Dr. Beverly Torok-Storb | RRID:CVCL_0335 | |
| Antibody | Mouse monoclonal anti-FGFR1 | Cell Signaling | 9740 | Dilution 1:1000 |
| Antibody | Rabbit polyclonal anti-FGF2 | Santa Cruz | Sc-79 | Dilution 1:500 |
| Antibody | Rabbit monoclonal anti-CD63 | ABCAM | ab134045 | Dilution 1:1000 |
| Antibody | Rabbit polyclonal anti-CD9 | Santa Cruz | Sc-9148 | Dilution 1:200 |
| Antibody | Mouse monoclonal anti-tsg-101 | Santa Cruz | Sc-7964 | Dilution 1:200 |
| Antibody | Mouse monoclonal anti-actin | Millipore | MAB1501 | Dilution 1:5000 |
| Peptide, recombinant protein | FGF2 (human) | Peprotech | ||
| Commercial assay or kit | Thermo Scientific lentiviral transfection kit | |||
| Chemical compound, drug | quizartinib (AC220) | LC labs | ||
| Chemical compound, drug | imatinib | LC labs | ||
| Chemical compound, drug | nilotinib | SelleckChem | ||
| Chemical compound, drug | PD173074 | SelleckChem | ||
| Chemical compound, drug | BGJ-398 | SelleckChem | ||
| Chemical compound, drug | doxycycline | Fisher | ||
| Software, algorithm | CellProfiler | Cell area |
Additional files
-
Supplementary file 1
Additional information on antibodies used in paper.
- https://doi.org/10.7554/eLife.40033.024
-
Transparent reporting form
- https://doi.org/10.7554/eLife.40033.025





