Unique morphogenetic signatures define mammalian neck muscles and associated connective tissues
Figures
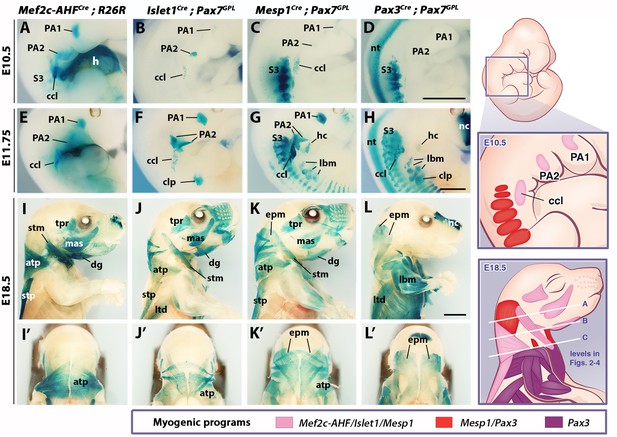
Genetic lineage tracing of neck muscle progenitors.
Whole-mount X-gal stainings of Mef2c-AHFCre;R26R, Islet1Cre;Pax7GPL, Mesp1Cre;Pax7GPL and Pax3Cre;Pax7GPL mice at E10.5 (A–D), E11.75 (E–H) and E18.5 (I–L’) (n = 3 for each condition). See associated Figure 1—supplements 1–3. (A–H) Note labeling of mesodermal core of pharyngeal arches (PAs) and cucullaris anlage (ccl) by Mef2c-AHF, Islet1 and Mesp1 lineage reporters; β-gal+ cells in anterior somites of Mesp1Cre embryos and in the clp anlagen of Islet1Cre embryos. Pax3 lineage marked somitic mesoderm. (I–L’) Mef2c-AHF, Islet1 and Mesp1 lineages marked branchiomeric (mas, tpr, dg) and cucullaris muscles (stm, atp and stp). Pax3Cre and Mesp1Cre labeled somitic epaxial neck muscles (epm). atp, acromiotrapezius; ccl, cucullaris anlage; clp, cutaneous maximus/latissimus dorsi precursor; dg, digastric; epm, epaxial musculature; h, heart; hc, hypoglossal cord; lbm, limb muscle anlagen and limb muscles; ltd, latissimus dorsi; mas, masseter; nc, nasal capsule; nt, neural tube; PA1-2, pharyngeal arches 1–2; S3, somite 3; stm, sternocleidomastoid; stp, spinotrapezius; tpr; temporal. Scale bars: in D for A-D and in H for E-H, 1000 µm; in L for I-L’, 2000 µm.
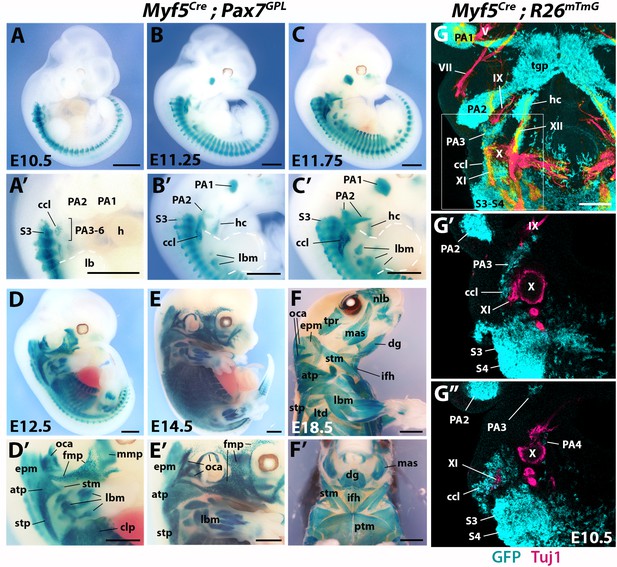
Ontogenetic analysis of Myf5 muscle progenitors at the head-trunk interface.
(A–F’) Temporal X-gal stainings of Myf5Cre;Pax7GPL embryos (n = 2–4 each condition). (G–G’’) Immunostaining for GFP and Tuj1 in E10.5 Myf5Cre;R26mTmG embryo (n = 2). Ventral 3D projection of cranial region (G) and two sections 60 µm apart (dorso-ventral direction) in region indicated in (G) are shown. The cucullaris is innervated by the accessory nerve XI. V, trigeminal nerve; VII, facial nerve; IX, glossopharyngeal nerve; X, vagal nerve; XI, accessory nerve; XII, hypoglossal nerve; atp, acromiotrapezius; ccl, cucullaris anlage; clp, cutaneous maximus/latissimus dorsi precursor; dg, digastric muscles; epm, epaxial neck musculature; fmp, facial muscle precursors; h, heart; hc, hypoglossal cord; ifh, infrahyoid muscles; lb, limb bud; lbm, limb muscle anlagen and limb muscles; ltd, latissimus dorsi; lvs, levator scapula; mas, masseter; mmp, masticatory muscle precursor; nlb, nasolabialis muscles; oca, occipito/cervico-auricularis anlagen and muscles; PA1-6, pharyngeal arches 1–6; ptm, pectoralis muscles; S3-S4, somites 3–4; stm, sternocleidomastoid; stp, spinotrapezius; tgp, tongue primordia; tpr; temporal. Scale bars: in A-E’, 1000 µm; in F-F’, 2000 µm, in G for G, 400 µm for G-G’, 200 µm.
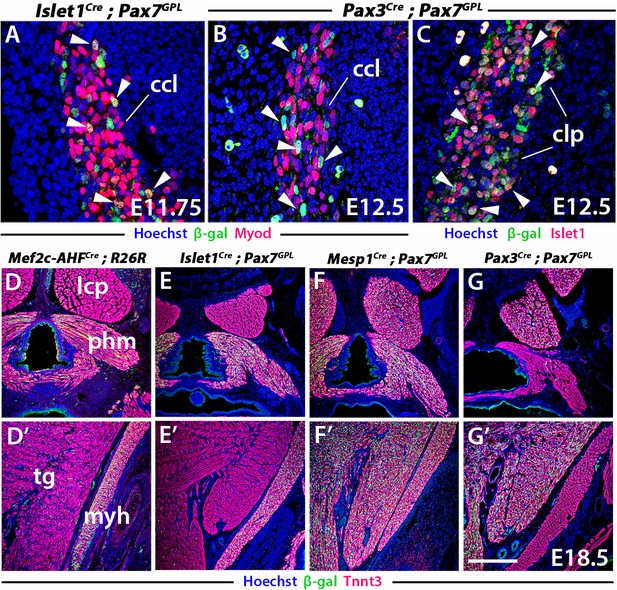
Mef2c-AHF, Islet1, Mesp1 and Pax3 lineage tracings using lacZ reporters.
(A–B) Immunostainings for Myod and β-gal reporter on coronal paraffin sections of E11.75 Islet1Cre;Pax7GPL and E12.5 Pax3Cre;Pax7GPL embryos in the cucullaris anlage (n = 2 each condition). (C) Immunostaining for Islet1 and β-gal reporter on coronal paraffin section of a E12.5 Pax3Cre;Pax7GPL embryo in the clp anlage (n = 2). White arrowheads in A-C indicate examples. (D–G’) Immunostainings for β-gal and Tnnt3 on coronal cryosections (n = 2 each condition). ccl, cucullaris anlage; clp, cutaneous maximus/latissimus dorsi precursor; ifh, infrahyoid muscles; lcp, longus capitis; myh, mylohyoid; phm; pharyngeal muscles; ssp, semispinalis; tg, tongue. Scale bars: In G’ for A-C 50 µm, for D-G 200 µm.
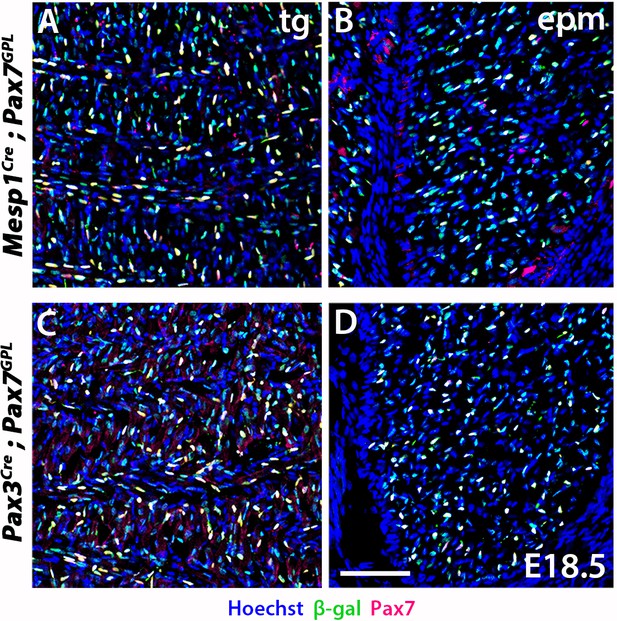
Mesp1 and Pax3 lineage tracings in somitic neck muscles using the Pax7GPL reporter.
(A–D) Immunostainings for β-gal and Pax7 on coronal cryosections of Mesp1Cre;Pax7GPL and Pax3Cre;Pax7GPL at E18.5 (n = 2 each condition). Note the white/yellow cells indicating colocalization of both markers. epm, epaxial muscle; tg, tongue. Scale bar in D for A-D 100 µm.
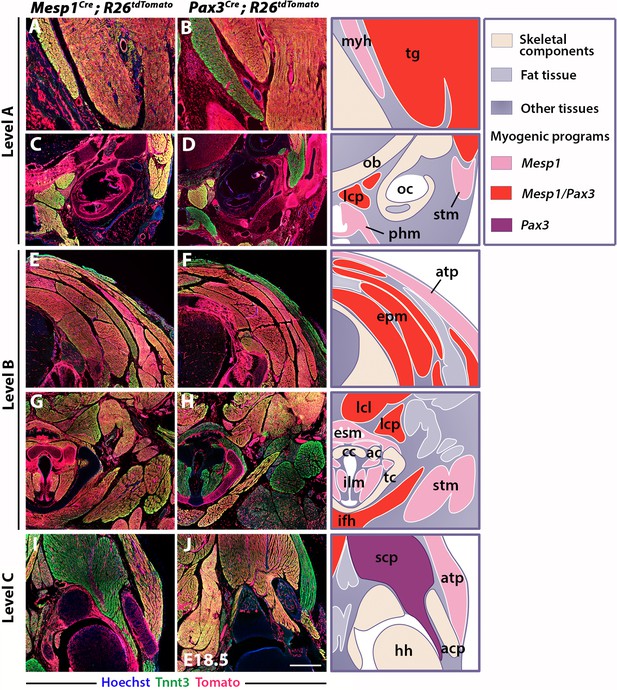
Differential contributions of Mesp1 and Pax3 lineages to neck and shoulders.
Immunostainings on coronal cryosections of E18.5 Mesp1Cre;R26tdTomato and Pax3Cre;R26tdTomato mice for the myofibre Tnnt3 and Tomato markers at levels indicated in Figure 1. Higher magnifications of selected areas in (A–J) are shown in Figure 2—figure supplement 2; (n = 2 for each condition). See also the atlas of neck musculature in Figure 2—figure supplement 1. (A–H) Mesp1Cre labeled all neck muscles including branchiomeric (myh, esm, phm and ilm), cucullaris (stm, atp), somitic epaxial (epm) and hypaxial (tg, lcp, lcl, ifh) muscles. Pax3Cre marked somitic muscles. (I–J) At shoulder level, Mesp1-derived cells did not contribute to posterior somitic myofibres including scapular muscles (scp) compared to that observed in Pax3Cre embryos. ac, arytenoid cartilage; acp, scapular acromion process; atp, acromiotrapezius; cc, cricoid cartilage; epm, epaxial musculature; esm, esophagus striated muscle; hh, humeral head; ifh, infrahyoid muscles; ilm, intrinsic laryngeal muscles; lcl, longus colli; lcp, longus capitis; myh, mylohyoid; ob, occipital bone; oc, otic capsule; phm, pharyngeal muscles; stm, sternocleidomastoid; scp, scapular musculature; tc, thyroid cartilage; tg, tongue. Scale bars: in J for A-B 200 µm, for C-J 400 µm.
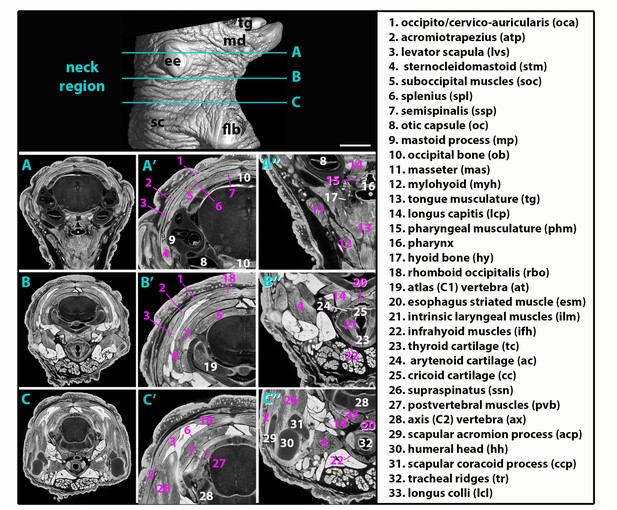
Atlas of neck musculature in mouse.
µCT scan analysis shows surface rendering of neck region of a E18.5 control fetus (n = 1). Levels A-C in the neck region (as reference, blue) are examined in Figures 2–5. Structures of interest are shown on virtual µCT sections at levels (A–C); higher magnifications on latero-dorsal (A’–C’) and latero-ventral regions (A”–C”). Neck structures are numbered for muscles in pink; other components in white. ee, external ear; flb, forelimb (distal part removed); md; mandible; sc, scapular region; tg, tongue. Scale bar in upper panel for upper panel and A-C 1000 µm, for A’-C’, A’-C’ 500 µm.
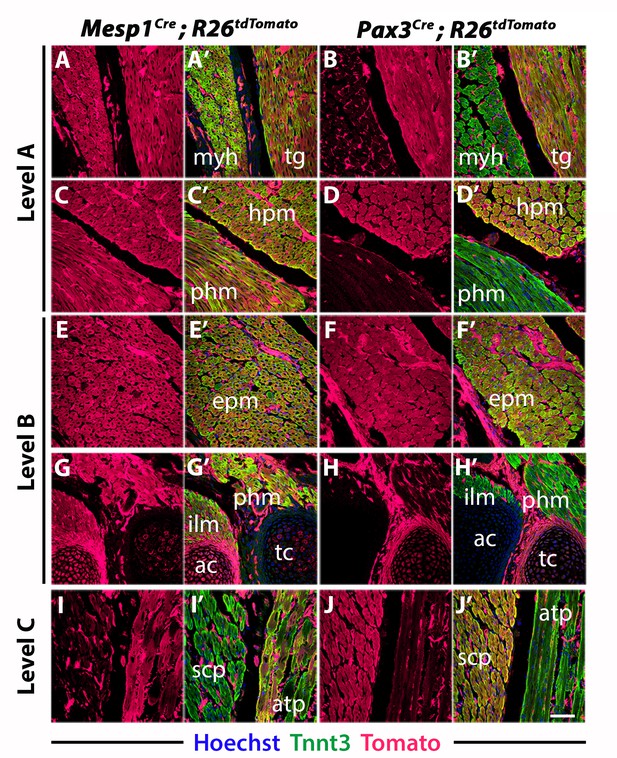
Mesp1 and Pax3 lineage contributions to neck and shoulder muscles.
High magnifications of selected areas of panels in Figure 2 at levels indicated in Figure 1. (A–J’) Immunostainings are indicated (n = 2 each condition). ac, arytenoid cartilage; atp, acromiotrapezius; epm, epaxial musculature; hpm, hypaxial musculature; ilm, intrinsic laryngeal muscles; myh, mylohyoid; phm, pharyngeal muscles; scp, scapular muscles; tc, thyroid cartilage; tg, tongue. Scale bars: in J’ for A-J’ 50 µm.
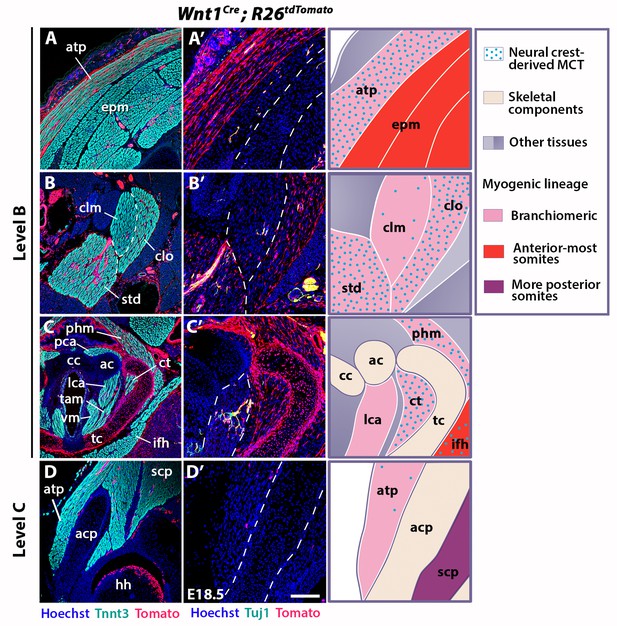
Neural crest contribution to neck muscle-associated tissue.
Immunostainings on coronal cryosections of E18.5 Wnt1Cre;R26tdTomato mice at levels indicated in Figure 1. Tnnt3/Tomato immunostainings are shown in (A–D) and immunostainings for Tuj1/Tomato on selected areas of (A–D) are shown with higher magnifications in (A’–D’). See associated Figure 3—figure supplement 1–4; (n = 2). (A–A’) Note high Wnt1 contribution in the acromiotrapezius but not in epaxial muscles where Wnt1-derived cells marked neuronal cells. (B–C’) Wnt1-derived cells marked differentially the distinct muscles composing the sternocleidomastoid and laryngeal musculatures. (D–D’) At shoulder level, the Wnt1 cells did not contribute to attachment of acromiotrapezius to scapula. ac, arytenoid cartilage; acp, scapular acromion process; atp, acromiotrapezius; cc, cricoid cartilage; clm, cleidomastoid; clo, cleido-occipitalis; ct, cricothyroid; epm, epaxial musculature; hh, humeral head; ifh, infrahyoid muscles; lca, lateral cricoarytenoid; MCT, muscle-associated connective tissue; pca, posterior cricoarytenoid; phm, pharyngeal muscles; scp, scapular musculature; std, sternomastoid; tam, thyroarytenoid muscle; tc, thyroid cartilage; vm, vocal muscle. Scale bars: in D’ for A-D 400 µm for A’-D’ 200 µm.
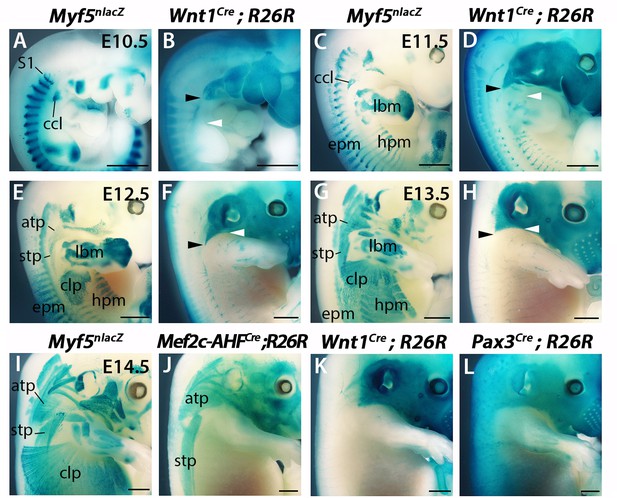
Distribution of developing neck muscles and neural crest cells.
(A–H) X-gal stainings in embryos from E10.5-E13.5. Note that the posterior limit of NCC distribution at the head-trunk interface (black arrowheads) corresponds to the acromiotrapezius at anterior edge of forelimb buds (white arrowheads). (I–L) X-gal stainings of embryos at E14.5. Note that the posterior trapezius muscles derived from the Mef2c-AHF lineage develop in a Wnt1-negative/Pax3-positive domain. (n = 2 each condition) atp, acromiotrapezius; ccl, cucullaris anlage; clp, cutaneous maximus/latissimus dorsi precursor; epm, epaxial musculature; hpm, hypaxial musculature; lbm, limb muscle anlagen; S1, somite 1; stp, spinotrapezius. Scale bars: 1000 µm.
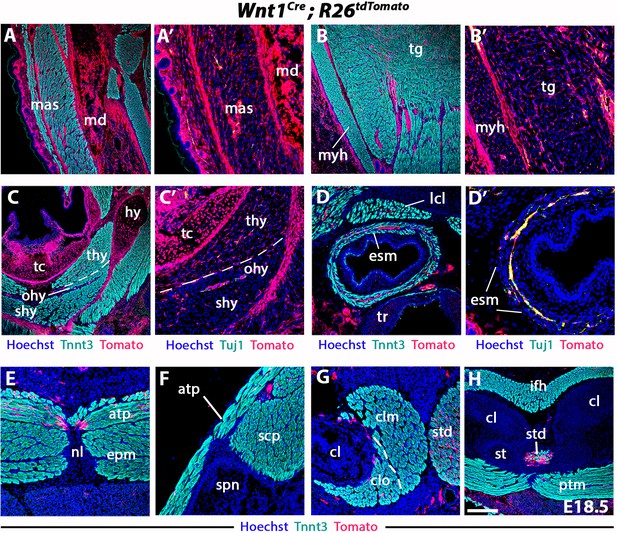
Neural crest contribution to neck and pectoral structures.
Contribution of Wnt1-derived NCCs to connective tissue of branchiomeric (mas, myh, esm) and somitic muscles (tg, ifh, ptm, epm, scp) (refer to Figure 3, n = 2) (A–H). Note that the esophagus (esm) and infrahyoid (thy, ohy, shy) muscles show little or no NCC contribution to connective tissue (C–D’). Only few Wnt1-derived cells are present at connection sites of cucullaris-derived muscles on the pectoral girdle (E–H). atp, acromiotrapezius; cl, clavicle; clm, cleidomastoid; clo, cleido-occipitalis; epm; epaxial neck musculature; esm, esophagus striated muscle; hy, hyoid bone; ifh, infrahyoid muscles; lcl, longus colli; mas, masseter; md; mandible; myh, mylohyoid; nl, nuchal ligament; ohy, omohyoid; ptm, pectoralis muscles; shy, sternohyoid; spn, scapular spine; scp, scapular muscles; st, sternum; std, sternomastoid; tc, thyroid cartilage; thy, thyrohyoid; tg, tongue; tr, tracheal ridge. Scale bars: in H for A-C, H 100 µm, for A’-C’, D-G 200 µm; for D’ 400 µm.
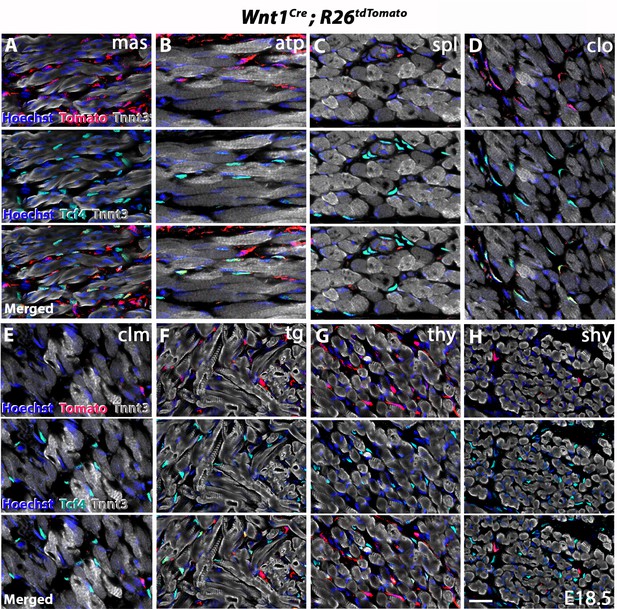
Wnt1 lineage contribution to connective tissue fibroblasts.
Immunostainings of coronal cryosections (n = 2) show selected muscles analysed in Figure 3 and Figure 3—figure supplement 2 including the masseter (A), acromiotrapezius (B), splenius (epaxial) (C), cleido-occipitalis (D), cleidomastoid (E), tongue (F), thyrohyoid (infrahyoid) (G) and sternohyoid (infrahyoid) (H). Note only little or no NCC contribution to cleidomastoid, infrahyoid and epaxial muscles. atp, acromiotrapezius; clm, cleidomastoid; clo, cleido-occipitalis; mas, masseter; shy, sternohyoid; spl, splenius; tg, tongue; thy, thyrohyoid. Scale bar in H for A-H 20 µm.
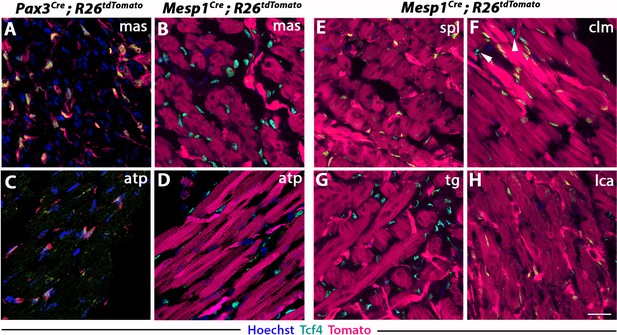
Contribution of Pax3 and Mesp1 lineages to connective tissue fibroblasts.
Immunostainings of coronal cryosections (n = 2 each condition). (A–D) Tcf4+ cells derive from Pax3 but not Mesp1 lineage in the masseter and acromiotrapezius muscles. (E–H) MCT fibroblasts in splenius (epaxial muscle) (E), cleidomastoid (F) and lateral cricoarytenoid (laryngeal muscle) (H) originate from Mesp1 lineage, but little or no contribution is observed in tongue MCT (G). atp, acromiotrapezius; clm, cleidomastoid; lca, lateral cricoarytenoid; mas, masseter; spl, splenius; tg, tongue. Scale bars: in H for A-H 20 µm.
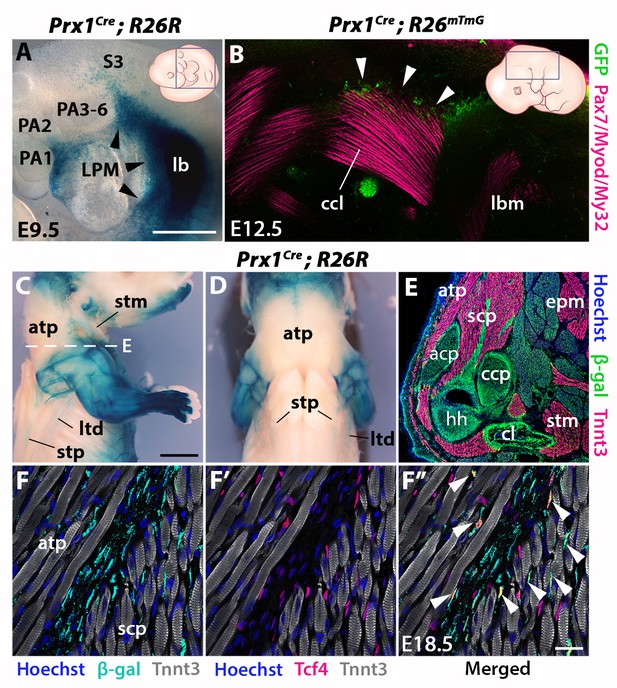
Prx1-LPM lineage contribution to neck and pectoral girdle.
See also Figure 4—figure supplement 1, 2. (A–D) X-gal stainings of Prx1Cre;R26R reporter mice at E9.5 (n = 3) (A) and E18.5 (n = 3) (C–D), and immunostaining for GFP and the Pax7/Myod/My32 myogenic markers in Prx1Cre;R26mTmG E12.5 embryo (n = 2) (B). Note Prx1-derived cells in postcranial LPM (A, black arrowheads) and Prx1-derived cells among, but not in, cucullaris myofibres (B–D). (E–F’’) Immunostaining for β-gal, Tnnt3 and Tcf4 on coronal cryosections of E18.5 Prx1Cre;R26R mice (n = 2) showed β-gal+ cells constituting the pectoral girdle (E, level C in Figure 1) and in MCT fibroblasts (F-F’’, white arrowheads), but not in trapezius myofibres. acp, scapular acromion process; atp, acromiotrapezius; ccl, cucullaris anlage; ccp, scapular coracoid process; cl, clavicle; epm, epaxial musculature; hh, humeral head; lb, forelimb bud; lbm, limb muscle anlagen; LPM, lateral plate mesoderm; ltd, latissimus dorsi; PA1-6, pharyngeal arches 1–6; S3, somite 3; scp, scapular muscles; stm, sternocleidomastoid; stp, spinotrapezius. Scale bars: in A for A, B 500 µm; in C for C-D 2000 µm, for E 500 µm; in F’’ for F-F’’ 20 µm.
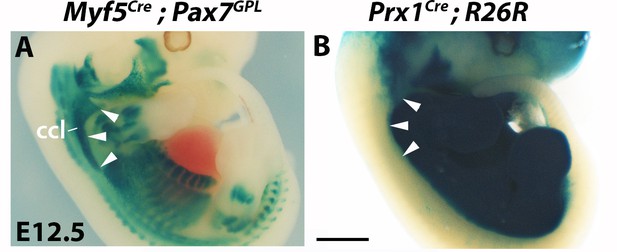
Comparison of the Myf5 and Prx1 lineage tracings.
X-gal stainings of E12.5 embryos (n = 3 each condition). Note the Myf5-derived cucullaris (ccl) excluded from the Prx1-derived LPM (A-B, white arrowheads). Scale bars in B for A-B 1000 µm.
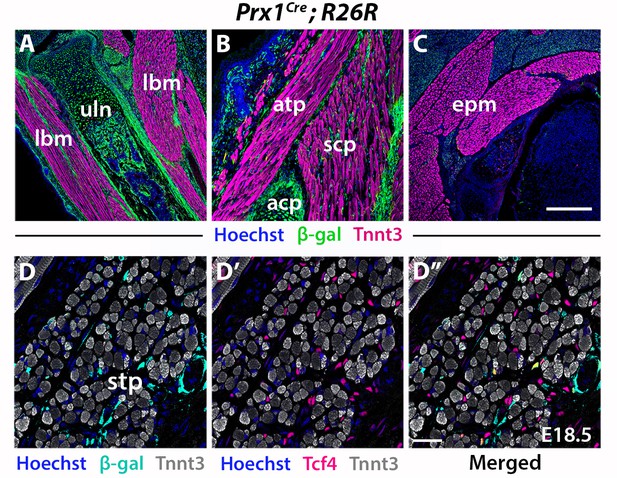
Prx1 lineage contribution to neck and limbs.
(A–C) Immunostainings on coronal cryosections (n = 2). Note Prx1-derived cells contributing to a great extent to form MCT, tendons and skeletal components of limb and shoulder (A–B); less β-gal+ cells are seen in acromiotrapezius (B); no contribution in neck epaxial muscles (C). (D–D”) Some Prx1-derived cells contribute to MCT fibroblasts of spinotrapezius, but not to myofibres. acp, scapular acromion process; atp, acromiotrapezius; ccl, cucullaris; epm, epaxial neck musculature; lbm; limb muscles; scp, scapular musculature; stp, spinotrapezius; uln, ulna. Scale bars: in C for A-C 200 µm, for D’ for D-D’ 50 µm.
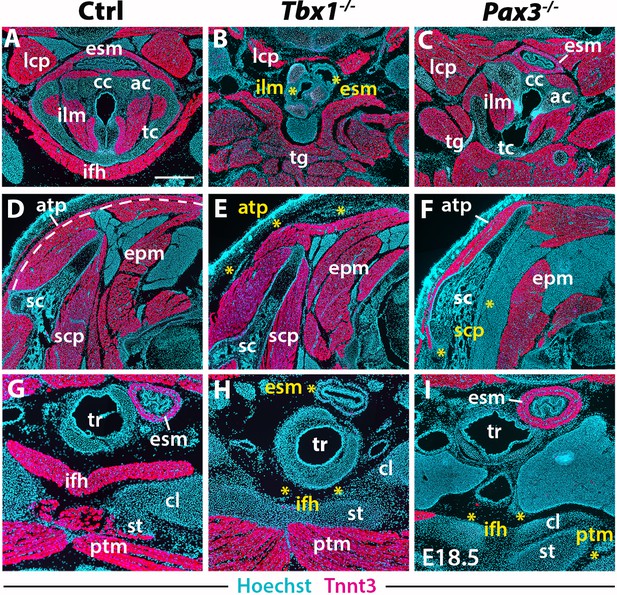
Neck muscle phenotypes in Tbx1 and Pax3 mutants.
(A–I) Immunostainings for Tnnt3 on coronal cryosections of control, Tbx1-null and Pax3-null fetuses at E18.5 (n = 3 each condition). Yellow asterisks indicate missing muscles. Note absence of branchiomeric laryngeal (ilm), esophagus (esm) and trapezius (atp) muscles and severe alteration of somitic infrahyoid muscles (ifh) in Tbx1 mutants. Scapular (scp) and pectoral (ptm) muscles are missing in Pax3 mutants. ac, arytenoid cartilage; atp, acromiotrapezius; cc, cricoid cartilage; cl, clavicle; epm, epaxial musculature; esm, esophagus striated muscle; ifh, infrahyoid muscles; ilm, intrinsic laryngeal muscles; lcp, longus capitis; ptm, pectoralis muscles; sc, scapula; scp, scapular muscles; st, sternum; tc, thyroid cartilage; tg, tongue. Scale bars: in A for A-I 500 µm.
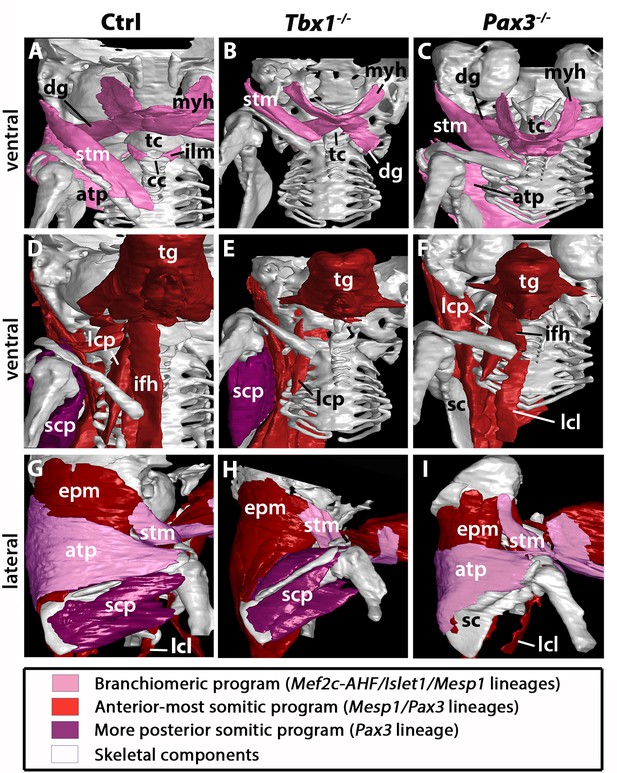
3D reconstructions of neck musculoskeletal system in Tbx1 and Pax3 mutants.
See interactive 3D PDFs in Supplementary file 1–3; control n = 1; mutants n = 2. (A–C) Branchiomeric and cucullaris-derived muscles marked by Mef2c-AHF/Islet1/Mesp1 lineages are indicated in pink. (D–F) Anterior somitic muscles (Mesp1, Pax3 lineages), in red. (G–I) Scapular muscles from more posterior somites (Pax3 lineage), in violet. atp, acromiotrapezius; cc, cricoid cartilage; dg, digastric muscles; epm, epaxial musculature; ifh, infrahyoid muscles; ilm, intrinsic laryngeal muscles; lcl, longus colli; lcp, longus capitis; myh, mylohyoid; sc, scapula; scp, scapular muscles; stm, sternocleidomastoid; tc, thyroid cartilage; tg, tongue.
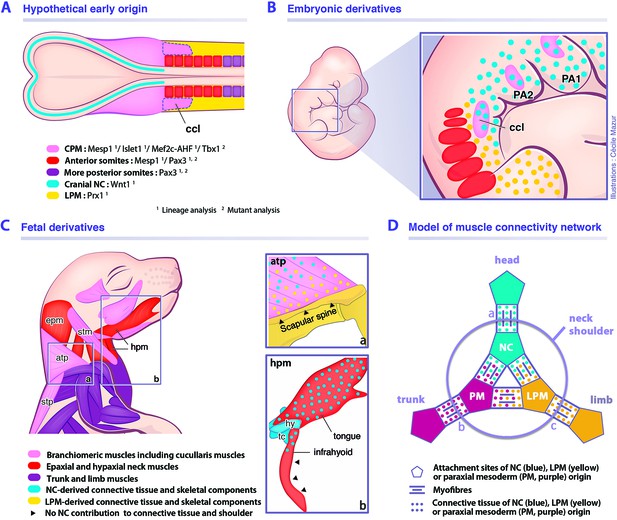
Model for musculoskeletal and connective tissue relationships during murine neck development.
See also Figure 7—figure supplement 1. (A, C) CPM (pink), anterior somites (red) and more posterior somites (violet) muscles are defined by three distinct myogenic programs. (B) Note that the cucullaris develops in a NC domain (blue dots), but is excluded from the postcranial LPM (yellow dots). (C) Dual NC/LPM origin of trapezius connective tissue is indicated in (a). NC contribution to connective tissue extends to tongue and anterior infrahyoid musculature (b). (D) Mixed origins of muscle connective tissues at the head-trunk-limb interface. Example of representative muscles: (a) masseter, (b) spinalis dorsi, (c) deltoid. atp, acromiotrapezius; ccl, cucullaris; CPM, cardiopharyngeal mesoderm; epm, epaxial neck musculature; hpm, hypaxial neck musculature; hy, hyoid bone; LPM, postcranial lateral plate mesoderm; NC, neural crest; PA1-2, pharyngeal arches 1–2; PM, paraxial mesoderm; stm, sternocleidomastoid; stp, spinotrapezius; tc, thyroid cartilage.
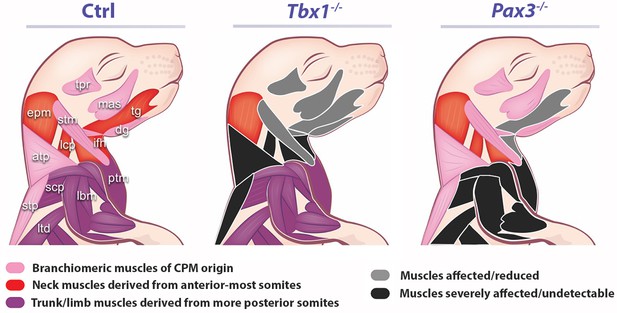
Muscles affected in Tbx1 and Pax3 mutants.
CPM (pink), anterior somites (red) and more posterior somites (violet) muscles are differentially affected (grey and black) in Tbx1 and Pax3 mutants. atp, acromiotrapezius; dg, digastric muscles; epm, epaxial musculature; ifh, infrahyoid muscles; lbm, limb muscles; lcp, longus capitis; ltd, latissimus dorsi; mas, masseter; ptm, pectoral muscles; scp, scapular muscles; stm, sternocleidomastoid; stp, spinotrapezius; tg, tongue; tpr, temporal.
Tables
Contribution of Mef2c-AHF, Islet1, Mesp1 and Pax3 lineages to neck and pectoral musculature.
https://doi.org/10.7554/eLife.40179.009| Mef2c/Islet1/Mesp1-derived muscles | Mesp1/Pax3-derived muscles | Pax3- derived muscles |
|---|---|---|
| Mylohyoid Digastric muscles Pharyngeal muscles Intrinsic laryngeal muscles Esophagus striated muscle Sternocleidomastoid Acromiotrapezius Spinotrapezius | Epaxial neck muscles (splenius, semispinalis, levator scapula, rhomboid occipitalis, suboccipital and postvertebral muscles) Hypaxial neck muscles (tongue muscles*, infrahyoid muscles, longus capitis, longus colli) | Scapular muscles (supraspinatus, Infraspinatus, subscapularis) Pectoralis Latissimus dorsi† Cutaneous maximus† |
| Branchiomeric myogenic program | Anterior-most somite myogenic program | More posterior somite myogenic program |
-
*Including intrinsic and extrinsic tongue muscles of somitic origin
†Also derived from an Islet1 lineage
Summary of the neck muscle phenotype observed in Tbx1- and Pax3-null fetuses.
https://doi.org/10.7554/eLife.40179.020| Tbx1-null | Pax3-null | |
|---|---|---|
| Branchiomeric muscles (Mef2c-AHF/Islet1/Mesp1 lineage) Mylohyoid Digastric muscles Intrinsic laryngeal muscles Esophagus striated muscle Sternocleidomastoid Acromiotrapezius | +/- +/- − − +/- − | ++ ++ + ++ + + |
| Anterior-most somite muscles (Mesp1/Pax3 lineage) Epaxial musculature Longus capitis Longus colli Infrahyoid muscles Tongue muscles* | ++ +/- − − + | + ++ ++ +/- + |
| More posterior somite muscles (Pax3 lineage) Scapular muscles Pectoralis | ++ ++ | − − |
-
++,normal; +, altered morphology; +/-, affected; -, severely affected or undetectable
*Including intrinsic and extrinsic tongue muscles of somitic origin
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus) | B6D2F1/JRj | Janvier | ||
| Genetic reagent (M. musculus) | Mef2c-AHFCre | PMID:16188249 | MGI:3639735 | Dr. Brian L Black (Cardiovascular Research Institute, University of California, USA) |
| Genetic reagent (M. musculus) | Islet1Cre | PMID:11299042 | MGI:2447758 | Dr. Thomas M Jessell (Howard Hughes Medical Institute, Columbia University, USA) |
| Genetic reagent (M. musculus) | Mesp1Cre | PMID:10393122 | MGI:2176467 | Pr. Yumiko Saga (National Institute of Genetics, Japan) |
| Genetic reagent (M. musculus) | Pax3Cre | PMID:22394517 | MGI:3573783 | Dr. Jonathan A. Epstein (Perelman Shool of Medicine, University of Pennsylvania, USA) |
| Genetic reagent (M. musculus) | Myf5Cre | PMID:17418413 | MGI:3710099 | Dr. Mario R Capecchi (Institute of Human Genetics, University of Utah, USA) |
| Genetic reagent (M. musculus) | Wnt1Cre | PMID:9843687 | MGI:J:69326 | Pr. Andrew P. McMahon (Keck School of Medicine of the University of Southern California, USA) |
| Genetic reagent (M. musculus) | Prx1Cre | PMID:12112875 | MGI: J:77872 | Dr. Clifford J Tabin (Department of genetics, Harvard Medical School, USA) |
| Genetic reagent (M. musculus) | Pax7GPL | PMID:19531352 | MGI:3850147 | Dr. Shahragim Tajbakhsh (Department of Developmental and Stem Cell Biology, Institut Pasteur, France) |
| Genetic reagent (M. musculus) | Rosa26R-lacZ | PMID:9916792 | MGI:1861932 | Pr. Philippe Soriano (Icahn School of Medicine at Mt. Sinai, USA) |
| Genetic reagent (M. musculus) | R26mTmG | PMID:17868096 | MGI:3716464 | Pr. Philippe Soriano (Icahn School of Medicine at Mt. Sinai, USA) |
| Genetic reagent (M. musculus) | R26tdTomato | PMID:20023653 | MGI:3809524 | Dr. Hongkui Zeng (Allen Institute for Brain Science, USA) |
| Genetic reagent (M. musculus) | Myf5nlacZ/+ | PMID:8918877 | MGI:1857973 | Dr. Shahragim Tajbakhsh (Department of Developmental and Stem Cell Biology, Institut Pasteur, France) |
| Genetic reagent (M. musculus) | Tbx1-null | PMID:11242110 | MGI:2179190 | Dr. Virginia Papaioannou (Department of Genetics and Development, Columbia University Medical Center, USA) |
| Antibody | Chicken polyclonal anti-β-gal | Abcam | Cat. #: ab9361 | IF (1:1000) |
| Antibody | Rabbit polyclonal anti-β-gal | MP Biomedicals | Cat. #: MP 559761 | IF (1:750) |
| Antibody | Chicken polyclonal anti-GFP | Aves Labs | Cat. #: 1020 | IF (1:500) |
| Antibody | Chicken polyclonal anti-GFP | Abcam | Cat. #: 13970 | IF (1:1000) |
| Antibody | Mouse monoclonal IgG1 anti-Islet1 | DSHB | Cat. #: 40.2D6 | IF (1:1000) |
| Antibody | Mouse monoclonal IgG1 anti-My32 | Sigma | Cat. #: M4276 | IF (1:400) |
| Antibody | Mouse monoclonal IgG1 anti-Myod | Dako | Cat. #: M3512 | IF (1:100) |
| Antibody | Mouse monoclonal IgG1 anti-Pax7 | DSHB | Cat. #: AB_528428 | IF (1:20) |
| Antibody | Rabbit polyclonal anti-Tcf4 | Cell Signalling | Cat. #: C48H11 | IF (1:150) |
| Antibody | Mouse monoclonal IgG1 anti-Tnnt3 | Sigma | Cat. #: T6277 | IF (1:200) |
| Antibody | Rabbit polyclonal anti-Tomato | Clontech | Cat. #: 632496 | IF (1:500) |
| Antibody | Mouse monoclonal IgG2a anti-Pax7 | Ozyme | Cat. #: BLE801202 | IF (1:1000) |
| Software, algorithm | GE phoenix datos|x 2.0 | GE Sensing and Inspection Technologies GmbH | ||
| Software, algorithm | 3D PDF maker | SolidWorks Corporation | ||
| Software, algorithm | Zen | Zeiss | ||
| Chemical compound, drug | X-gal | Fisher | Cat. #: 10554973 | |
| Chemical compound, drug | paraformaldehyde | Electron Microscopy Sciences | Cat. #: 15710 | |
| Chemical compound, drug | Triton X-100 | Sigma | Cat. #: T8787 | |
| Chemical compound, drug | Tween 20 | Sigma | Cat. #: P1379 | |
| Chemical compound, drug | Histoclear II | National Diagnostics | Cat. #: HS-202 |
Additional files
-
Supplementary file 1
Interactive 3D neck reconstruction of a E18.5 control fetus.
Download PDF for full details.
- https://doi.org/10.7554/eLife.40179.023
-
Supplementary file 2
Interactive 3D neck reconstruction of a E18.5 Tbx1-null fetus.
Download PDF for full details.
- https://doi.org/10.7554/eLife.40179.024
-
Supplementary file 3
Interactive 3D neck reconstruction of a E18.5 Pax3-null fetus.
Download PDF for full details.
- https://doi.org/10.7554/eLife.40179.025
-
Transparent reporting form
- https://doi.org/10.7554/eLife.40179.026





