Point of View: Managing a sustainable deep-sea ‘blue economy’ requires knowledge of what actually lives there
- Article
- Figures and data
- Abstract
- Introduction
- What animals actually live in regions targeted for industrial activity?
- The historic failure of taxonomy on the high seas
- Without faunistic knowledge, there is no knowledge
- Setting new goals
- References
- Decision letter
- Author response
- Article and author information
- Metrics
Abstract
Ensuring that the wealth of resources contained in our oceans are managed and developed in a sustainable manner is a priority for the emerging 'blue economy'. However, modern ecosystem-based management approaches do not translate well to regions where we know almost nothing about the individual species found in the ecosystem. Here, we propose a new taxon-focused approach to deep-sea conservation that includes regulatory oversight to set targets for the delivery of taxonomic data. For example, a five-year plan to deliver taxonomic and genomic knowledge on a thousand species in regions of the ocean earmarked for industrial activity is an achievable target. High-throughput, integrative taxonomy can, therefore, provide the data that is needed to monitor various ecosystem services (such as the natural history, connectivity, value and function of species) and to help break the regulatory deadlock of high-seas conservation.
Introduction
The growth of industrial activity in our oceans is astonishing. Deep-sea mining, offshore energy, underwater cables, high-seas fisheries and marine biotechnology are just some sectors of the ‘blue economy’ (European Commission, 2018). This economy is targeted for growth to satisfy our ever-increasing appetite for food, energy, technology and wealth. But as we dive deeper into our unexplored oceans to search for new resources and new ideas, there is a growing need to make these efforts sustainable.
To date, major projects associated with a sustainable approach to deep-sea exploitation have, in our opinion, been overly ecosystem-based (Pikitch et al., 2004). Attempting a holistic understanding of communities and habitats might work well in a terrestrial environment, where most of the species in a given ecosystem are known. But in our deep oceans, they are not. The vast majority of species have only recently been identified, and most remain undiscovered (Bouchet et al., 2016). We contend that sustainability and growth for the blue economy will only be achieved with an initial ‘taxon-focused approach’ to conservation. We cannot, essentially, model the ecosystem-wide impacts of deep-sea exploitation without any knowledge of the animals that live there.
Deep-sea mining is an example of a major industrial activity that has been severely constrained by a lack of basic scientific knowledge, even though the cost of obtaining this information would be small relative to the potential size of the industry. Ever since the 1960s, when John Mero first enthused the geological world with a ‘call to arms’ to reap the riches of the Pacific (manganese, or polymetallic nodules being the primary goal), the industry has been an emerging one, always ‘just ten years away’ from reality (Mero, 1965).
The engineering challenges are considerable, but not fundamentally a problem, and as far back as 1978, riser pipes were used to recover some 800 tonnes of nodules from the remarkable depth of 5,500 m (Nimmo et al., 2013). The progress of deep-sea mining since those early explorations has been well-reviewed elsewhere (Secretariat of the Pacific Community, 2013). In summary, the constraints on commercial exploitation have been driven by a remarkable global effort to regulate the deep-sea floor, thanks to the United Nations Convention on the Law of the Sea (UNCLOS) and the body that was set up under that legislation – the International Seabed Authority (ISA). Together, UNCLOS and the ISA have, commendably, put the brakes on deep-sea mining until a suitable legal, financial and environmental set of rules that are fair to all has been agreed. And it is for these environmental rules that we require this missing scientific knowledge.
What animals actually live in regions targeted for industrial activity?
The most active area for industrial seafloor activity in the high seas is a region called the Clarion-Clipperton Zone (CCZ) in the central Pacific Ocean. It is a region of approximately six million km2 with typical depths of 4,000-5,500 m and a sedimented, abyssal plain seafloor rich in polymetallic nodules – small potato-sized mineral accretions that sit on top of, or just slightly beneath, the sediment-water interface. Over 21 billion tonnes of nodules have been estimated to exist in the CCZ; moreover, for nine metals critical to industry (including manganese, cobalt and nickel essential for modern green technologies such as battery-powered cars) the reserves in the CCZ nodules exceed the entire terrestrial reserve base (Hein et al., 2013). As of July 2018, 16 exploration contracts with the ISA have been signed for nodule mining in the CCZ, including five with European Union states (Germany, France, Belgium and two with the UK). In recent years, between 10 and 20 exploration cruises have taken place every year to the CCZ (ISA, 2015). An extremely conservative estimate for the total number of research expeditions to the CCZ is at least 200, considering that it has been actively explored for the last 40 years (Glover et al., 2015).
Given that survey and exploration of the CCZ is at such astonishing levels relative to any other high-seas seafloor ecosystem, it might be expected that such basic things as a list of the animals that live there is relatively easy to obtain. But a simple analysis using the Ocean Biogeographic Information System, the most up-to-date source of taxonomic records in our oceans that pulls data from most major museum collections and databases, shows the complete opposite (Figure 1; OBIS, 2018). For example, we searched for records of annelid worms (a dominant animal in both shallow and abyssal sedimented habitats) in a five-degree box (~300,000 km2) in the shallow North Sea, recovering over 80,000 records of 1,500 taxa. However, a five-degree box centered over the eastern CCZ (the best-explored part of the CCZ) recovers just nine records from five taxa. Remarkably, even extending the search area to the entire CCZ (~6,000,000 km2) still recovers only three additional records (Figure 1).
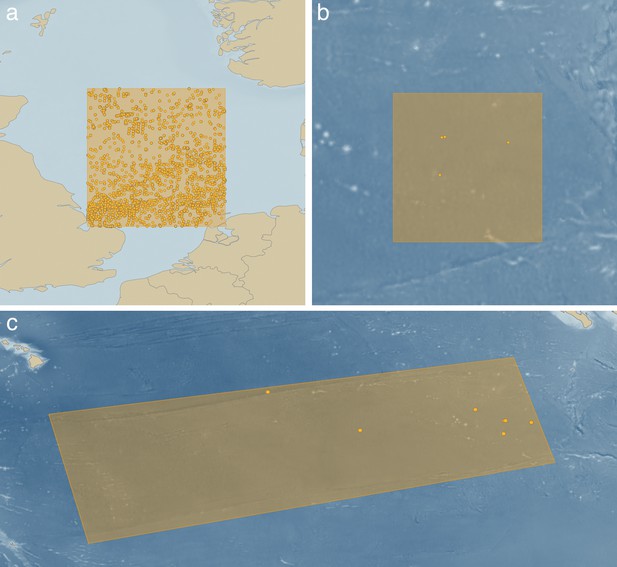
Highlighting the absence of faunistic data in deep-sea mining exploration regions using the Ocean Biogeographic Information System.
(a) A 5° (300,000 km2) search box centered on the shallow North Sea with over 80,000 records from 1,500 annelid worm taxa. (b) The same size search box centered on the eastern Clarion-Clipperton Zone with just nine records from five taxa. (c) An expanded search box for the entire six million km2 CCZ showing only 12 records (OBIS, 2018). Criteria used: Phylum: Annelida, Sample Depth >500 m.
So, is the CCZ particularly depauperate in annelid worms? It is not, and we know this from the few broad-scale studies of community ecology, which show that the abyssal CCZ is one of the most biodiverse sedimented marine habitats on our planet (Glover et al., 2002; Neal et al., 2011). Abyssal plain annelid worm biodiversity is typically two to three times the diversity of shallow slope environments along our continental margins (Neal et al., 2011). The reason for the lack of faunal lists of the CCZ is that there has been almost no taxonomy done, and no systematic archiving of faunal data with accessible, vouchered and databased material in open, curated collections.
The historic failure of taxonomy on the high seas
It is worth reflecting briefly on the reasons for this taxonomic failure, before discussing the implications and some potential solutions. There has certainly been no lack of collecting effort; we know that many hundreds of CCZ expeditions have taken place and perhaps tens of thousands of biological samples have been collected (ISA, 2015; Glover et al., 2015). Almost certainly, the reason is the lack of resources to complete post-expedition analysis and archiving of samples and data. Typically, a deep-sea expedition has a much higher relative cost compared to an inshore or terrestrial operation. A typical 30-day expedition with 20 scientists and 20 crew to the CCZ on a modern research vessel would cost $1–2m, depending on equipment used and excluding the salary costs of the researchers. Given that most wealthy nations funding large-scale science projects typically work on a three to five year set budget of per year, it is obvious to see that a deep-sea expedition will have a much lower relative budget for post-expedition analysis.
The issue is exacerbated by what is probably best described as a ‘fear of the scale of the problem’. Or, the job is so big that nobody actually wants to start it. If there are 10,000 species to describe and archive from the CCZ, and a funded project can only realistically do 100 species, a 1% target may not suffice to impress a grant-awarding body. Furthermore, taxonomic assessment is generally not seen as hypothesis-driven research, and can as such be lower-ranked than other projects (House of Lords, 2008). Interestingly, in recent years, almost all taxonomic studies in the CCZ that include full archiving of samples and genetic data have been funded by private contractors. These investors have realized how important baseline taxonomic data are, and are not perhaps as concerned with the trending fashions of ecosystem-based science (Dahlgren et al., 2016; Glover et al., 2016; Wiklund et al., 2017; Gooday et al., 2018; Lim et al., 2017). Nevertheless, these studies represent informal and formal descriptions of only 54 taxa (none of which are annelids) out of perhaps several thousand left to describe.
Without faunistic knowledge, there is no knowledge
The father of modern taxonomic nomenclature – Carl Linnaeus – recognized in the 18th century that in order to make better use of biological diversity it was essential to systematize and standardize the naming system of species. The voyaging of Daniel Solander, James Cook, Joseph Banks and others during the Enlightenment was largely driven by a desire to categorize and make use of the world – an early form of a sustainable blue economy. As we explore newly-discovered parts of our planet today, this need remains. However, the modern obsession with the ecosystem-based approach to conservation and sustainability – commendable in regions where we know the components of those ecosystems – does not translate well to the deep oceans where our knowledge is so poor.
It is a central dogma of modern conservation that one of the main reasons we seek to conserve the world around us is for the essential services it provides. Some of these, soil quality or inshore fisheries for example, are quite easy to understand and hence can be used as clear policy frameworks. Translating these types of services into a deep-sea context is challenging, but has been attempted. For example, Thurber et al. list carbon sequestration, methane oxidation, hydrocarbon extraction, deep-sea mining, deep-sea fisheries and many other examples of services provided by the deep seas; they also make a noble attempt to list cultural benefits (such as inspiration and education) that are provided by research into the deep seas (Thurber et al., 2014). But combining abiotic services such as carbon sequestration or mineral wealth, which are not essentially reliant on seafloor animals, with biotic ones such as fisheries, leads to a rather confusing conservation message. The truth is that the ecosystem function, let alone service, of most deep-sea animals is completely unknown, as the animals themselves are undiscovered.
We contend that in order to understand the functioning of, and services provided by our deep-ocean ecosystems, we must study the integrative taxonomy and natural history of the animal components of that ecosystem. A fully integrative taxonomy that includes all available information on the biology and traits of new organisms coupled to archived specimens is a clear necessity for the study of ecosystem function and service (Will et al., 2005; Wheeler, 2018). Such an approach applied to conservation in the deep sea would open up both new discoveries of ecosystem services and new approaches to management, based on hard evidence. We present here two contrasting examples.
In 2001, a remarkable new species of animal called the ‘scaly-foot gastropod’ was discovered at the hydrothermal vents of the deep Indian Ocean (Warén et al., 2003). Now formally known as Chrysomallon squamiferum (Chen et al., 2015a), this animal is typical of charismatic vent fauna in its evolutionary novelty and remarkable adaptations – the only known metazoan to use iron in its skeleton (Figure 2a). The discovery and detailed taxonomic study of this single animal has opened up a vast wealth of new knowledge on both the ecosystem value of hydrothermal vents and the challenges of management in an extraordinarily spatially-constrained and at-risk habitat (Chen et al., 2015a; Chen et al., 2015b; Chen et al., 2015c; Sigwart et al., 2017). It is hard to imagine how it would be possible to ‘value’ the Indian Ocean vent sites without knowledge of this taxon – it is this iconic species that defines the vent in its scientific and public worth.
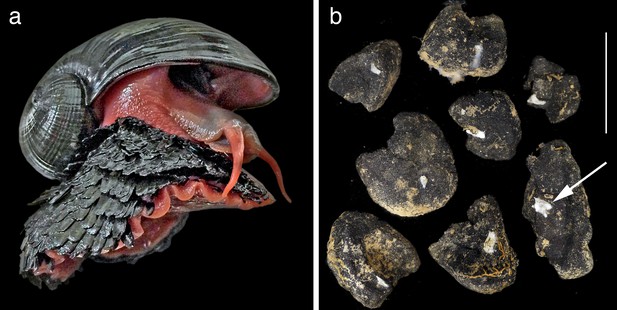
Two examples of a taxon-focused approach to conservation in the deep sea that identify both new discoveries of ecosystem services and new approaches to management based on hard evidence.
(a) The ‘scaly-foot gastropod’ Chrysomallon squamiferum is the ‘signature’ taxon discovered at Indian Ocean hydrothermal vents. As the only known animal to use iron in its skeleton, its discovery opens up new biological knowledge and the ability to ‘value’ environments such as hydrothermal vents (Chen et al., 2015c). Shell length 4 cm. (b) The small sponge Plenaster craigi is probably the most common animal living on nodules in the Clarion-Clipperton Zone, and was only described in 2017 (Lim et al., 2017). It is also a potentially useful monitoring taxon given our new knowledge of its distribution and functional role in filter-feeding on the small potato-sized nodules targeted for deep-sea mining. Scale bar 5 cm.
The second example is the newly-described deep sea sponge Plenaster craigi (Figure 2b; Lim et al., 2017). Contrary to the constrained vent habitat of the scaly-foot gastropod, P. craigi lives in what must be the largest continuous and open ecosystem on the planet – the eastern Pacific abyssal plain. Despite over 40 years of intensive sampling in the CCZ, the most abundant animal living on nodules had been completely overlooked. As a slow-growing, widespread filter-feeding sponge with perhaps a rich microbiome with biotechnological spin-offs, it would seem be exactly the sort of animal that one would want to know about before deciding on a management strategy for the region. Yet, it is only now that we have taxonomic information on it, and it is only a single taxon out of so many more.
Faunistic knowledge gained through a taxon focus provides more than an intrinsic value to ecosystems. In the case of P. craigi, new microsatellite DNA data demonstrate the potential value of a new protected area to the south of the mining zones (Taboada et al., 2018). And more broadly, taxonomic knowledge that includes spectacular images or videos of deep-sea animals is invaluable in connecting broader society to the intrinsic value of our wilderness regions. Ideally, sustainable development should lead to both economic benefits and natural benefits to people – and if people are unaware of what they are conserving, they do not realize the benefit.
Setting new goals
The ISA has made commendable efforts to regulate industrial activity in the deep sea, and in recent years, a raft of documents and recommendations has been produced, outlining both the process of gaining an exploitation contract (ISA, 2018) and a broader regional environmental management plan for some areas, including the CCZ (ISA, 2011). But we think that there has been an over-emphasis on a ‘holistic’ ecosystem approach to environmental data and ecosystem services, when what is most urgently needed are things as basic and fundamental as a list of the species that live in a contracted region, linked to archived samples and genetic material. The universally recognized International Union for Conservation of Nature (IUCN) Red List, for example, is widely used to manage terrestrial ecosystems. But information required for assessment is lacking for the vast majority of the named deep-sea animals, and those undescribed are not even eligible for evaluation.
A reasonable solution would be for the regulator (the ISA) to set targets for the provision of new taxonomic data that includes accessible vouchered material and genetic data. Deep-sea contractors are already funding some taxonomic work. For example, in the period between June 2017 to June 2018, eight peer-reviewed papers on the taxonomy of CCZ animals were published (Glover, 2018). These efforts should be recognized and encouraged by the ISA, and perhaps a minimum target set of 100 taxonomic descriptions per year (95 appeared in the period described above). This could be relatively easily achieved with high-throughput, integrative DNA taxonomy approaches (Glover et al., 2015) and use of existing quality samples. This would mean that in ten years’ time (when some might quip that deep-sea mining will finally start), 1,000 species from the CCZ would be described. With a very modest funding boost that could be shared jointly amongst contractors and stakeholders, this could easily be delivered within five years. Knowledge of a thousand new species would be a revolutionary leap in our understanding of the biodiversity, connectivity, community ecology, ecosystem function and services of this vast region of our planet. Globally, a new emphasis on taxon-focused conservation may be the only way to develop the sustainable blue economy that we all desire.
References
-
BookHow many species of molluscs are there in the world’s oceans, and who is going to describe them?In: Strong E, Bouchet P, editors. Tropical Deep-Sea Benthos 29. Paris: Muséum National d'Histoire Naturelle. pp. 9–24.
-
Polychaete species diversity in the central Pacific abyss: local and regional patterns, and relationships with productivityMarine Ecology Progress Series 240:157–170.https://doi.org/10.3354/meps240157
-
An end-to-end DNA taxonomy methodology for benthic biodiversity survey in the Clarion-Clipperton Zone, central Pacific abyssJournal of Marine Science and Engineering 4:2.https://doi.org/10.3390/jmse4010002
-
Abyssal fauna of the UK-1 polymetallic nodule exploration claim, Clarion-Clipperton Zone, central Pacific Ocean: EchinodermataBiodiversity Data Journal 25:e7251.
-
WebsiteNew scientific data from exploration contract areas relevant to the management of deep-sea miningAccessed November 13, 2018.
-
Five new species and two new genera of xenophyophores (Foraminifera: Rhizaria) from part of the abyssal equatorial Pacific licensed for polymetallic nodule explorationZoological Journal of the Linnean Society 183:723–748.https://doi.org/10.1093/zoolinnean/zlx093
-
WebsiteDraft regulations on the exploitation of mineral resources in the AreaAccessed November 13, 2018.
-
Polychaete species diversity on the West Antarctic Peninsula deep continental shelfMarine Ecology Progress Series 428:119–134.https://doi.org/10.3354/meps09012
-
WebsiteGlobal biodiversity indices from the Ocean Biogeographic Information SystemAccessed November 13, 2018.
-
BookDeep Sea Minerals: Manganese Nodules, a Physical, Biological, Environmental, and Technical ReviewBaker E, Beaudoin Y, editors. Secretariat of the Pacific Community.
-
Ecosystem function and services provided by the deep seaBiogeosciences 11:3941–3963.https://doi.org/10.5194/bg-11-3941-2014
-
Blank canvas: the case for descriptive taxonomyIntegrative and Comparative Biology.https://doi.org/10.1093/icb/icy067
-
The perils of DNA barcoding and the need for integrative taxonomySystematic Biology 54:844–851.https://doi.org/10.1080/10635150500354878
Decision letter
-
Helga GrollReviewing Editor; eLife, United Kingdom
-
Peter A RodgersSenior Editor; eLife, United Kingdom
In the interests of transparency, eLife includes the editorial decision letter and accompanying author responses. A lightly edited version of the letter sent to the authors after peer review is shown, indicating the most substantive concerns; minor comments are not usually included.
Thank you for submitting your article "Managing a sustainable deep-sea 'blue economy' requires knowledge of what actually lives there" to eLife for consideration as a Feature Article. Your article has been reviewed by three peer reviewers. The following individuals involved in review of your submission have agreed to reveal their identity: Derek Tittensor (Reviewer #1); Ann Vanreusel (Reviewer #2); Nelia Mestre (Reviewer #3).
We invite you to submit a revised version of the manuscript that addresses the essential revisions listed below.
Summary:
The reviewers find that Glover et al. present an excellent and compelling opinion feature that describes the serious gaps in our deep-sea taxonomic knowledge and expertise. It is very well written and the manuscript provides an excellent series of arguments to support the point the authors want to make: stimulate taxonomic research in the CCZ area. While the 'taxonomic impediment' of the deep ocean is reasonably well-known within the deep-sea scientific community, the authors link it to the rapid ramping-up of the 'blue economy' and how it hinders our attempts to effectively manage such ecosystems. The authors provide clear and correct arguments, which may have a major impact on the future research and management of the area so generating a significant impact. It may also become a highly cited paper for a large and still growing community that is contributing in one way or another to the development of an environmental management plan for the area.
Essential revisions:
1) The authors suggest that ecosystem-based management requires knowledge of species identities and their taxonomic description. It is not clear to me that this is the case. Why could such management not proceed simply with species functioning identified – i.e. is the role that they play in the ecosystem not more important for ensuring management for ecosystem function rather than taxonomic description? If the role of a species, or a functional group of species, can be identified, why do they additionally need to go through the process of formal description? Having rapid automated approaches or coarse taxonomic or morphological/genetic approaches to identifying ecosystem function (e.g. through key traits) would surely be a more rapid way to respond to commercial pressures on the deep-sea?
I am not a taxonomist or geneticist, but it is also not clear why using OTUs (and storing genetic data) cannot take the place of full taxonomic identification? Surely the pressing urgency of growing industrial usage requires creative solutions that can bypass the slow description process? Can the authors not suggest additional novel solutions to be coupled with the centuries-old (though undeniably important) process of taxonomic description?
Perhaps the authors could look into the literature on why species identity is important over and above species traits or functioning for EBM. For example, a partial answer might be that having multiple species fulfilling the same role can provide a buffer against changing conditions, but there is more out there on the benefits of species diversity vs. functional diversity.
The Red List argument given later on in the manuscript is more compelling as a reason for accelerating taxonomic description – but naming species is not enough for Red List assessment. Fairly good knowledge of ranges and population abundance is also required for an assessment of extinction risk. Is this really feasible simply with somewhat more intensive sampling of the CCZ in the next decade, or is it realistically out of reach in the near future?
Improving our taxonomic knowledge of deep-sea ecosystems is clearly a worthwhile cause. But for a robust argument, the 'added value' over new or alternative approaches – or even identifying traits and functioning – needs to be more clearly articulated. Ideally within the context of an integrated approach that couples taxonomy with more rapid-response or precautionary approaches to deep-sea stewardship outlined herein.
2) Major concerns relate to the highlights of the absence of faunistic data, demonstrated by Figure 1, where for CCZ only 5 taxa of annelid worms are registered. However, in the next heading, the authors report a massive effort in the last years where 54 taxa have been described. This is rather confusing to the reader, and raises the questions: How many of these are annelid worms? Only the 5 taxa reported previously? If there are more, why is this missing from the OBIS database? If more taxa of annelid worms in the CCZ is available, the data should either be included in the OBIS maps in Figure 1, or another map should be added to the figure including the taxa, denoting that it was described in the last 2-3 years, or at least a good justification should be made to make it clearer. At least, it would be useful to report the previous example of annelid worms, otherwise, the important point of the message of this article gets a bit lost.
3) In the second paragraph, the authors suggest that with modest funding 1,000 species could be described in 5 years. I think that following this sentence it would be very useful to point if new cruises are still necessary or not, and if not make it clear that there are already enough specimens preserved in museums, etc, just waiting to be described thus emphasising the idea that no extra cruises are needed, as this reassures that indeed with modest funding this is achievable.
https://doi.org/10.7554/eLife.41319.004Author response
Essential revisions:
1) The authors suggest that ecosystem-based management requires knowledge of species identities and their taxonomic description. It is not clear to me that this is the case. Why could such management not proceed simply with species functioning identified – i.e. is the role that they play in the ecosystem not more important for ensuring management for ecosystem function rather than taxonomic description? If the role of a species, or a functional group of species, can be identified, why do they additionally need to go through the process of formal description? Having rapid automated approaches or coarse taxonomic or morphological/genetic approaches to identifying ecosystem function (e.g. through key traits) would surely be a more rapid way to respond to commercial pressures on the deep-sea?
I am not a taxonomist or geneticist, but it is also not clear why using OTUs (and storing genetic data) cannot take the place of full taxonomic identification? Surely the pressing urgency of growing industrial usage requires creative solutions that can bypass the slow description process? Can the authors not suggest additional novel solutions to be coupled with the centuries-old (though undeniably important) process of taxonomic description?
Perhaps the authors could look into the literature on why species identity is important over and above species traits or functioning for EBM. For example, a partial answer might be that having multiple species fulfilling the same role can provide a buffer against changing conditions, but there is more out there on the benefits of species diversity vs. functional diversity.
The Red List argument given later on in the manuscript is more compelling as a reason for accelerating taxonomic description – but naming species is not enough for Red List assessment. Fairly good knowledge of ranges and population abundance is also required for an assessment of extinction risk. Is this really feasible simply with somewhat more intensive sampling of the CCZ in the next decade, or is it realistically out of reach in the near future?
Improving our taxonomic knowledge of deep-sea ecosystems is clearly a worthwhile cause. But for a robust argument, the 'added value' over new or alternative approaches – or even identifying traits and functioning – needs to be more clearly articulated. Ideally within the context of an integrated approach that couples taxonomy with more rapid-response or precautionary approaches to deep-sea stewardship outlined herein.
The reviewers have raised some important points that relate to both how taxonomy has changed over the years and the value of taxonomy to other areas of biology, such as ecosystem function. There is rather a long debate in the general biology and philosophy of science literature on this and it is beyond the scope of our short piece to go into great detail. So, what we have done is insert a new sentence and references to two key papers, the first on ‘integrative taxonomy’ by Will et al., 2005, and the second a recent update to this argument by Wheeler, 2018. It is quite hard to express the importance of descriptive, integrative taxonomy better than this quote from the Wheeler, 2018, paper so I reproduce it here:
“The case for descriptive taxonomy. Imagine walking into a gallery of a major art museum and being disappointed to see all the paintings have been hung face-to-the-wall, each with a barcode glued to the reverse of the canvas (Figure 1). Handed a barcode reader, you are told by the docent that you can confidently identify each and every painting. Reading barcodes, you can tell a da Vinci from a de la Tour, but to what end? It is the unique combinations of subject, technique, colors, composition, and the emotions evoked by the works in their totality, that we seek. A taxonomy based on DNA alone has great utilitarian value, but falls far short of its full potential when combined with descriptive taxonomy to reveal in vivid detail the diversity, origins, and history of complex anatomical attributes of species and clades.”
In specific response to our reviewer, they have questioned whether taxonomy is needed if one can identify taxa using OTUs, learn about their traits, their functions and apply that to conservation issues in the deep sea. Our response is that identifying taxa using DNA (the OTU method), describing the traits of those taxa (e.g. morphology, size, fecundity, dispersal ability, feeding mode, symbioses, associations, biological interactions etc.) and working out what role they play in an ecosystem is exactly what integrative and descriptive taxonomy actually is. This is what we are proposing. We call it taxonomy because a name must be applied to these OTUs in order to link all the information (e.g. traits) that we know about them, and they must be linked to a voucher specimen (type) and where the animal was identified with DNA – the sequences and tissue sample so that can be checked if needed. The linkages of the name to the data to the original source information (the specimen) is just standard best scientific practice across any discipline. There is nothing particularly special about it.
In regards to the points raised by the reviewer that we are not pushing forward ‘alternatives’ to taxonomy (e.g. rapid-throughput barcoding, metabarcoding etc.) is that we do not think these are ‘alternatives’ to taxonomy, they are just a new way of doing taxonomy. We are fully behind them, and in fact the recent papers we cite use a rapid-throughput method (described in detail in Glover et al., 2015) that delivers archived and accessible taxonomic information that can be built upon in the future. These are ‘turbo-taxonomy’ approaches based primarily on DNA that deliver the necessary taxonomic data for deep-sea stewardship in a more rapid-way than individual species descriptions.
We have added sentences and references to these comments in the last two sections of the manuscript which we think deal with these useful comments and improve the manuscript considerably.
2) Major concerns relate to the highlights of the absence of faunistic data, demonstrated by Figure 1, where for CCZ only 5 taxa of annelid worms are registered. However, in the next heading, the authors report a massive effort in the last years where 54 taxa have been described. This is rather confusing to the reader, and raises the questions: How many of these are annelid worms? Only the 5 taxa reported previously? If there are more, why is this missing from the OBIS database? If more taxa of annelid worms in the CCZ is available, the data should either be included in the OBIS maps in Figure 1, or another map should be added to the figure including the taxa, denoting that it was described in the last 2-3 years, or at least a good justification should be made to make it clearer. At least, it would be useful to report the previous example of annelid worms, otherwise, the important point of the message of this article gets a bit lost.
We realise this is a bit confusing and we have fixed it. Firstly, we have updated the map in Figure 1C which had excluded a couple of data points (thus addressing the issue of why the distribution of the points in the maps looked a bit different – something picked up by another reviewer). There are now 12 records (not 9) across the whole CCZ for benthic annelids. We have also made it clear that this is for records below 500m in order to remove some surface plankton tows (which account for quite a few records on OBIS). Secondly, in regards the 54 taxa described in the papers listed, none are annelids. We are working furiously on annelid papers, but it’s a huge job and we have not finished yet. We have published only on cnidaria, echinoderms and molluscs so far – those are the 54 taxa. We have clarified this in the text. The data in Figure 1 is correct, as is the 54 taxa mentioned in the text. Thanks for the help here.
3) In the second paragraph, the authors suggest that with modest funding 1,000 species could be described in 5 years. I think that following this sentence it would be very useful to point if new cruises are still necessary or not, and if not make it clear that there are already enough specimens preserved in museums, etc, just waiting to be described thus emphasising the idea that no extra cruises are needed, as this reassures that indeed with modest funding this is achievable.
We have added a sentence here that addresses this and links to the concept above of high-throughput taxonomy and mentions the need for working on existing samples. Some additional sampling is likely to be needed. We think it is precautionary to not rule out additional sampling as particularly for megafauna (large animals) this is still urgently needed. But it is correct that working on existing DNA-fixed samples could deliver perhaps the majority of what is needed. Thanks for the comment.
https://doi.org/10.7554/eLife.41319.005Article and author information
Author details
Funding
UK Seabed Resources Ltd
- Adrian G Glover
- Thomas G Dahlgren
European Commission (603418)
- Adrian G Glover
- Thomas G Dahlgren
Gordon and Betty Moore Foundation
- Adrian G Glover
- Thomas G Dahlgren
Mohamed bin Zayed Species Conservation Fund (182518473)
- Chong Chen
Svenska Forskningsrådet Formas (2014-12285-29251-29)
- Thomas G Dahlgren
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
The authors acknowledge the many colleagues in deep-sea biology who have contributed to informal discussions relevant to this manuscript, in particular Craig R Smith, Daniel Jones, Pedro Martinez Arbizu, Jeff Drazen, Nick Higgs, Diva Amon, Magdalena Georgieva, Julia Sigwart, Sergi Taboada and Andy Gooday. We also thank the reviewers for their helpful comments. The authors are funded by a range of sources that have assisted in developing these ideas including UK Seabed Resources Ltd, the Gordon and Betty Moore Foundation, the European Commission, JPI Oceans, the Mohamed bin Zayed Species Conservation Fund and the Swedish Research Council FORMAS.
Publication history
- Received:
- Accepted:
- Version of Record published:
Copyright
© 2018, Glover et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 3,171
- views
-
- 461
- downloads
-
- 76
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Citations by DOI
-
- 76
- citations for umbrella DOI https://doi.org/10.7554/eLife.41319






