Timing mechanism of sexually dimorphic nervous system differentiation
Figures
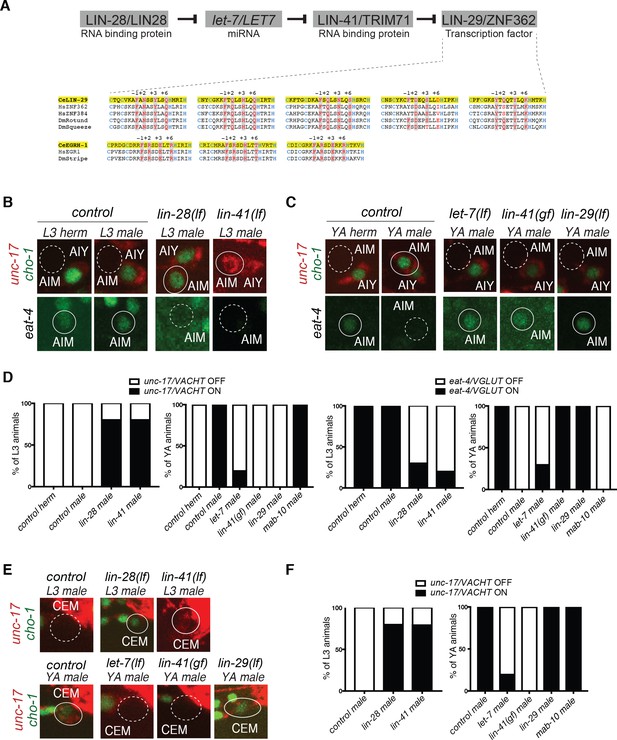
Heterochronic pathway effects on AIM and CEM neuronal differentiation during sexual maturation.
(A) A simplified schematic of the heterochronic pathway, deduced from the genetic analysis of hypodermal cell lineage division events. The LIN-28 RNA binding protein prevents the maturation of miRNA let-7. When LIN-28 protein levels go down at the end of the second larval stage (L2), mature miRNA let-7 levels rise and bind to the 3’UTR of let-7 targets. One let-7 target is the RNA-binding protein LIN-41, whose levels are downregulated at the onset of sexual maturation (L4). This allows for its negative regulator, the zinc-finger transcription factor lin-29, to be expressed at the L4 stage to induce the larval to adult transition. Sequence homology alignment of the five Zn-finger domains shows that LIN-29 is an orthologue of human ZNF362 and ZNF384, while EGRH-1 is an orthologue of human EGR. The Zn fingers of worm, fly and human are shown. Zn coordinating Cys and His residues are colored in blue and DNA-contacting residues (at position −1,+2,+3 and+6 of the helixes preceding the His residues) are colored in red. Conservation is indicated in grey shading. (B) AIM neuronal differentiation is precocious in lin-28(n719lf) and lin-41(bch28lf). unc-17/VACHT (ot907) and cho-1/CHT (otIs534) cholinergic reporter expression (cytoplasmic for unc-17 and nuclear for cho-1) is not observed in AIM in control animals at the L3 stage (dotted circles, top panels). lin-28(n719lf) and lin-41(bch28lf) mutant males show precocious cholinergic gene expression in AIM at the L3 stage (top panels). eat-4/VGLUT (otIs388) glutamatergic reporter expression is observed in AIM in control animals at the L3 stage (circled in white, bottom panels). lin-28(n719lf) and lin-41(bch28lf) mutant males show precocious loss of AIM glutamatergic identity (dotted circles, bottom panels). The AIY cholinergic neuron is located next to AIM and is used as a positional reference. Solid circles indicate expression of the reporter, stippled circle indicates loss of expression. (C) Male-specific AIM differentiation is blocked in let-7(n2853ts), lin-41(xe8gf) and lin-29a/b(n333lf) mutants. unc-17/VACHT (ot907) and cho-1/CHT (otIs354) cholinergic reporter expression (cytoplasmic for unc-17 and nuclear for cho-1) is observed in young adult (YA) control males but not in AIM neurons in hermaphrodites nor let-7(n2853ts), lin-41(xe8gf) and lin-29a/b(n333lf) mutant males (dotted circles, top panels). eat-4/VGLUT (otIs388) glutamatergic reporter expression is observed in hermaphrodites and in let-7(n2853ts), lin-41(xe8gf) and lin-29a/b(n333lf) mutant males, where it fails to be downregulated (circled in white, bottom panels). L1 let-7(n2853ts) animals were shifted to the restrictive temperature (25°C) and imaged after 48hs. The incomplete penetrance of the let-7 mutants is likely because the allele used, n2853, a point mutation in the miRNA seed region, is hypomorphic. The AIY cholinergic neuron is located next to AIM and is used as a positional reference. (D) Quantification for the AIM neurotransmitter switch in heterochronic mutants lin-28(n719lf), lin-41(bch28lf), let-7(n2853ts), lin-41(xe8gf), lin-29a/b(n333lf) and mab-10(xe44) (n = 15). Expression of unc-17/VACHT (ot907) and eat-4/VGLUT (otIs388) was quantified as ON or OFF. (E) CEM neuronal differentiation defects in heterochronic mutants. CEM cholinergic gene expression is precocious in lin-28(n719lf) and lin-41(bch28lf) males compared to control. unc-17/VACHT (ot907) and cho-1/CHT (otIs354) cholinergic reporter expression is not observed in CEM neurons in wild-type males at the L3 stage (dotted circle). lin-28(n719lf) and lin-41(bch28lf) males show precocious cholinergic gene expression in CEM neurons at the L3 stage (circled in white, top panels). CEM cholinergic gene expression is lost in let-7(n2853ts) and lin-41(xe8gf) mutants (dotted circles). lin-29a/b(n333lf) mutants showed no defect in cholinergic gene expression in CEM neurons (circled in white). L1 let-7(n2853ts) animals were shifted to the restrictive temperature (25°C) and imaged after 48hs. The incomplete penetrance of the let-7 mutants is likely because the allele used, n2853, a point mutation in the miRNA seed region, is hypomorphic. (F) Quantification for CEM cholinergic gene expression in heterochronic mutants lin-28(n719lf), lin-41(bch28lf), let-7(n2853ts), lin-41(xe8gf), lin-29a/b(n333lf) and mab-10(xe44) (n = 15). Expression of unc-17/VACHT (ot907) was quantified as ON or OFF.

Expression pattern for the endogenously tagged unc-17/VACHT allele during development.
We used CRISPR/Cas9 genome editing to place an mKate2::3xFLAG tag at the C-terminal end of the unc-17/VACHT locus. The endogenously tagged cholinergic locus (ot907) recapitulates our previously described unc-17/VACHT expression, assessed with a fosmid-based reporter. The confocal images show that unc-17/VACHT is expressed in AIM sex-shared neurons only in the males (bottom panels), but not in hermaphrodites (top panels), starting at the L4 stage. Each image square inset is zoomed in at the top right corner. AIM neurons are circled inside each inset at the different developmental stages. CEM male-specific neurons showed unc-17/VACHT expression also starting at the L4 stage (circled at the different larval stages). The cho-1/CHT fosmid-based yfp reporter (otIs534) was used to label the neuronal nuclei of cholinergic neurons facilitating their identification.
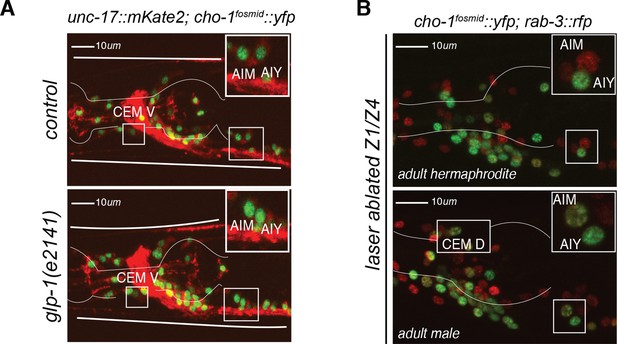
AIM and CEM neuronal differentiation does not require the gonad nor the germline.
(A) Germline loss has no effect on AIM nor CEM cholinergic gene expression in males. glp-1(e2141) mutants showed no difference in expression of cholinergic genes unc-17/VACHT and cho-1/CHT in AIM and CEM neurons compared to control adult males. Confocal images for a young adult hermaphrodite (top panel) and young adult male (bottom panel) are shown. (B) Laser ablation of gonad precursors has no effect on AIM and CEM cholinergic gene expression in males. Z1/Z4 gonad and germline precursors were ablated at the L1 stage. No difference was observed in the expression of the cholinergic gene cho-1/CHT (otIs534) in the AIM and CEM neurons of Z1/Z4 ablated adult male animals compared to control animals. All neurons are labeled with a red pan-neuronal rab-3 reporter (otIs355).
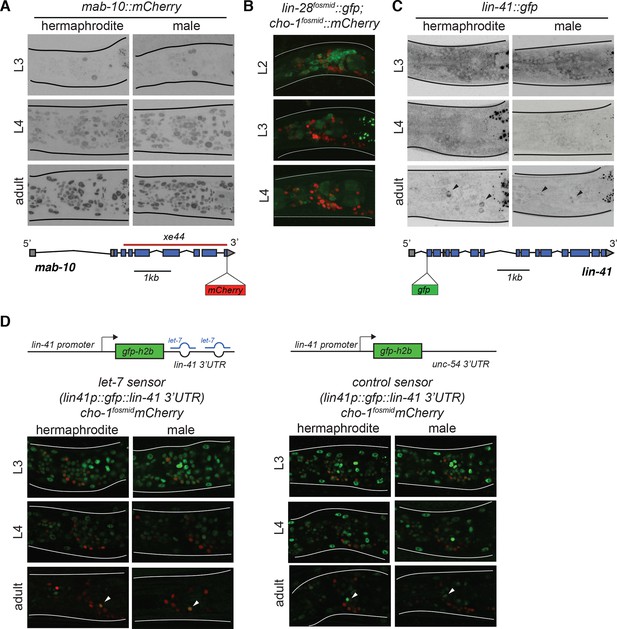
Temporal expression pattern of heterochronic pathway genes in the nervous system.
(A) mab-10::mCherry expression pattern during larval development in both sexes. An endogenously tagged mab-10 allele (xe75) showed ubiquitous expression starting in the L4 stage, including in the nervous system. Reporter expression was maintained in the adult. No sexually dimorphic differences were observed for mab-10 expression. A cartoon for the mab-10 locus is shown below the images. (B) lin-28::gfp expression pattern during early larval development. A lin-28::gfp fosmid-based reporter (wgIs535) showed ubiquitous expression at the L2 stage, including in the nervous system. Reporter expression was broadly downregulated at the L3 and L4 stages. The fosmid-based cho-1/CHT reporter (otIs544) was used to label cholinergic neurons. No sexually dimorphic differences were observed for lin-28 expression. (C) lin-41 expression pattern during larval development in both sexes. An endogenously tagged lin-41 allele (tn1541) showed ubiquitous expression at the L3 stage, including in the nervous system. Reporter expression is broadly downregulated at the L4 stage. Expression of lin-41::gfp was maintained in two neuronal pairs in the head (black arrows). No sexually dimorphic differences were observed for lin-41 expression. A cartoon for the lin-41 locus is shown below the images. (D) let-7 activity sensor and control sensor expression during larval development in both sexes. A lin-41 promoter fusion driving gfp and fused to the lin-41 3’UTR (xeSi182), which is directly targeted by let-7 miRNA, showed ubiquitous expression of gfp in the nervous system from the L1 to the L3 stage. At the L4 stage, reporter expression is broadly downregulated and only a few cells are labeled by GFP in the adult head, recapitulating the lin-41::gfp expression. The same lin-41 promoter fusion driving gfp and fused to the unc-54 3’ UTR (xeSi202) failed to be normally downregulated at the L4 stage showing that lin-41 downregulation requires its 3’UTR being bound by miRNA let-7. Cholinergic neurons were labeled by a cho-1/CHT fosmid reporter (otIs544) driving mCherry allowing us to correlate the lin-41 downregulation with AIM neurotransmitter switch in males at the L4 stage. No sexually dimorphic differences in gfp expression were observed for the let-7 sensor and its control. Cartoons for each construct are shown above the images.
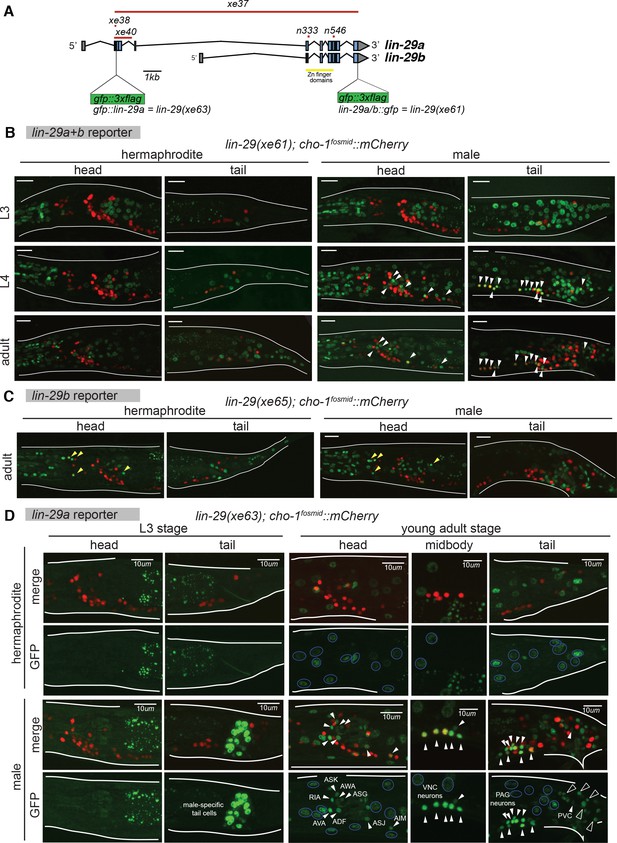
Temporal expression pattern of lin-29 in the nervous system of both sexes.
(A) Cartoon showing the lin-29 locus. Alternative promoter usage generates two LIN-29 protein isoforms. Exons 1 – 4 are LIN-29A specific while exons 5 – 11, that include the Zn-finger DNA binding domain, are shared by both -A and -B isoforms. The lin-29 locus was tagged using CRISPR/Cas9 genome engineering. gfp was inserted either at the C-terminal end (lin-29a/b::gfp; xe61 allele) to tag both LIN-29A and B protein isoforms or at the N-terminal end (gfp::lin-29a; xe63 allele) to tag only the LIN-29A isoform. Canonical alleles n333 and n546 as well as the newly generated alleles xe37 (null), xe38 (A-specific) and xe40 (A-specific) are indicated. (B) lin-29a/b expression pattern during larval development in both sexes. Confocal images for endogenously tagged lin-29a/b::gfp (xe61) show that GFP is expressed in the pharynx at the L3 stage and onwards in both sexes (examination of young animals showed that pharyngeal expression starts at the L1 stage). Hypodermal GFP expression all along the body started at the end of the L3 stage in both sexes. At the L4 stage, we also detected GFP expression in neurons only in the male, many of which were also labeled by a cholinergic marker, a cho-1/CHT mCherry expressing fosmid (marked by arrows). Expression in these male neurons persisted in the adult stage. No neuronal expression was observed in the head nor tail in hermaphrodite animals. Scale bar: 10 µm. (C) lin-29b expression pattern at the young adult stage in both sexes. Confocal images for gfp tagged lin-29b(xe40) (this allele is given a new name, xe65, since it contains the xe40 lesion plus the gfp insertion) show that GFP is expressed in head glia in both sexes (marked by yellow arrowheads). GFP is also observed in the pharynx and in tail cells in both sexes. Cholinergic cho-1/CHT mCherry expressing fosmid (otIs544) did not co-localize with GFP showing that LIN-29B is not expressed in neurons in the head nor tail in either sex (Note that LIN-29B is expressed in midbody neurons in both sexes not shown in this image). Scale bar: 10 µm. (D) gfp::lin-29a expression pattern during larval development in both sexes. Confocal images for endogenously tagged gfp::lin-29a (xe63) show that GFP is expressed in male-specific non-neuronal tail cells at the end of the L3 stage and onwards. No expression was observed in the head or tail of the hermaphrodite at this stage. No overlap between GFP and the cholinergic fosmid based reporter cho-1/CHT (otIs544) was observed at this stage. At the young adult stage, we observed GFP expression in neurons only in males (expression in male neurons started at the L4 stage), indicated by white arrows. No neuronal expression was observed in hermaphrodite neurons. Many GFP positive neurons in the male were also labeled by the cho-1/CHT mCherry expressing fosmid (otIs544). Cholinergic neurons expressing lin-29a included AIM, ASJ, AVA, ASK, AWA, ASG, RIA and ADF in the head, ventral nerve cord (VNC) motor neurons of the A, B, D and AS classes and the PVC interneuron in the tail. Neuronal GFP expression is indicated by arrows and neuronal identity is indicated for head and tail neurons in the GFP panels. GFP expression was observed throughout the hypodermis in both sexes, indicated with blue circles in the GFP panels. Hypodermis nuclei are larger. Cholinergic retrovesicular ganglion (RVG), ventral nerve cord (VNC) and pre-anal ganglion (PAG) neurons expressing lin-29a are shown in the head, midbody and tail respectively. Male-specific tail cells expressing lin-29a are marked with black arrows. Scale bar: 10 µm.
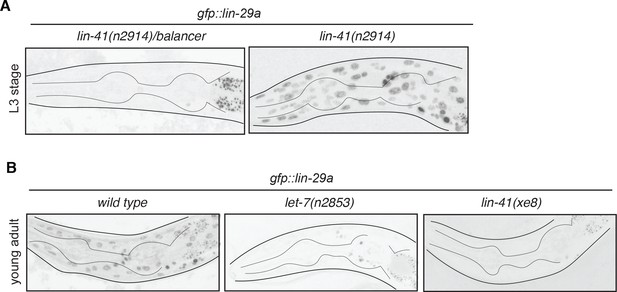
lin-29a neuronal expression is temporally regulated by lin-41 and let-7.
(A) gfp::lin-29a expression in the lin-29a(xe63) strain is precocious in the absence of lin-41. Expression was examined in synchronized L3 animals and compared between lin-41(n2914)/balancer control versus lin-41(n2914) loss-of-function males. Neuronal nuclei are more compact than the larger hypodermal nuclei. (B) gfp::lin-29a expression in the lin-29a(xe63) strain is downregulated in let-7(lf) and lin-41(gf) mutants. Expression was examined at the young adult stage in let-7(n2853ts) loss-of-function mutants and lin-41(xe8) gain-of-function mutants, compared to control males. While control males showed expression of gfp::lin-29a in the hypodermis and nervous system (more compact nuclei), both mutants showed a severe downregulation of gfp::lin-29a expression in the hypodermis and neurons at the young adult stage. L1 let-7(n2853ts) animals were shifted to the restricted temperature (25°C) and imaged as adults, after 48hs.
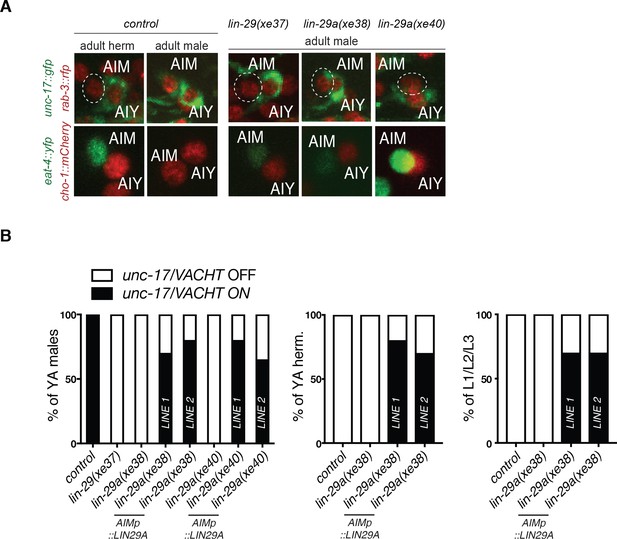
Cell-autonomy and sufficiency of lin-29a for the AIM neurotransmitter switch.
(A) The neurotransmitter switch is blocked in three newly generated lin-29 mutants. In adult control animals, the fosmid-based cholinergic reporters unc-17/VACHT (otIs576) and cho-1/CHT (otIs544) are expressed in AIM neurons of adult males but not hermaphrodites while the eat-4/VGLUT (otIs518) glutamatergic reporter is expressed in AIM neurons of adult hermaphrodites but not males. The AIM neurotransmitter switch is blocked in a newly generated lin-29a/b(xe37) null allele and in two lin-29a-specific mutants (xe38 and xe40). In these lin-29 mutant males, AIM fails to turn on cholinergic markers unc-17/VACHT (top panels) and cho-1/CHT (bottom panels) and expresses a pan neuronal maker rab-3::rfp (top panels) and the glutamatergic marker eat-4/VGLUT (bottom panels). A pan-neuronal rab-3::rfp (otIs355) reporter was used to label all neurons in the top panels. (B) AIM-specific LIN-29A expression is sufficient to cell-autonomously rescue the neurotransmitter switch in males and induce it in young larvae and the opposite sex. LIN-29A expression was driven under an AIM-specific promoter in lin-29a mutant animals: LIN-29A was expressed in lin-29a(xe38) (otEx7316 and otEx7317) and in lin-29a(xe40) (otEx7318 and otEx7319). Expression of cholinergic gene reporter unc-17/VACHT (otIs576) was examined to assay rescue and ectopic induction of the AIM neurotransmitter switch (n = 15).
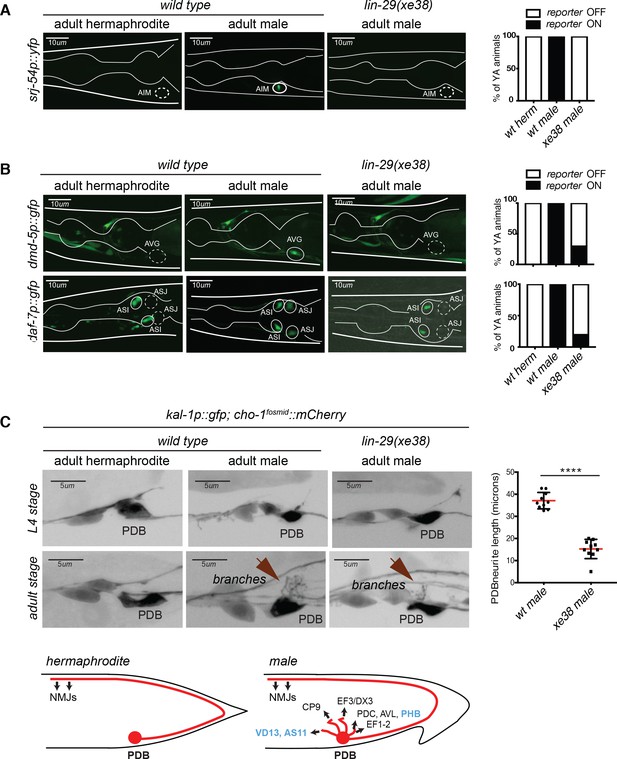
Male-specific molecular differentiation programs are lost in lin-29a mutants.
(A) Male-specific expression of srj-54 in AIM requires lin-29a. Expression of the GPCR srj-54 (fsIs5) promoter fusion is observed in adult males but not hermaphrodites in the AIM interneurons. Expression of srj-54 was lost in all the lin-29a(xe38) mutant males. Quantification is shown on the right (n = 15). Scale bar: 10 µm. (B) Male-specific expression of dmd-5 in AVG requires lin-29a. Expression of the DM-containing transcription factor promoter fusion dmd-5 (pUL#JS9B3) is observed in adult males but not hermaphrodites in the AVG interneuron. In the absence of lin-29a, expression is lost from AVG in 80% of the male worms (top panels); quantification is shown on the right (n = 15). The expression of the daf-7/TGFβ-like molecule promoter fusion is observed in adult males but not hermaphrodites and this expression is lost in the lin-29a(xe38) mutant males (bottom panels). Quantification is shown on the right (n = 15). Scale bar: 10 µm. (C) lin-29a is required for normal PDB branching. A cis-regulatory element from the kal-1 locus is expressed in a number of neurons in the tail region in both sexes, including the PDB –inter and motorneuron (Wenick and Hobert, 2004). kal-1::gfp reporter expression showed that the PDB neuron process exhibits elaborate branching in the male after sexual maturation but not at earlier larval stages. In the absence of lin-29a, PDB branching is lost from adult males (bottom panels). Quantification of total neurite length is shown on the right (n = 10). PDB neurites establish extensive electrical and chemical synapses with sex-shared and male-specific neurons exclusively in adult males (schematized based on data from (Jarrell et al., 2012); synaptic partners of PDB present in both sexes are in blue and in black if sex-specific) . Scale bar: 5 µm.
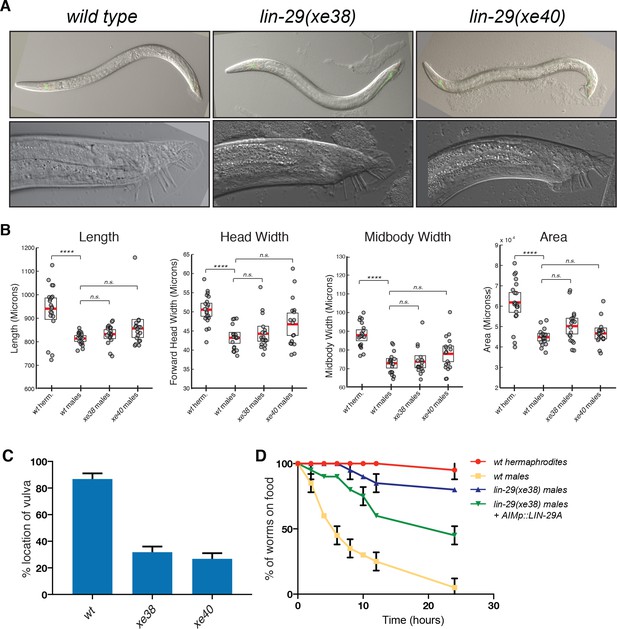
lin-29a is required for male mating behaviors.
(A) lin-29A mutants show a normal male tail morphology. DIC images for young adult wild type and lin-29a mutants xe38 and xe40. No morphological defects were detected in the young adult lin-29a mutant males compared to wild type. 20x images for young adult males are shown on the top panels. 63x images of the male tail are shown on the bottom panels. (B) WormTracker analysis of sexually dimorphic postural features showed no difference between wild-type and lin-29a mutant males. Hermaphrodites and males were tracked for 5 min and features describing body posture were analyzed using the WormTracker software. Adult hermaphrodites and males showed sex-specific differences in posture including length, head width, midbody width and area. When wild-type males were compared to lin-29a xe38 and xe40 mutant males no significant differences were found. (C) lin-29a is required for male mating. The ability of the of lin-29a(xe38) and lin-29a(xe40) mutant males to locate the hermaphrodite vulva is significantly reduced compared to lin-29(+) control males (n = 15). (D) lin-29a is required for male-specific mate searching behavior. Young adult males are followed over time and scored for movement beyond 3 cm away from the food source. lin-29a(xe38) mutant males failed to leave the food and search for mates and behaved similarly to wild-type hermaphrodites. lin-29a mate-searching defect could be partially rescued by restoring LIN-29A expression in AIM interneurons (otEx7316). Values plotted are an average of two independent experiments (n = 15 for each experiment).
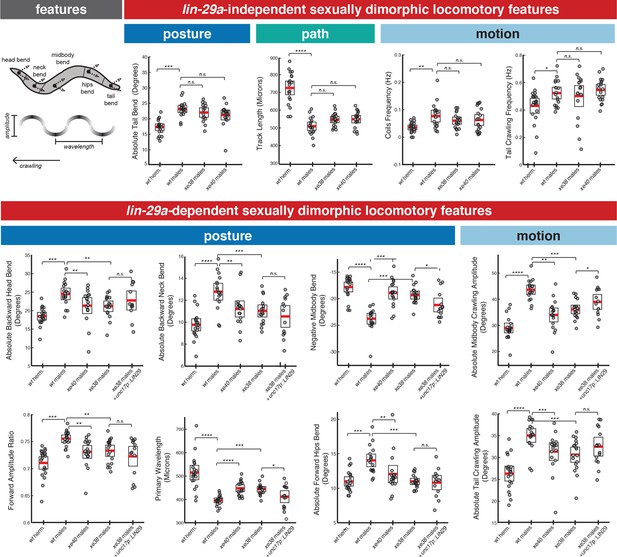
lin-29a is required for the establishment of male-specific features of locomotor behavior.
There are lin-29a dependent and lin-29a independent adult-specific sexually dimorphic locomotory features. Cartoon of an adult C. elegans depicts the location of the body parts used for feature computation. The arrows for each body part represent the bend angle. Wavelength and amplitude while crawling are shown. Adult hermaphrodites and males showed sex-specific differences in posture, path and locomotory features that were not affected by lin-29a absence (top panel). A complete list of sexually dimorphic features not affected by lin-29a is provided in Supplementary file 1. In lin-29a mutant males, a number of sexually dimorphic features were feminized compared to wild-type males (bottom panel), including posture and locomotory features. Restoration of lin-29a expression in command interneuron AVA and ventral nerve cord motor neurons (otEx7320) partially rescued the feminization of two postural features (midbody bend and wavelength) as well as one locomotory feature (midbody crawling amplitude) (n = 20).
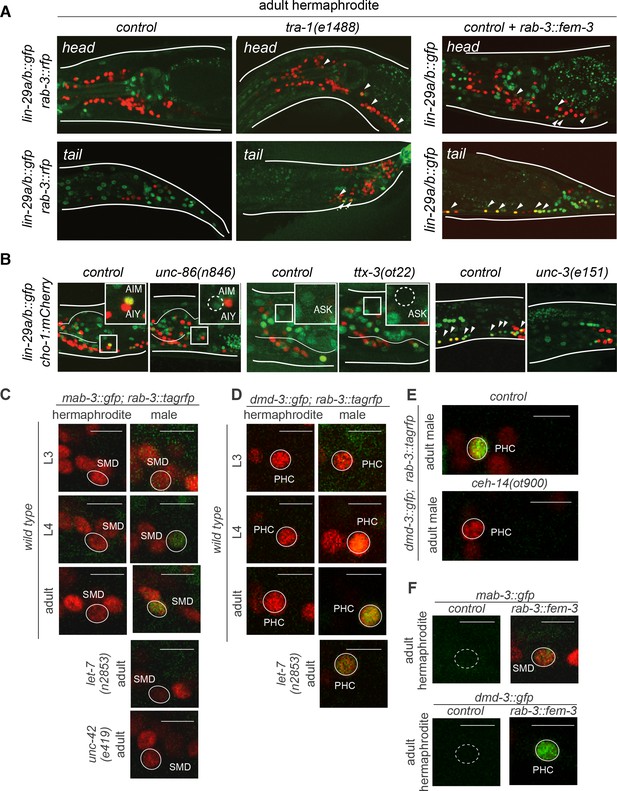
Intersectional control of distinct lin-41 effectors via heterochronic, sexual (tra-1) and spatial (terminal selector) inputs.
(A) LIN-29 neuronal expression is regulated by the sex-determination pathway. Mutant hermaphrodites for the downstream effector of the sex-determination pathway tra-1 showed lin-29a/b::gfp (xe61) expression in hermaphrodite neurons in L4 and adult stages. Masculinization of the hermaphrodite nervous system by a pan-neuronal promoter driving fem-3 (otEx7321), that represses tra-1 function, induced lin-29a/b::gfp (xe61) expression in hermaphrodite neurons in L4 and onwards. (B) lin-29a/b::gfp neuronal expression is lost in neuron-specific transcription factor mutants. The POU homeobox transcription factor unc-86 is required for lin-29a/b::gfp expression in AIM interneurons. The LIM-HD transcription factor ttx-3 is required for lin-29a/b::gfp expression in ASK. The COE/EBF transcription factor unc-3 is required for lin-29a/b::gfp expression in AVA, SAB, DA, DB, VA, VB, PDA, PDB and PVC neurons. Cholinergic neurons are labeled with the cho-1/CHT mCherry fosmid reporter. (C) mab-3::gfp expression in the SMD neurons in control hermaphrodites, males and mutant animals. mab-3::gfp (ot931) expression was determined during larval development. GFP was first observed in the SMD neurons at the L4 stage and onwards, only in males. mab-3::gfp expression was lost from SMD neurons in a let-7(n2853ts) mutant male. L1 let-7(n2853ts) animals were shifted to the restricted temperature (25°C) and imaged as adults, after 48hs. mab-3::gfp expression was lost from SMD neurons in an unc-42(e419) mutant male. Scale bar: 5 µm. (D) dmd-3::gfp expression in the PHC neuron in control hermaphrodites, males and mutant animals. GFP was first observed in the PHC neurons at the L4 stage and onwards, only in males. dmd-3::gfp expression was decreased from PHC neurons in a let-7(n2853) mutant male. L1 let-7(n2853) animals were shifted to the restricted temperature (25°C) and imaged as adults, after 48hs. (E) dmd-3::gfp expression in PHC was lost in ceh-14 null mutant males and in let-7(n2853ts) mutant males. L1 let-7(n2853ts) animals were shifted to the restricted temperature (25°C) and imaged as adults, after 48hs. (F) Masculinization of the hermaphrodite nervous system by a pan-neuronal promoter driving fem-3 (otEx7322 and otEx7323, respectively), that promotes TRA-1 degradation, induced mab-3::gfp (ot931) expression in hermaphrodite SMD neurons and dmd-3::gfp (ot932) expression in hermaphrodite PHC neurons in L4 and onwards.
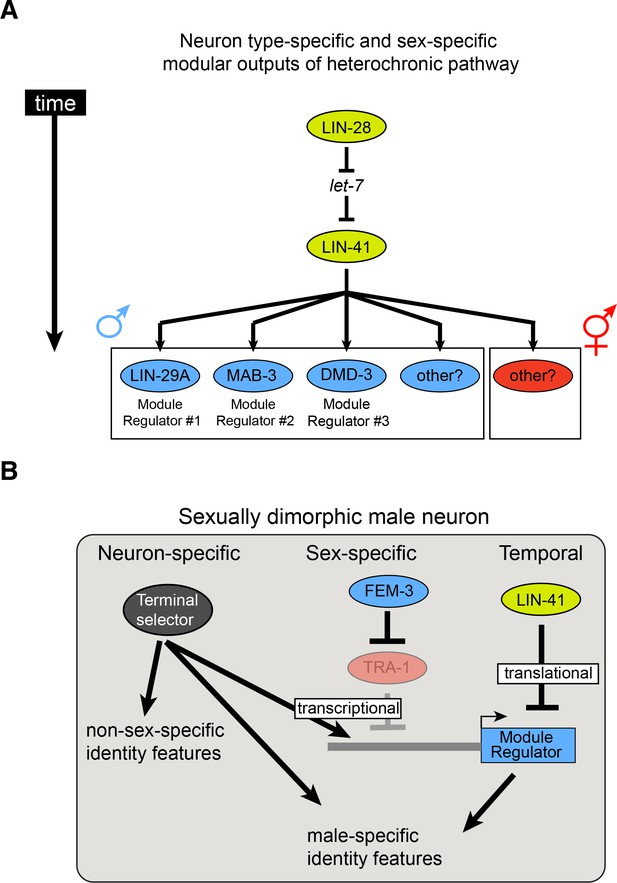
Timing mechanisms controlling sexually dimorphic nervous system differentiation during sexual maturation.
(A) The evolutionarily conserved lin-28/let-7/lin-41 heterochronic pathway controls the timing of the expression of multiple neuron-specific effectors in the male nervous system. The RNA-binding protein LIN-28 controls the expression of the miRNA let-7, which in turn controls expression of the RNA-binding protein LIN-41. LIN-41 let-7-mediated downregulation at the onset of sexual maturation de-represses LIN-41 targets LIN29A, MAB-3 and DMD-3. Expression of these LIN-41 effectors in specific neurons in males, at the onset of sexual maturation, constitute effector modules directing the precisely timed nervous system masculinization. We hypothesize that similar LIN-41 effectors, not identified yet, could be acting in other neurons in the male nervous system and in the hermaphrodite nervous system. (B) Temporal, sex-specific and neuron-specific intersectional control of nervous system differentiation during sexual maturation. A hypothetical male neuron is shown. As discussed above, LIN-41 repression of the heterochronic pathway is relieved at the onset of sexual maturation, allowing neuron-specific effector proteins to be expressed. In males, degradation of the ubiquitously expressed transcriptional repressor TRA-1 allows expression of male-specific regulatory effectors (blue boxes same as panel A). The neuron type-specificity of regulatory effector gene expression is determined by terminal selector transcription factors that control the expression of many other neuronal identity features. Hence, terminal selectors define a neuron type-specific, but permissive state whose sex- and time-specificity is controlled by factors that antagonize the effect of terminal selectors on regulatory effector genes (transcriptionally – TRA-1; translationally – LIN-41). Note that features that are controlled by sex/time-specific regulatory effectors, exemplified by LIN-29A regulation of neurotransmitter pathway genes, are also controlled by terminal selectors, thereby constituting a ‘feedforward’ regulatory architecture.
Tables
Strain list.
https://doi.org/10.7554/eLife.42078.015| Strain name | Genotype | Relevant DNA on array or single copy transgene |
|---|---|---|
| DR466 | him-5(e1490) | |
| MT1524 | lin-28(n719) | |
| MT7897 | lin-41(n2914)/unc-29(e1072); lin-11(n1281) | |
| MT7626 | let-7(n2853) | |
| HW1814 | lin-41(xe8/bch28); him-5(e1490) 1 | |
| VT132 | lin-29a/b(n333)/mnC1; sqt-1(sc13) | |
| CB4037 | glp-1(e2141) | |
| HW1672 | lin-29a/b(xe37) | |
| HW1693 | lin-29a(xe38) | |
| HW1695 | lin-29a(xe40) | |
| HW1698 | mab-10(xe44) | |
| CB2823 | tra-1(e1488)/eDp6 | |
| MT1859 | unc-86(n846) | |
| OH161 | ttx-3(ot22) | |
| CB151 | unc-3(e151) | |
| CB419 | unc-42(e419) | |
| OH15422 | ceh-14(ot900) 2 | |
| OH15568 | unc-17(ot907[unc-17::mKate2::3xflag]) | |
| DG3913 | lin-41(tn1541[lin-41::gfp]) | |
| HW1822 | lin-29(xe61[lin-29a/b::gfp::3xflag]) | |
| HW2224 | lin-29(xe63 [gfp::3xflag::lin-29a]); him-5(e1490) | |
| HW1835 | lin-29(xe65 [lin-29b::gfp::3xflag; lin-29(xe40)]) | |
| HW2047 | mab-10(xe75[mab-10::flag::mCherry]) | |
| HW2225 | lin-29(xe63); him-5(e1490); let-7(n2853) | |
| HW2342 | lin-29(xe63); him-5(e1490); lin-41(n2914)/unc-29(e1072); lin-11(n1281) | |
| HW2344 | lin-29(xe63); him-5(e1490); lin-41(xe8/bch28) | |
| OH15732 | mab-3(ot931[mab-3::3xflag::gfp]) | |
| OH15733 | dmd-3(ot932[dmd-3::3xflag::gfp]) | |
| OH10689 | otIs355 | rab-3::nls::tag_rfp |
| OH12543 | otIs534 | cho-1 fosmid::sl2::yfp::h2b |
| OH12655 | otIs544 | cho-1 fosmid::sl2::mCherry::h2b |
| OH13083 | otIs576 | unc-17 fosmid::gfp |
| OH11124 | otIs388 | eat-4 fosmid::sl2::yfp::h2b |
| OH12496 | otIs518 | eat-4 fosmid::sl2::mCherry::h2b |
| OP535 | wtIs535 | lin-28 fosmid::gfp |
| HW2172 | xeSi182 | plin-41::gfp(pest)::h2b::lin-41 3’UTR |
| HW2173 | xeSi202 | plin-41::gfp(pest)::h2b::unc-54 3’UTR |
| UR219 | fsIs5 | srj-54p::yfp |
| UL2497 | Ex(dmd-5p::gfp) | dmd-5p::gfp (pUL#JS9B3 plasmid) |
| FK181 | ksIs2 | daf-7p::gfp |
| EB2509 | dzIs75 II | kal-1p::gfp 3 |
| OH15741 | lin-29(xe38) + otEx7316 | eat-4p::lin-29a |
| OH15742 | lin-29(xe38) + otEx7317 | eat-4p::lin-29a |
| OH15743 | lin-29(xe40) + otEx7318 | eat-4p::lin-29a |
| OH15744 | lin-29(xe40) + otEx7319 | eat-4p::lin-29a |
| OH15745 | lin-29(xe38) + otEx7320 | unc-17p::lin-29a |
| OH15746 | lin-29(xe61) + otEx7321 | rab-3p::fem-3::mCherry |
| OH15748 | mab-3(ot931) + otEx7322 | rab-3p::fem-3::mCherry |
| OH15749 | dmd-3(ot921) + otEx7323 | rab-3p::fem-3::mCherry |
-
1The xe8 gain of function and bch28 null alleles have been described in Ecsedi et al. (2015) and in Katic et al. (2015). These alleles were maintained in trans to improve health of the animals. Offspring homozygous for either allele were scored for phenotypic traits
-
3The kal-1 gfp promoter fusion corresponds to ‘promoter G’ from Wenick and Hobert, 2004
Additional files
-
Supplementary file 1
List of sexually dimorphic, but lin-29A independent locomotory features.
- https://doi.org/10.7554/eLife.42078.016
-
Transparent reporting form
- https://doi.org/10.7554/eLife.42078.017





