Dystroglycan is a scaffold for extracellular axon guidance decisions
Figures
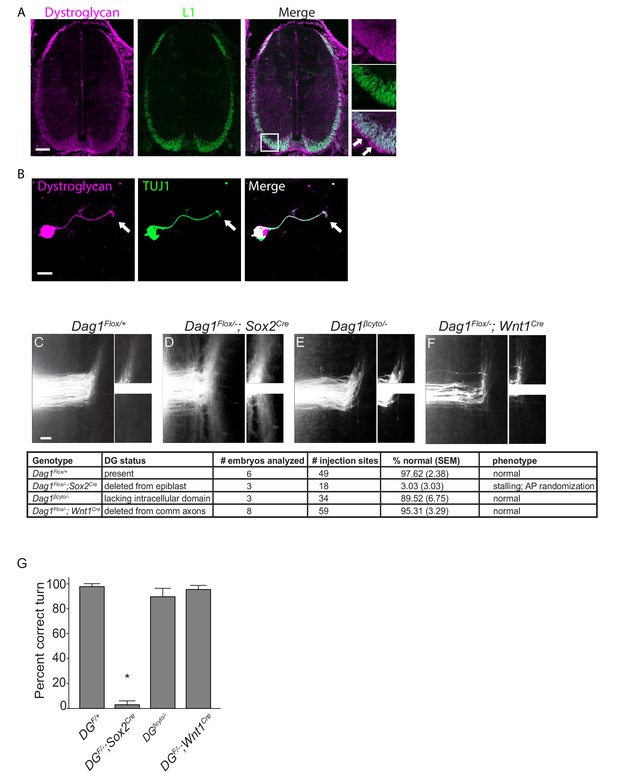
Dystroglycan functions non-cell autonomously to guide spinal commissural axons.
(A) Immunostaining of E12.5 spinal cord shows Dystroglycan protein (magenta, left panel) expression in commissural axons (L1, green, middle panel). In the high magnification insets, arrows indicate the enriched expression of Dystroglycan in the basement membrane of the spinal cord proximal to the axons. (B) Commissural neurons from E12 dorsal spinal cord cultured for two days in vitro (2DIV) were stained with antibodies to Dystroglycan (magenta, left panel), TUJ1 (green, middle panel). Dystroglycan is present throughout the cell body, axon and growth cone (arrow). (C–F) DiI injections in open-book preparations of E12 spinal cords were used to examine the trajectory of commissural axons. In controls (C), axons extend through the floor plate, then execute an anterior turn (n=6 animals, 49 total injection sites). In Dag1F/-;Sox2Cre mice (D), axons stall within the floor plate and post-crossing axons exhibit anterior-posterior randomization (n=3 animals, 18 total injection sites). (E) Commissural axons in mice lacking the intracellular domain of Dystroglycan (Dag1βcyto/-) show normal crossing and anterior turning (n=3 animals, 34 total injection sites). Conditional deletion of Dystroglycan from commissural neurons in Dag1F/-;Wnt1Cre mice (F) did not affect floor plate crossing or anterior turning (n=8 animals, 59 total injection sites). Higher magnification insets for each image show the anterior (top) and posterior (bottom) trajectories of post-crossing commissural axons. (G) Quantification of open book preparations. On average, 97.62 3.39% of controls, 3.03 4.80% of Dag1F/-;Sox2Cre mutants, 89.52 4.80% of Dag1βcyto/- mutants, and 95.31 2.94% of Dag1F/-;Wnt1Cre mutants showed normal crossing and anterior turning. All of the Dag1F/-;Sox2Cre mutants with turning defects also showed stalling within the floor plate. *p< 0.001, one-way ANOVA, Tukey’s post hoc test. Scale bar = 100μm (A), 10μm (B) and 50μm (F–H).
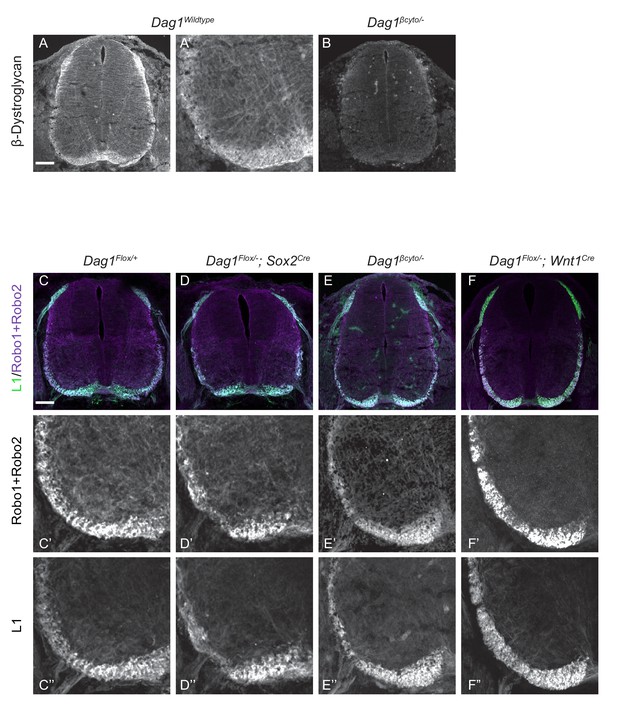
Analysis of Dystroglycan expression and commissural axon phenotypes in spinal cord sections.
(A) An antibody raised against the intracellular domain of Dystroglycan shows staining in the basement membrane and in both pre-crossing and post-crossing commissural axons (A’). (B) Lack of staining in spinal cord sections from Dag1βcyto/- mutants verifies the specificity of the Dystroglycan antibody. (C–F) L1, Robo1 and Robo2 antibodies were used to label commissural axons in E12 spinal cord sections from Dag1F/+ (C–C”), Dag1F/-;Sox2Cre (D–D”), Dag1βcyto/- (E–E”), and Dag1F/-;Wnt1Cre (F–F”) mutants. Post-crossing axons are disorganized and the ventrolateral funiculus appears fragmented in Sox2Cre mutants (D–D”), but appears normal in Dag1βcyto/- (E–E”), and Dag1F/-;Wnt1Cre (F–F”) mutants. Scale bar = 100 μm.
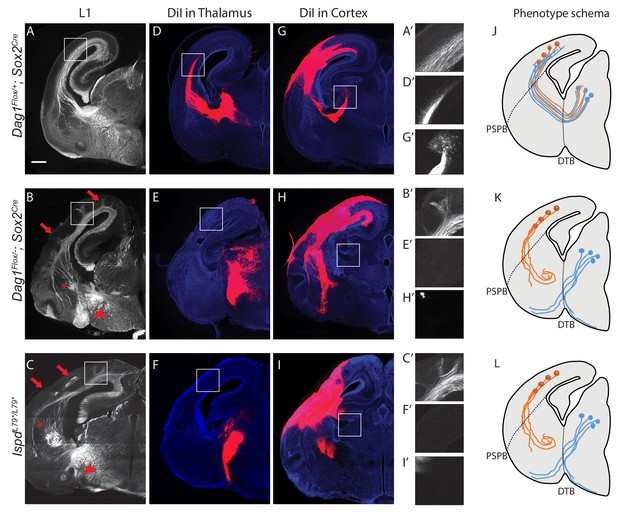
Dystroglycan is required for axon tract formation in the forebrain.
(A) L1 immunohistochemistry on P0 brain sections from Dag1F/+;Sox2Cre controls (n = 3 animals) labels descending CTAs and ascending TCAs in the internal capsule. In Dag1F/-;Sox2Cre (n = 4 animals) (B) and IspdL79*/L79* (n = 5 animals) (C) mutants, the internal capsule is highly disorganized, with axons projecting into the upper layers of the cortex (red arrows), forming ectopic bundles in the ventral telencephalon (red asterisks), and abnormal projections extending ventrally (red arrowheads). High magnification insets show L1 +axons in the intermediate zone of the cortex of controls (A’) and ectopic axonal projections into the upper cortical layers in Dag1F/-;Sox2Cre (B’) and IspdL79*/L79* (C’) mutants. DiI injection in the thalamus of Dag1F/+;Sox2Cre controls (n = 4 animals) labels TCAs as they cross the DTB, extend through the ventral telencephalon, across the PSPB, and into the intermediate zone of the cortex. In Dag1F/-;Sox2Cre (n = 4 animals) (E) and IspdL79*/L79* (n = 4 animals) (F) mutants, TCAs fail to cross the DTB, and instead project ventrally out of the diencephalon. High magnification insets show DiI-labeled TCAs extending into the intermediate zone of the cortex of controls (D’), and a lack of labeled TCAs in the cortex of Dag1F/-;Sox2Cre (E’) and IspdL79*/L79* (F’) mutants. DiI injection in the cortex of Dag1F/+;Sox2Cre controls (n = 3 animals) labels CTAs as they extend across the PSPB, through the ventral telencephalon, and across the DTB into the thalamus. CTAs in Dag1F/-;Sox2Cre (n = 4 animals) (H) and IspdL79*/L79* (n = 5 animals) (I) mutants fail to cross the PSPB or take abnormal trajectories through the ventral telencephalon. High magnification insets show DiI-labeled CTAs extending into the thalamus in controls (G’), and a lack of labeled CTAs in the thalamus of Dag1F/-;Sox2Cre (I’) and IspdL79*/L79* (F’) mutants. (J–L) Schematic summarizing CTA (brown) and TCA (blue) axon trajectories in controls (J), Dag1F/-;Sox2Cre (K) and IspdL79*/L79* (L). Scale bar = 500 μm.
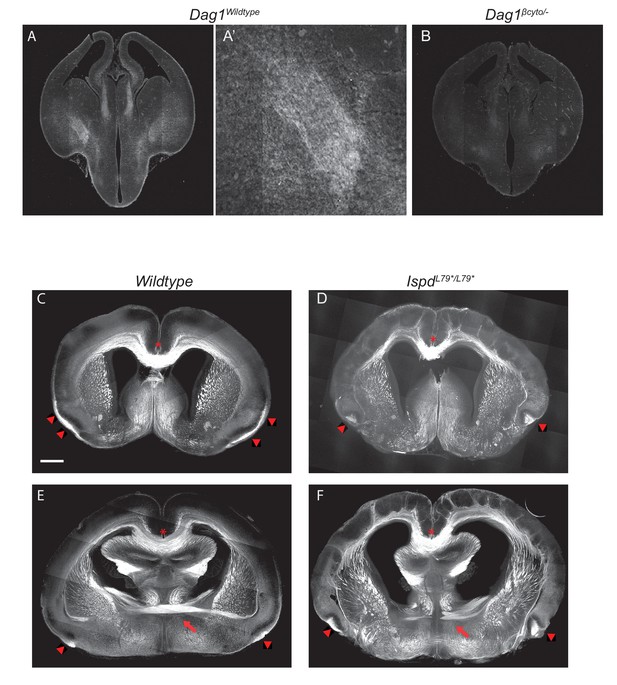
Anterior commissure, lateral olfactory tract and corpus callosum phenotypes in IspdL79*/L79* mutants.
(A) Dystroglycan is expressed in the basement membrane surrounding the brain at E14.5. Dystroglycan is also expressed in the developing thalamus and in the axons that form the internal capsule (A, A’ inset). (B) Lack of staining in sections from Dag1βcyto/- mutants verifies the specificity of Dystroglycan staining in the cortex. (C–F) L1 staining was used to label forebrain axon tracts in P0 wildtype (n = 3 animals) (C,E) and IspdL79*/L79* mutants (n = 3 animals) (D,F). The corpus callosum (red asterisk) in IspdL79*/L79* mutants appears largely normal compared to controls. In contrast, the anterior commissure (red arrows) is thinner and disorganized in IspdL79*/L79* mutants (D). The lateral olfactory tract (red arrowheads) extends along the pial surface of the ventrolateral telencephalon in controls (C,E), whereas it appears hyperfasciculated and projects deeper into the piriform cortex as a disorganized bundle in IspdL79*/L79* mutants (D,F). Scale bar = 500 μm.
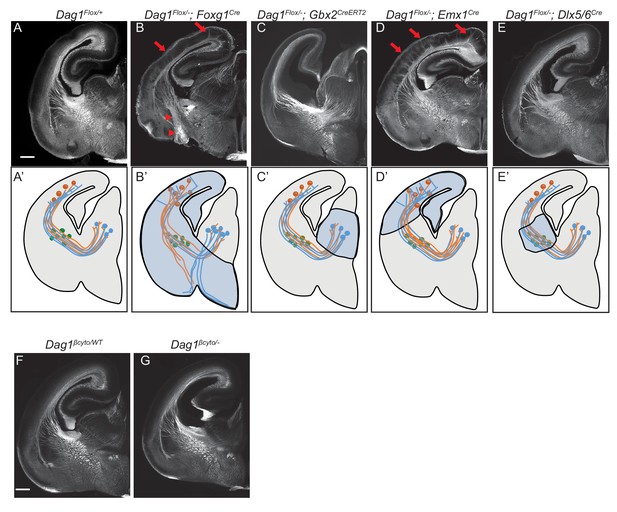
Dystroglycan is required in ventral telencephalon neuroepithelial cells to guide corticothalamic and thalamocortical axons.
L1 staining of P0 brain sections from Dag1F/+ controls (n = 3 animals) (A, F), Dag1F/-;Foxg1Cre (n = 4 animals) (B), Dag1F/-;Gbx2CreERT2 (n = 4 animals) (C), Dag1F/-;Emx1Cre (n = 3 animals) (D), Dag1F/-;Dlx5/6Cre (n = 3 animals) (E), and Dag1βcyto/- (n = 5 animals) (G). A’-E’ illustrate the recombination patterns in each Cre/CreERT2 line in the blue shaded area. Deletion of Dystroglycan throughout the neuroepithelium of the dorsal and ventral telencephalon in Dag1F/-;Foxg1Cre mutants (B, B’) results in abnormal projections in the internal capsule (red arrowheads) and abnormal axonal projections into the upper layers of the cortex (red arrows). Deletion of Dystroglycan from the neuroepithelium of the dorsal telencephalon with Emx1Cre mutants (D) results in abnormal axonal projections into the upper layers of the cortex (red arrows), but normal internal capsule formation. Deletion of Dystroglycan from the thalamus with Gbx2CreERT2 (C) or ‘corridor’ cells with Dlx5/6Cre (E) did not affect axon guidance. Deletion of the intracellular domain of Dystroglycan in Dag1βcyto/- mutants (G) did not affect formation of the internal capsule compared to control littermates (F). A-G Scale bar = 500 μm.
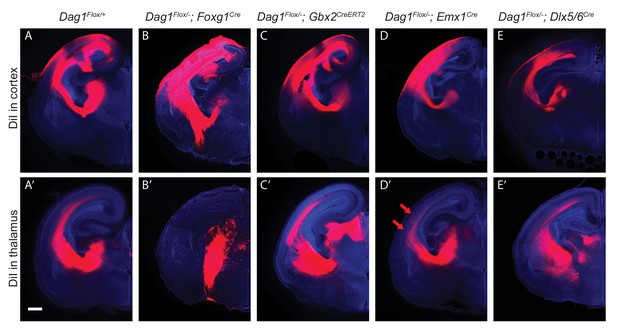
DiI labeling of CTAs and TCAs in Dystroglycan conditional mutants.
(A–E) DiI injections into the cortex (top row) or thalamus (bottom row) of Dystroglycan conditional mutants labels CTAs and TCAs, respectively. CTAs (B) in Dag1F/-;Foxg1Cre mutants (n = 3 animals) take an abnormal trajectory through the ventral telencephalon, and TCAs (n = 4 animals) (B’) fail to cross the DTB and instead extend ventrally out of the diencephalon. CTAs in Dag1F/-;Gbx2CreERT2 (n = 3 animals) (C), Dag1F/-;Emx1Cre (n = 5 animals) (D) and Dag1F/-;Dlx5/6Cre (n = 3 animals) (E) mutants are normal, as are TCAs in Dag1F/-;Gbx2CreERT2 (n = 4 animals) (C’), and Dag1F/-;Dlx5/6Cre (n = 3 animals) (E’) mutants. TCAs in Dag1F/-;Emx1Cre (n = 4 animals) (D’) mutants project through the internal capsule normally, but project into the upper layers prematurely upon entering the cortex (red arrows). Scale bar = 500 μm.
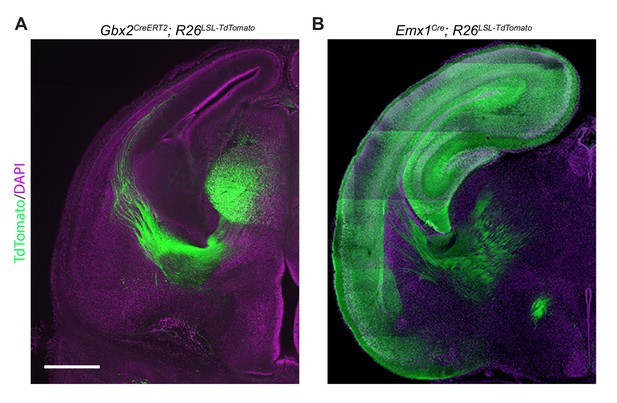
Recombination pattern in Gbx2CreERT2 and Emx1Cre mice.
(A) Gbx2CreERT2 mice crossed to the AI9; Rosa26lox-stop-lox-tdTomato reporter (n = 2 animals) were dosed with 2.5 mg tamoxifen at e10.5. Analysis of brains at E16 showed recombination of the tdTomato reporter (green) in thalamic neurons/axons. (B) Emx1Cre mice crossed to the AI9; Rosa26lox-stop-lox-tdTomato reporter (n = 2 animals) showed recombination of the tdTomato reporter (green) in cortical neurons/axons at P0. Scale bar = 500 μm.

Dystroglycan interacts with the LG1 domain of Celsr3.
(A) Schematic of Celsr3 protein structure, highlighting the location of Cadherin, Laminin G (LG), EGF, Hormone Receptor Domain (HRM) and GPCR Proteolytic Site (GPS) domains. (B) Fc-tagged α-Dystroglycan (Fc-DG) secreted from 293 T cells was incubated with Alkaline Phosphatase (AP)-tagged Celsr3-LG1, Celsr3-LG2, or AP-tag alone, and complexes were isolated with Protein A/G beads. DG-Fc interacts selectively with Celsr3-LG1, but not Celsr3-LG2. (C) AP-Celsr3-L1, AP-Celsr3-LG2, or AP-tag alone were incubated with WGA enriched brain lysate, and complexes were purified with Ni-NTA beads. AP-Celsr3-LG1 binds endogenous glycosylated Dystroglycan, whereas AP-Celsr3-LG2 and AP-tag do not. (D) COS7 cells transfected with full-length Dystroglycan were incubated with 5 nM AP-tag, AP-Celsr3-LG1, AP-Celsr3-LG2, or AP-Slit-Cterm. Both AP-Celsr3-LG1 and AP-Slit-Cterm exhibited selective binding. Scale bar = 50 μm.

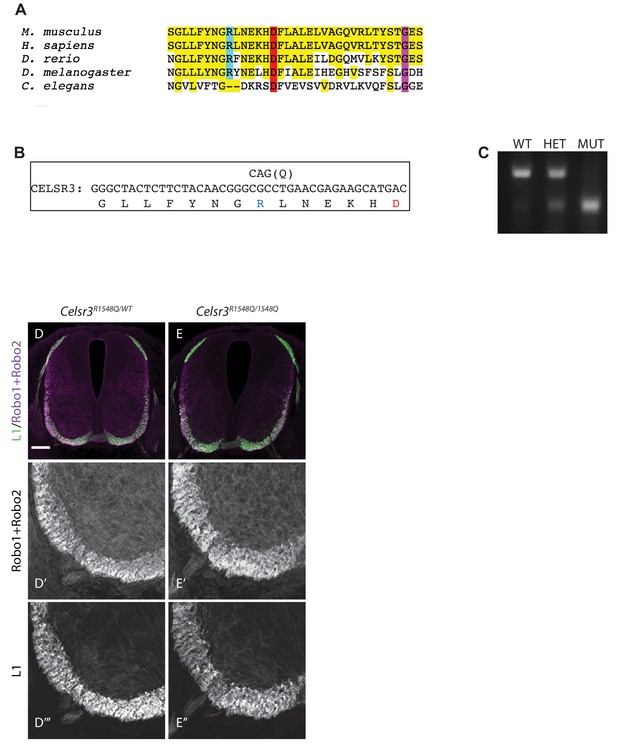
Dystroglycan binding requires specific motifs in Celsr3 LG1.
(A) Top: schematic showing the structure of the LG4 domain of Laminin-α2 (PDB:1OKQ), highlighting conserved residues critical for Dystroglycan binding: Arginine2803 (blue), Aspartate2808 (red) and Glycine2826 (purple). Bottom: Partial sequence alignment of murine Celsr3-LG1 (amino acids 1540–1574) with murine Laminin-α2-LG4 (amino acids 2795–2828) shows conservation at Arginine1548 (blue), Aspartate1564 (red) and Glycine1572 (purple) of Celsr3. (B–C) 293 T cells transfected with Celsr3-GFP or mutant Celsr3R1548Q-GFP showed no differences in expression levels or cell surface localization by immunocytochemistry (B) or western blotting (C). (D) Mutation of Celsr3-LG1 at Arginine1548 (AP-LG1R1548Q) results in loss of binding to FC-tagged Dystroglycan. (E–J) Section binding assay with 5 nM AP alone (E–F), AP-Celsr3-LG1 (G–H), or AP-Celsr3-LG1R1548Q (I–J). Inset panels show higher magnification of the ventrolateral funiculus (top panels E’, (G’, I’) and the internal capsule (bottom panels, (F’, H’, J’). AP-Celsr3-LG1 binds to commissural axons in the ventrolateral funiculus (arrow, G,G’) and the internal capsule (arrow, H,H’). AP-Celsr3-LG1R1548Q shows minimal binding in either region and is almost indistinguishable from AP alone. Scale bar = 10 μm (B), 100 μm (E,G,I), 500 μm (F,H,J).

Dystroglycan:Celsr3 interactions are required for spinal commissural axon guidance.
(A) Western blotting of brain lysates from CelsrR1548Q/R1548Q mutants and wildtype littermates show no difference in size or expression level of Celsr3 or Celsr1 protein. Brain lysate from Celsr3-/- mutants is included as a control for antibody specificity. (B, C). In Celsr3WT/R1548Q heterozygous controls (B), DiI labeling of open book preparations shows that commissural axons extend through the floor plate, then execute an anterior turn in 86.5 ± 2.52% of injection sites (n = 7 animals, 49 total injection sites). In contrast, only 22.32 ± 6.35% of injection sites in Celsr3R1548Q/R1548Q mutants (n = 6 animals, 48 total injection sites) (C) show normal anterior turning, with the remaining 77.68% exhibiting AP randomization after crossing the floor plate, similar to Dag1F/-;Sox2Cre, IspdL79*/L79*, and Celsr3-/- mice. Higher magnification insets for each image show the anterior (top) and posterior (bottom) trajectories of post-crossing commissural axons. (D) Quantification of open book preparations, *p<0.001, Student’s T-test. (E–J) L1 immunohistochemistry (E, H) and DiI labeling of thalamocortical (F, I) and corticothalamic (G, J) axons show no defects in internal capsule formation in Celsr3R1548Q mutants. High magnification insets show DiI-labeled thalamocortical axons extending into the intermediate zone of the cortex (F’, I’) and DiI-labeled corticothalamic axons entering the thalamus (G’,J’). (K) Proposed model for Dystroglycan:Celsr3 interactions in guiding commissural axons. Scale bar = 50 μm (B,C), 500 μm (E–J).
Analysis of CelsrR1548Q mutants.
(A) Evolutionary conservation of Celsr3-LG1 region that forms a putative binding interface with Dystroglycan. The conserved Arginine (blue), Aspartic Acid (red) and Glycine (purple) residues critical for LG domain binding are highlighted. (B) Schematic of the Celsr3 nucleotide and amino acid sequence highlighting the specific sequence targeted to generate CelsrR1548Q knock-in mice. (C) Genotyping of wildtype, CelsrR1548Q/+, and CelsrR1548Q/R1548Q mutants. (D–E) L1, Robo1 and Robo2 antibodies were used to label commissural axons in E12 spinal cord sections from CelsrR1548Q/+ (D–D”) and CelsrR1548Q/R1548Q (E–E”), mutants. Post-crossing axons appear normal in CelsrR1548Q/R1548Q mutants. Scale bar = 100 μm (D,E).
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (M. musculus) | Dag1Flox | Jackson Labs | stock: 009652 | |
| Genetic reagent (M. musculus) | DagBcyto | PMID: 19846701 | Dr. Kevin Campbell, HHMI, University of Iowa | |
| Genetic reagent (M. musculus) | IspdL79* | PMID: 23217742, Jackson Labs | stock: 022019 | Dr. Kevin Wright, Vollum Institute |
| Genetic reagent (M. musculus) | Celsr3R1548Q | generated de novo | Dr. Kevin Wright, Vollum Institute | |
| Genetic reagent (M. musculus) | Sox2Cre | Jackson Labs | stock: 008454 | |
| Genetic reagent (M. musculus) | Wnt1Cre | Jackson Labs | stock: 022137 | |
| Genetic reagent (M. musculus) | Foxg1Cre | Jackson Labs | stock: 006084 | |
| Genetic reagent (M. musculus) | Gbx2CreERT2 | Jackson Labs | stock: 022135 | |
| Genetic reagent (M. musculus) | Emx1Cre | Jackson Labs | stock: 005628 | |
| Genetic reagent (M. musculus) | Dlx5/6Cre | Jackson Labs | stock: 008199 | |
| Genetic reagent (M. musculus) | R26-LSL-TdTomato | Jackson Labs | stock: 007909 | |
| Cell line (H. sapiens) | 293T | ATCC | CRL-11268, RRID:CVCL_1926 | |
| Cell line (C. aethiops) | COS7 | ATCC | CRL-1651, RRID:CVCL_0224 | |
| Antibody | L1 (rat monoclonal) | Millipore | RRID: AB_2133200 | 1:500 dilution |
| Antibody | Robo1 (goat polyclonal) | R and D Systems | RRID: AB_354969 | 1:250 dillution |
| Antibody | Robo2 (goat polyclonal) | R and D Systems | RRID: AB_2181857 | 1:250 dilution |
| Antibody | Dystroglycan (rabbit polyclonal) | Santa Cruz Biotech | RRID: AB_1118902 | 1:50 dilution |
| Antibody | myc (mouse monoclonal) | Thermo Fisher | RRID: AB_2533008 | |
| Antibody | IIH6 glcosylated dystroglycan (mouse monoclonal) | Millipore | RRID: AB_309828 | |
| Antibody | Celsr3 (rabbit poyclonal) | Fadel Tissir | Dr. Fadel Tissir, UC Louvain | |
| Antibody | Celsr1 (guinea pig polyclonal) | Fadel Tissir | Dr. Fadel Tissir, UC Louvain | |
| Transfected construct (O. cuniculus) | DG-Fc | PMID: 11604425 | Dr. Kevin Campbell, HHMI, University of Iowa | |
| Transfected construct (M. musculus) | Celsr3-GFP | PMID: 25108913 | Dr. Fadel Tissir, UC Louvain | |
| Transfected construct (M. musculus) | Celsr3-GFP-R1548Q | generated de novo | Dr. Kevin Wright, Vollum Institute | |
| Transfected construct (synthetic vector) | AP-Tag5-COMP | generated de novo | Dr. Kevin Wright, Vollum Institute | |
| Transfected construct (M. musculus) | AP-Tag5-COMP-Celsr3-LG1 | generated de novo | Dr. Kevin Wright, Vollum Institute | |
| Transfected construct (M. musculus) | AP-Tag5-COMP-Celsr3-LG2 | generated de novo | Dr. Kevin Wright, Vollum Institute | |
| Transfected construct (M. musculus) | AP-Tag5-COMP-Celsr3-LG1-R1548Q | generated de novo | Dr. Kevin Wright, Vollum Institute | |
| Chemical compound, drug | DiI | Thermo Fisher | D-3911 | |
| Chemical compound, drug | BCIP | Roche Applied Science | Cat: 11383221001 | |
| Chemical compound, drug | NBT | Roche Applied Science | Cat: 11383213001 | |
| Commercial assay or kit | cell surface biotinylation kit | Thermo Fisher | Cat: 89881 |
Additional files
-
Supplementary file 1
Raw data for open book preparation in Dag1 mutants.
E12.5 spinal cords were processed for open book preparations and each well-isolated DiI injection site was assessed as showing either normal anterior turning or anterior-posterior randomization.
- https://doi.org/10.7554/eLife.42143.013
-
Supplementary file 2
Raw data for open book preparation in Celsr3R1548Q mutants.
E12.5 spinal cords were processed for open book preparations and each well-isolated DiI injection site was assessed as showing either normal anterior turning or anterior-posterior randomization.
- https://doi.org/10.7554/eLife.42143.014
-
Transparent reporting form
- https://doi.org/10.7554/eLife.42143.015





