Arterial smooth muscle cell PKD2 (TRPP1) channels regulate systemic blood pressure
Figures
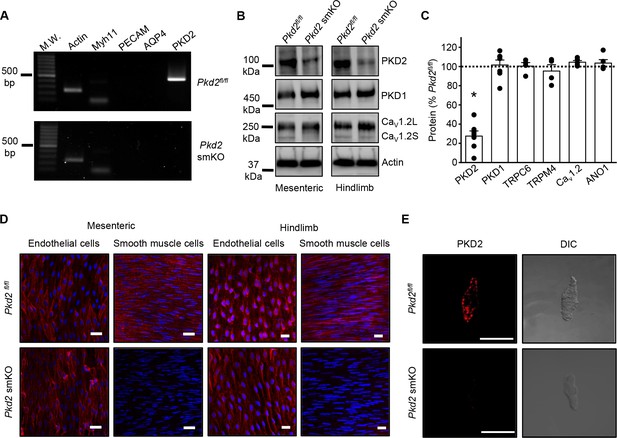
Activation of Cre recombinase abolishes PKD2 in arterial myocytes of Pkd2fl/fl:myh11cre/ERT2 mice.
(A) RT-PCR showing the absence of PKD2 transcript in isolated myocytes from tamoxifen-treated Pkd2fl/fl:myh11-cre/ERT2 mice. (B) Western blots illustrating the effect of tamoxifen-treatment in Pkd2fl/fl and Pkd2fl/fl:myh11-cre/ERT2 mice on PKD2, CaV1.2L (full-length CaV1.2) and CaV1.2S (short CaV1.2) proteins in mesenteric and hindlimb arteries. (C) Mean data for proteins in mesenteric arteries of tamoxifen-treated Pkd2fl/fl:myh11-cre/ERT2 mice when compared to those in tamoxifen-treated Pkd2fl/fl mice. n = 4–7. * indicates p<0.05 versus Pkd2fl/fl. (D) En-face immunofluorescence imaging illustrating that PKD2 protein (red, Alexa Fluor 555) is abolished in myocytes of mesenteric and hindlimb arteries in tamoxifen-treated Pkd2fl/fl:myh11-cre/ERT2 mice (representative of 6 mesenteric and six hindlimb arteries). In contrast, PKD2 protein in endothelial cells is unaltered. Nuclear staining (DAPI) is also shown. Scale bars = 20 µm. (E) Confocal and DIC images illustrating that PKD2 protein (Alexa Fluor 555) is abolished in isolated mesenteric artery myocytes of tamoxifen-treated Pkd2fl/fl:myh11-cre/ERT2 mice (representative data from 5 Pkd2fl/fl and 5 Pkd2fl/fl:myh11-cre/ERT2 mice). Scale bars = 10 µm.
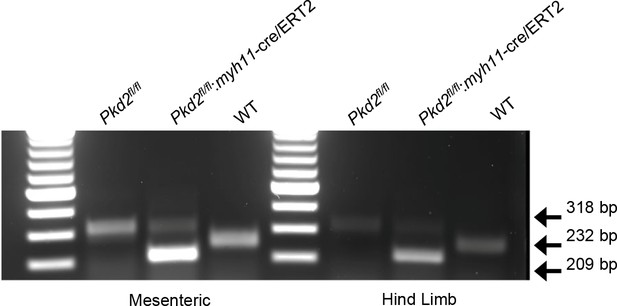
Genotyping of mouse lines.
Ethidium bromide gel illustrating PCR products in vasculature of C57BL/6J (WT) mice and tamoxifen-treated Pkd2fl/fl and Pkd2fl/fl:myh11cre/ERT2 mice.
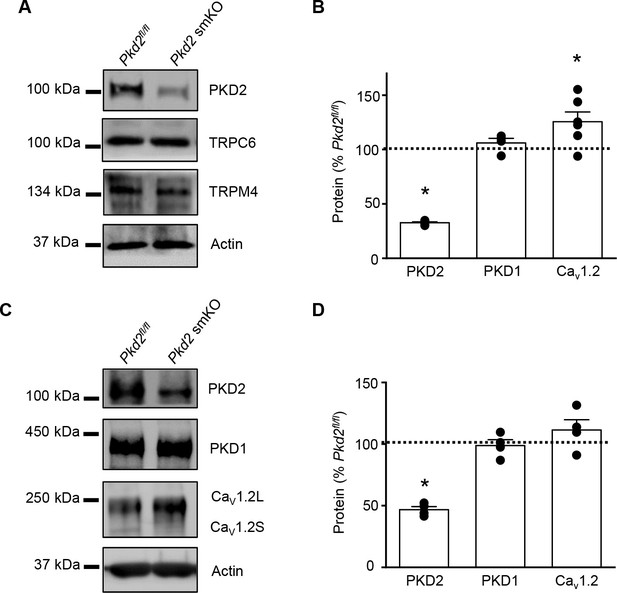
PKD2 protein is lower in aorta and mesenteric and hindlimb arteries from tamoxifen-treated Pkd2fl/fl:myh11-cre/ERT2 mice.
(A) Western blots illustrating PKD2 protein was lower in mesenteric arteries of tamoxifen-treated Pkd2fl/fl:myh11-cre/ERT2 mice, whereas other proteins were similar. (B) Mean data for proteins in hindlimb arteries of Pkd2 smKO mice (n = 4–6). (C) Western blots of proteins in aorta. Cav1.2L, full-length Cav1.2; Cav1.2S, short Cav1.2. (D) Mean data from aorta (n = 4). * indicates p<0.05 versus Pkd2fl/fl.
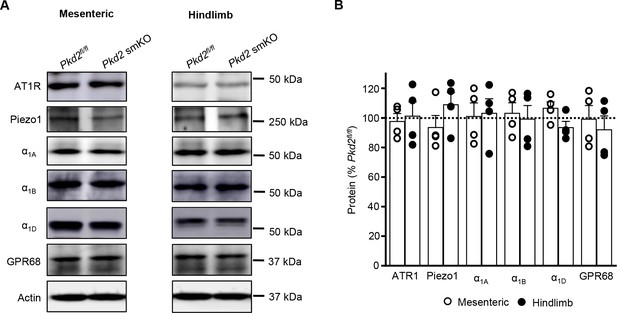
Several proteins that regulate arterial contractility are unchanged in tamoxifen-treated Pkd2fl/fl:myh11-cre/ERT2 mice.
(A) Western blots illustrating Angiotensin II type one receptor (AT1R), Piezo1, α1-adrenergic receptor A (α1A), α1-adrenergic receptor B (α1B), α1-adrenergic receptor D (α1D) and G protein-coupled receptor 68 (GPR68) protein levels in mesenteric and hindlimb arteries of Pkd2fl/fland Pkd2fl/fl:myh11-cre/ERT2 mice. (B) Mean data from mesenteric and hindlimb arteries (n = 4 per group).
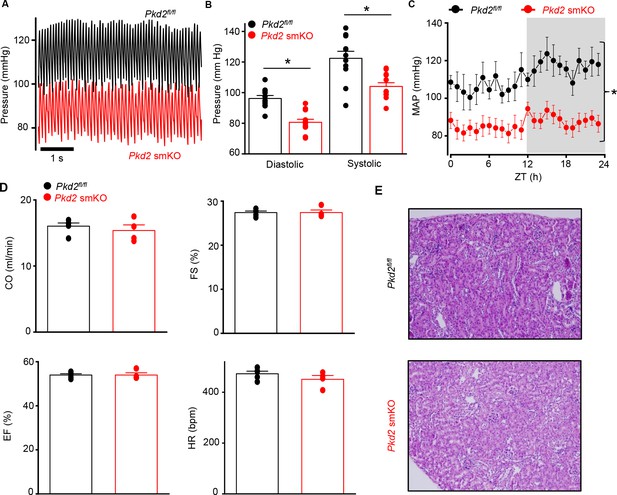
Pkd2 smKO mice are hypotensive with normal cardiac function and renal histology.
(A) Original telemetric blood pressure recordings from Pkd2 smKO and Pkd2fl/fl mice. (B) Mean systolic and diastolic blood pressures in Pkd2fl/fl (n = 11) and Pkd2 smKO (n = 12) mice. * indicates p<0.05 versus Pkd2fl/fl. (C) Mean arterial blood pressures (MAP) in Pkd2fl/fl (n = 11) and Pkd2 smKO (n = 12) mice during day and night (gray) cycles. ZT: Zeitgeber Time. * indicates p<0.05 versus Pkd2fl/fl for all data points. (D) Mean echocardiography data. Cardiac output (CO), fractional shortening (FS), ejection fraction (EF) and heart rate (HR). (Pkd2fl/fl, n = 5; Pkd2 smKO mice, n = 4). (E) Representative images of H and E stained kidney cortex used for histological assessment (n = 3 mice used for for each group).
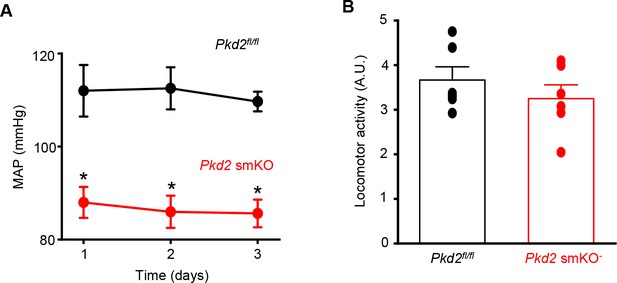
Lower blood pressure is sustained in Pkd2 smKO mice.
(A) Mean arterial blood pressure (MAP) in Pkd2 smKO and Pkd2fl/fl mice (n = 6 per group). * indicates p<0.05 versus Pkd2fl/fl. (B) Mean data of locomotor activity, n = 6 per group.
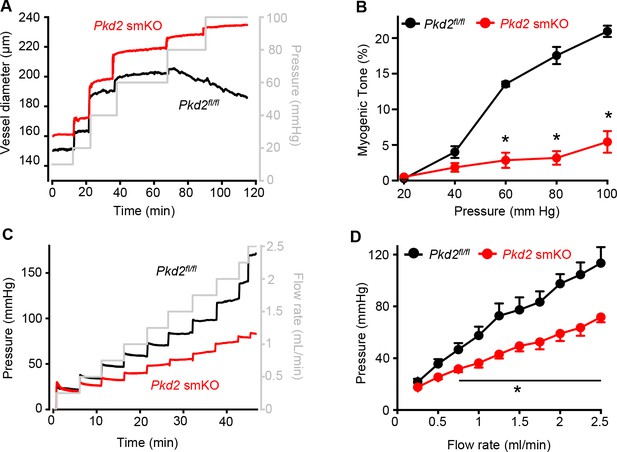
Pressure-induced vasoconstriction is attenuated in Pkd2 smKO mouse hindlimb arteries.
(A) Representative traces illustrating diameter responses to intravascular pressure in gastrocnemius arteries of Pkd2fl/fl and Pkd2 smKO mice. (B) Mean data for myogenic tone in gastrocnemius arteries (Pkd2fl/fl, n = 5; Pkd2 smKO, n = 6). * indicates p<0.05 versus Pkd2fl/fl. (C) Representative traces illustrating hindlimb perfusion pressure in response to increasing flow. (D): Mean data for hindlimb perfusion pressure (Pkd2fl/fl, n = 6; Pkd2 smKO, n = 4). * indicates p<0.05 versus Pkd2fl/fl.
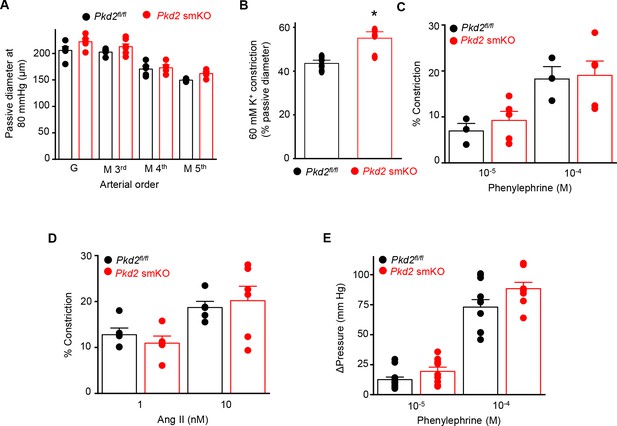
Myocyte PKD2 knockout does not alter phenylephrine or angiotensin II-induced vasoconstriction in hindlimb arteries.
(A) Mean passive diameter at 80 mmHg of first-order gastrocnemius arteries (G) and third-, fourth-and fifth-order mesenteric arteries (M) (Pkd2fl/fl: G, n = 5; M3rdn = 4; M4thn = 5; M5thn = 5 and Pkd2 smKO: G, n = 5; M3rd n = 7; M4th n = 4; M5th n = 5). (B) Mean data for 60 mM K+-induced constriction in pressurized (100 mmHg) gastrocnemius arteries from Pkd2fl/fl (n = 4) and Pkd2 smKO (n = 4) mice. * indicates p<0.05 versus Pkd2fl/fl. (C) Mean data of phenylephrine-induced constriction in pressurized gastrocnemius arteries (Pkd2fl/fl n = 4, Pkd2 smKO n = 5). (D) Mean data of angiotensin II-induced constriction in gastrocnemius arteries pressurized to 100 mmHg (Pkd2fl/fl, n = 5 and Pkd2 smKO, n = 5–6). (E) Mean data of phenylephrine-induced pressure responses in intact hindlimb (Pkd2fl/fl, n = 11–13 and Pkd2 smKO, n = 8–9).
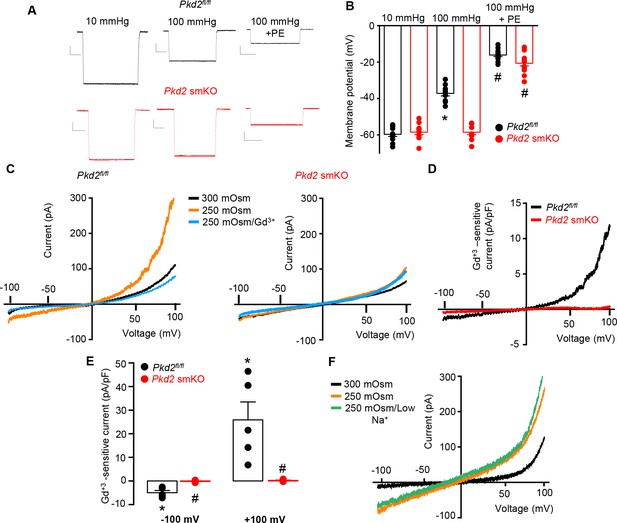
PKD2 channels contribute to pressure-induced hindlimb artery depolarization and swelling-activated Na + currents in hindlimb artery myocytes.
(A) Representative traces of microelectrode impalements under indicated conditions illustrating that pressure-induced depolarization is attenuated in gastrocnemius arteries of Pkd2 smKO mice. Phenylephrine (PE) = 1 µM. Scale bars: Y = 10 mV, X = 20 s. (B) Mean data for membrane potential recordings in pressurized hindlimb arteries in the absence or presence of PE (Pkd2fl/fl: 10 mmHg, n = 11; 100 mmHg, n = 10; 100 mmHg + PE, n = 13 and Pkd2 smKO: 10 mmHg, n = 11; 100 mmHg, n = 10; 100 mmHg + PE, n = 14). * indicates p<0.05 versus 10 mmHg in Pkd2fl/fl. # indicates p<0.05 versus 100 mmHg in the same genotype. (C) Representative ICats recorded between −100 and +100 mV in isotonic (300 mOsm), hypotonic (250 mOsm) and hypotonic bath solution with Gd3+ (100 µM) in the same Pkd2fl/fl and Pkd2 smKO mouse hindlimb artery myocytes. (D) Representative I-V relationships of Gd+3 -sensitive Icats activated by hypotonic solution in Pkd2fl/fl and Pkd2 smKO hindlimb myocytes. (E) Mean data for Gd3+-sensitive ICats recorded in hypotonic solution in Pkd2fl/fl and Pkd2 smKO myocytes (n = 5 for each). * indicates p<0.05 versus 250 mOsm, # p<0.05 versus Pkd2fl/fl. (F) Representative I-V relationships between −100 and +100 mV in isotonic (300 mOsm), hypotonic (250 mOsm) and hypotonic bath solution with low (40 mM) Na+ in the same Pkd2fl/fl mouse hindlimb artery myocyte.
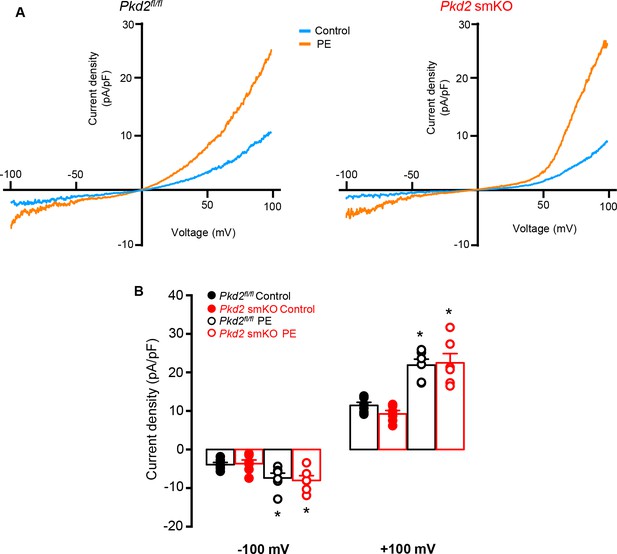
PKD2 knockout does not alter phenylephrine (PE)-activated ICat in isolated hindlimb artery myocytes.
(A) Representative I-V relationships recorded between −100 and +100 mV in the same hindlimb artery myocytes of Pkd2fl/fl or Pkd2 smKO mice in control and PE (10 µM). (B) Mean data for current density at −100 and +100 mV (Pkd2fl/fl, n = 6 and Pkd2 smKO, n = 6). * indicates p<0.05 versus control in the same genotype.
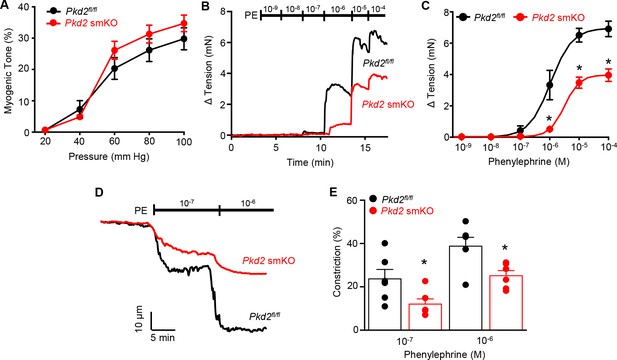
Pressure-induced vasoconstriction is unaltered, whereas phenylephrine-induced vasoconstriction is attenuated, in mesenteric arteries of Pkd2 smKO mice.
(A) Mean vasoconstriction over a range of pressures in resistance-size mesenteric arteries from Pkd2fl/fl (n = 7) and Pkd2 smKO (n = 9) mice. (B) Original recordings of concentration-dependent, phenylephrine (PE)-induced contraction in mesenteric artery rings. (C) Mean PE-induced contraction (Pkd2fl/fl, n = 5; Pkd2 smKO, n = 6; *p<0.05 versus Pkd2fl/fl). (D) Representative phenylephrine-induced vasoconstriction in pressurized (80 mmHg) fifth-order mesenteric arteries. (E) Mean PE-induced vasoconstriction in pressurized (80 mmHg) fourth-and fifth-order mesenteric arteries (Pkd2fl/fl, n = 6; Pkd2 smKO, n = 6; *p<0.05 versus Pkd2fl/fl at the same PE concentration).
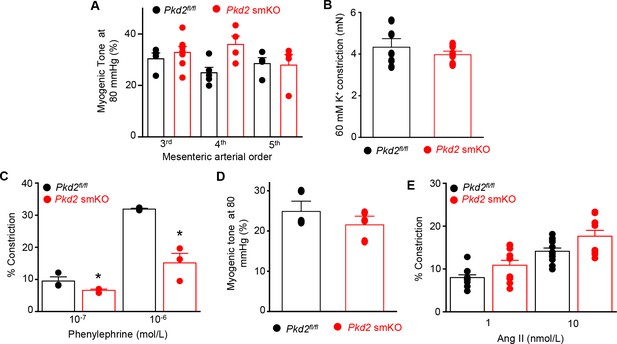
Myocyte PKD2 knockout attenuates phenylephrine-induced vasoconstriction, but does not alter pressure or angiotensin II-induced vasoconstriction in mesenteric arteries.
(A) Mean myogenic tone at 80 mmHg illustrating that myogenic tone is similar in third-, fourth-and fifth-order mesenteric arteries and unaltered by PKD2 knockout (Pkd2fl/fl: 3rd n = 4; 4th n = 5; 5th n = 4 and Pkd2 smKO: 3rd n = 7; 4th n = 4; 5th n = 4). (B) Mean data for 60 mM K+-induced constriction in first-and second order mesenteric artery rings (Pkd2fl/fln = 5; Pkd2 smKO n = 6). (C) Mean data for phenylephrine-induced vasoconstriction in pressurized, endothelium-denuded 4th order mesenteric arteries (Pkd2fl/fl, n = 3 and Pkd2 smKO, n = 3). * indicates p<0.05 versus Pkd2fl/fl. (D) Mean myogenic tone at 80 mmHg in endothelium-denuded 4th order mesenteric arteries (Pkd2fl/fl, n = 3 and Pkd2 smKO, n = 3). (E) Mean data for angiotensin II-induced vasoconstriction in mesenteric arteries (Pkd2fl/fl, n = 11–12 and Pkd2 smKO, n = 10–11).
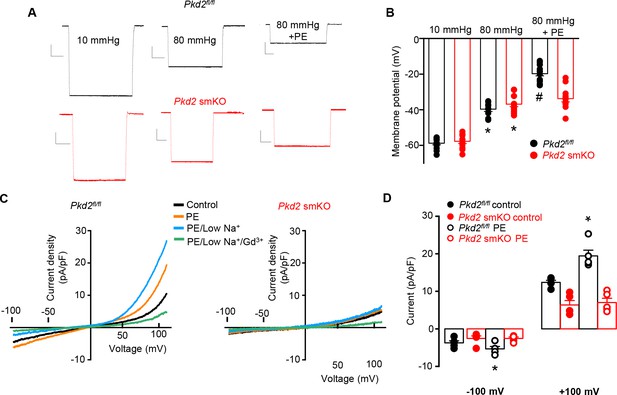
PKD2 channels contribute to phenylephrine-induced mesenteric artery depolarization and INa in mesenteric artery myocytes.
(A) Representative traces of microelectrode impalements illustrating that phenylephrine (PE, 1 µM)-induced depolarization is attenuated in mesenteric arteries of Pkd2 smKO mice. Scale bars: Y = 10 mV, X = 20 s. (B) Mean membrane potential recordings in pressurized (10 and 80 mmHg) mesenteric arteries and in PE at 80 mmHg (Pkd2fl/fl: 10 mmHg, n = 13; 80 mmHg, n = 9; 80 mmHg + PE, n = 15. Pkd2 smKO: 10 mmHg, n = 11; 80 mmHg, n = 12; 80 mmHg + PE, n = 12). *p<0.05 versus 10 mmHg in the same genotype. # p<0.05 versus 80 mmHg in the same genotype. (C) Original current recordings obtained between −100 and +100 mV in the same Pkd2fl/fl and Pkd2 smKO myocytes in control, PE (10 μM), low Na+ (40 mM)+PE and low Na+ (40 mM)+PE + Gd3+ (100 µM). (D) Mean paired data (Pkd2fl/fl, n = 5; Pkd2 smKO, n = 5; *p<0.05 versus control in the same genotype).
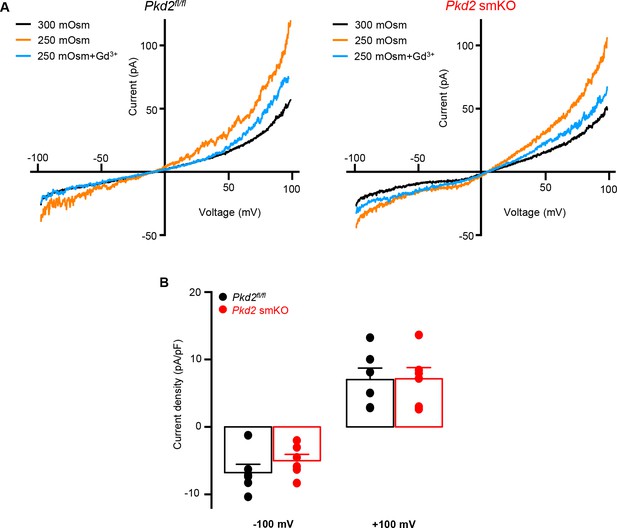
PKD2 knockout does not alter swelling-activated Icat in isolated mesenteric artery myocytes.
(A) Representative I-V relationships from the same isolated mesenteric artery myocytes of Pkd2fl/fl or Pkd2 smKO mice in isosmosmotic (300 mOsm), hyposmotic (250 mOsm) and hyposmotic (250 mOsm) + Gd3+ (100 µM) solutions. (B) Mean data for hyposmoticactivated Gd3+ (100 µM)-sensitive cationic current density at −100 and +100 mV (Pkd2fl/fl, n = 6 and Pkd2 smKO, n = 6).
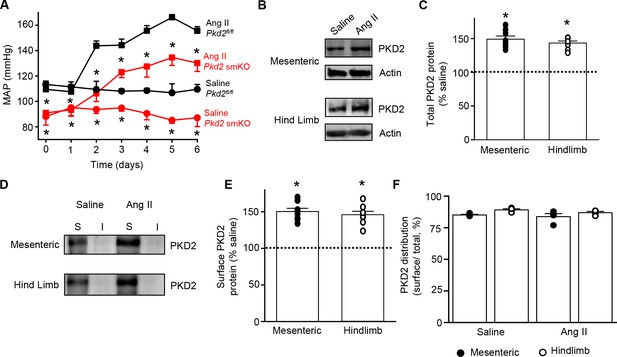
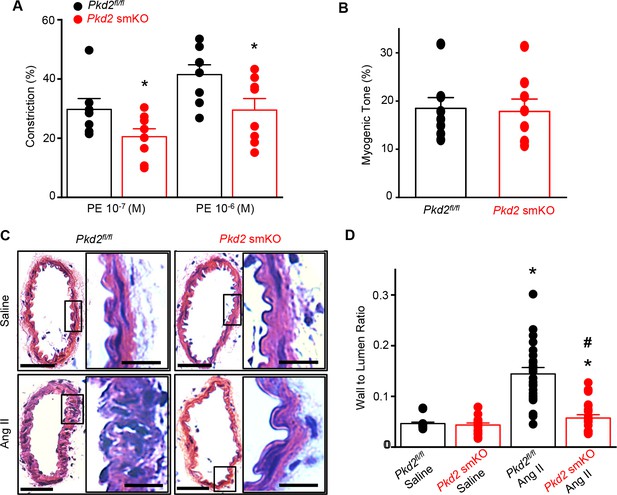
Angiotensin II-induced hypertension is attenuated in Pkd2 smKO mice.
(A) Telemetric blood pressure time course showing the development of angiotensin II-induced hypertension in Pkd2fl/fl (n = 6) and Pkd2 smKO mice (n = 9). Osmotic minipumps containing either saline or angiotensin II were implanted one day prior to day 0. * indicates p<0.05 versus Pkd2fl/fl in the same condition. (B) Western blots illustrating total PKD2 protein in mesenteric and hindlimb arteries of saline-and angiotensin II-treated control mice. (C) Mean total PKD2 protein in mesenteric and hindlimb arteries of angiotensin II-treated mice compared to saline control (n = 8 for each group). * indicates p<0.05 versus saline in the same arterial preparation. (D) Western blots showing surface and intracellular PKD2 protein in arteries of saline-and angiotensin II-treated mice. (E) Mean surface PKD2 protein in mesenteric and hindlimb arteries of angiotensin II-treated mice compared to saline control (n = 8 for each group). * indicates p<0.05 versus saline in the same arterial preparation. (F) Mean data illustrating the percentage of total PKD2 located at the surface in mesenteric and hindlimb arteries of saline-and angiotensin II-treated mice (n = 4 for each group).
Tables
Plasma hormones and plasma and urine electrolytes.
| Pkd2fl/fl | Pkd2 smKO | |
|---|---|---|
| Plasma hormones (pg/ml) | ||
| Angiotensin II | 202.5 ± 17.2 (n = 16) | 204.7 ± 12.1 (n = 15) |
| Aldosterone | 341.0 ± 18.2 (n = 16) | 365.6 ± 14.0 (n = 10) |
| ANP | 107.2 ± 9.4 (n = 18) | 118.3 ± 12.3 (n = 18) |
| Plasma electrolytes (mM) | ||
| Na+ | 142.3 ± 1.0 (n = 7) | 152.0 ± 5.5 (n = 6) |
| K+ | 6.4 ± 0.4 (n = 7) | 6.8 ± 0.3 (n = 6) |
| Cl- | 78.8 ± 0.7 (n = 7) | 84.1 ± 4.3 (n = 6) |
| Urine electrolytes (mM) | ||
| Na+ | 136.9 ± 9.3 (n = 15) | 147.0 ± 16.2 (n = 15) |
| K+ | 563.2 ± 30.2 (n = 15) | 548.6 ± 37.6 (n = 15) |
| Cl- | 455.4 ± 24.7 (n = 15) | 479.1 ± 42.0 (n = 15) |
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Strain, strain background (Mus musculus) | Pkd2fl/fl | John Hopkins PKD Core | PMID:20862291 | Mice with Pkd2 gene flanked by loxP regions |
| Strain, strain background (M. musculus) | SMMHC-CreERT2Myh11-cre/ERT2 | Jackson Laboratories | Stock # 019079 PMID:18084302 | Mice with tamoxifen-inducible Cre recombinase that is expressed under the smooth muscle myosin heavy polipeptide 11 (Myh11) promoter. |
| Strain, strain background (M. musculus) | Pkd2fl/fl:smCre+ | This paper | Mouse line created in-house by mating Pkd2fl/fl with SMMHC-CreERT2. Mice with inducible smooth muscle-specific deletion of PKD2. | |
| Antibody | anti PKD2 (rabbit polyclonal) | Baltimore PKD Core | Rabbit mAB 3374 CT-14/4 | IF 1:200 dilution |
| Antibody | anti PKD2 (mouse monoclonal) | Santa Cruz | Cat# sc-47734 RRID:AB_672380 | WB 1:100 dilution, IF 1:100 dilution |
| Antibody | anti PKD2 (mouse monoclonal) | Santa Cruz | Cat# sc-28331 RRID:AB_672377 | WB 1:100 dilution, IF 1:100 dilution |
| Antibody | anti PKD1 (mouse monoclonal) | Santa Cruz | Cat# sc-130554 | WB 1:100 dilution |
| Antibody | anti CaV1.2 (mouse monoclonal) | UC Davis/NIH NeuroMab | Cat# 73–053 RRID:AB_10672290 | WB 1:100 dilution |
| Antibody | anti ANO1 (rabbit monoclonal) | Abcam | Cat# ab64085 | WB 1:100 dilution |
| Antibody | anti TRPC6 (rabbit polyclonal) | Abcam | Cat# ab62461 | WB 1:1000 dilution |
| Antibody | anti TRPM4 (rabbit polyclonal) | Abcam | Cat# ab106200 | WB 1:500 dilution |
| Antibody | anti Actin (mouse monoclonal) | Millipore Sigma | Cat# MAB1501 | WB 1:5000 dilution |
| Antibody | Alexa 555 secondary antibodies (anti rabbit and anti mouse) | Thermofisher | Cat# A-21429 and # A-31570 | IF 1:400 dilution |
| Other | Nuclear staining (DAPI) | Thermofisher | Cat# 3571 | IF 1:1000 dilution |
| Commercial assay or kit | EZ-Link Sulfo-NHS -LC-LC-Biotin | Thermofisher | Cat# 21338 | |
| Commercial assay or kit | EZ-Link Maleimide-PEG2-Biotin | Thermofisher | Cat# 21901BID | |
| Commercial assay or kit | Mouse Angiotensin II ELISA kit | Elabscience | Cat# E-EL-M2612 | |
| Commercial assay or kit | Mouse Atrial Natriuretic Peptide ELISA kit | Elabscience | Cat# E-EL-M0166 | |
| Commercial assay or kit | Mouse Aldosterone ELISA kit | Mybiosource | Cat# MBS775626 | |
| Chemical compound, drug | Angiotensin II | Sigma-Aldrich | Cat# A9525 |
Additional files
-
Supplementary file 1
Primers used for RT PCR.
The PKD2 forward primer recognizes nucleotides in exon 9 and 10 and the reverse primer was aligned with a sequence in exon 13.
- https://cdn.elifesciences.org/articles/42628/elife-42628-supp1-v4.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/42628/elife-42628-transrepform-v4.pdf