Stereotyped terminal axon branching of leg motor neurons mediated by IgSF proteins DIP-α and Dpr10
Figures
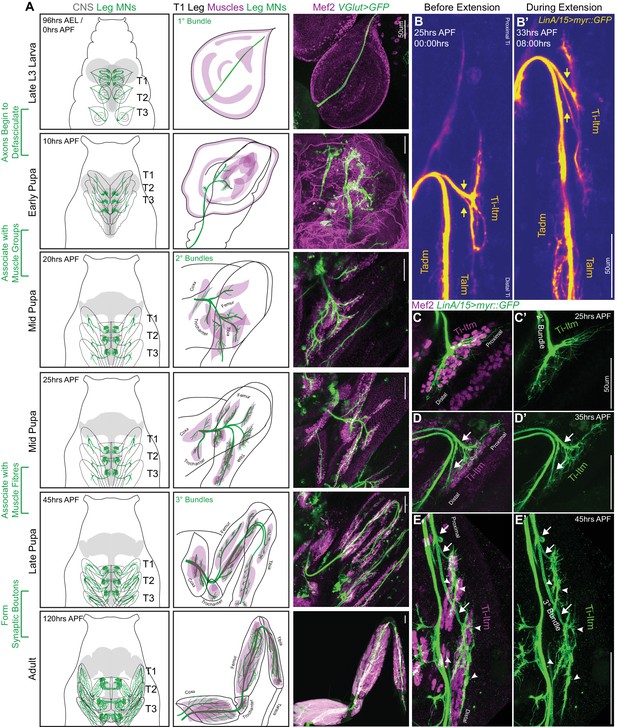
Sequential defasciculation and branching of developing Drosophila adult leg motor neurons.
(A) Development of Drosophila adult leg motor neurons across six distinct time points during pupariation – Late L3 (96 hr AEL/0 hr APF), Early Pupa (10 hr APF), Mid Pupa (20 hr and 25 hr APF), Late Pupa (45 hr APF) and Adult (120 hr APF). Left Column: Schematic representation of Drosophila larval to adult stages denoting the locations of adult leg MN cell bodies and dendrites (green) in the CNS (gray) along with axons (green) targeting ipsilateral legs (T1 - forelegs, T2 - midlegs and T3 - hindlegs). Middle Column: Schematic representation of the developing T1 leg denoting the locations of muscle precursors (magenta) and leg MN axons (green). Locations of muscles within the four leg segments (Coxa, Trochanter, Femur and Tibia) are denoted from 20 hr APF onwards. Right Column: Leg MN axons in the developing T1 leg labeled by VGlut-QF >10XUAS-6XGFP (green) and stained for Mef2 (magenta) to label muscle precursors. Mature MNs and muscles in the Adult T1 leg are labeled using OK371-Gal4 > 20XUAS-6XGFP and Mef2-QF > 10XQUAS-6XmCherry respectively. (scale Bar: 50 μm) (B) Snapshots from a time-lapse series of developing LinA/15 leg MNs expressing myr::GFP at 25 hr APF (B); before extension) and 35 hr APF (B’); after extension) (see also Video 1). Arrows denote distinct axon bundles within the Ti-ltm-targeting bundle. Axon bundles are labeled according to muscle targeting – Ti-ltm: Tibia-long tendon muscle, Tadm: Tarsal depressor muscle, Talm: Tarsal levator muscle. (scale Bar: 50 μm) (C–E) Confocal images of LinA/15 Ti-ltm-targeting leg MN axons expressing myr::GFP (green) and muscles stained for Mef2 (magenta) at 25 hr APF (C–C’), 35 hr (D–D’) and 45 hr APF (E–E’). Arrows point to defasciculating tertiary bundles and arrowheads (E–E’) point to terminal axon branches. (scale Bar: 50 μm).
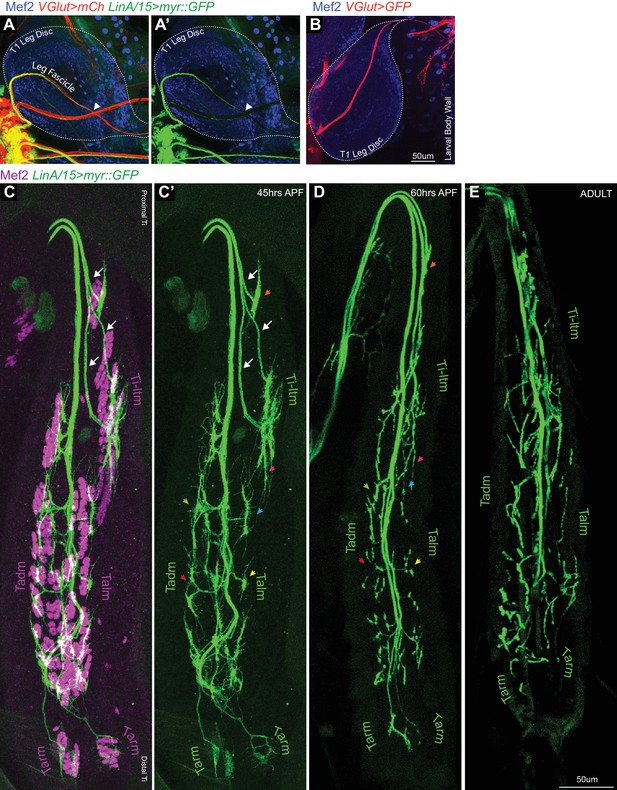
Sequential defasciculation and branching of developing Drosophila adult leg motor neurons.
(A, A’) Maximum projection confocal images of a T1 leg disc denoting the terminal position (white arrowhead) of LinA/15 leg MNs expressing myr::GFP (green; generated by lineage tracing) and all MNs labeled with VGlut-QF >10XQUAS-6XmCherry (red). Note that VGlut+ axons extend beyond the leg disc, while the LinA/15 MNs are within this same fascicle but stop in the middle of the leg disc (arrowhead). Muscle precursors in the leg disc are identified by Mef2 expression (blue). (B) Maximum projection confocal image of the glutamatergic axon bundle labeled with VGlut-QF >10XQUAS-6XGFP (red), comprising of adult leg and larval body wall MNs, passing through the T1 leg disc and innervating mature larval body wall muscles. Muscle precursors in the leg disc and mature body wall muscles are identified by Mef2 expression (blue). (scale bar: 50 μm). (C–E) Maximum projection confocal images of LinA/15 leg MNs expressing myr::GFP (green) targeting Tibia muscles, labeled by staining against Mef2 (magenta), at 45 hr APF (C) and leg MNs alone at 45 hr APF (C’), 60 hr APF (D) and in the adult (E). White arrows denote distinct axon bundles within the Ti-ltm-targeting bundle and colored arrowheads represent examples of secondary branches whose stereotyped morphologies are retained between 45–60 hr APF. (scale Bar: 50 μm).
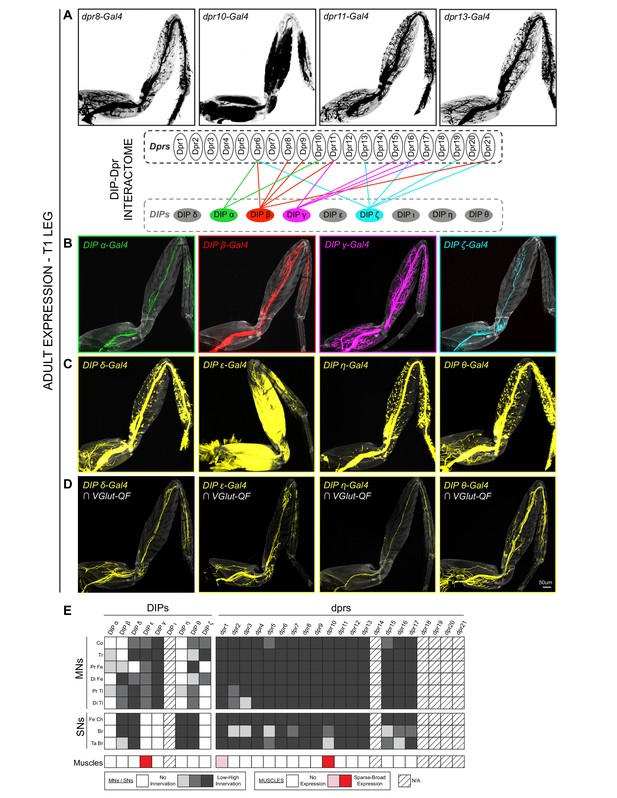
Expression patterns of DIPs and dprs in Drosophila T1 adult leg neuro-musculature.
(A–B) dpr (A) and DIP (B) expression patterns in the Drosophila T1 adult leg for a subset of heterophilic binding partners identified by DIP-Dpr ‘interactome’ studies (Carrillo et al., 2015; Özkan et al., 2013): DIP-α (green) and dpr10 (black); DIP-β (red) and dpr8 (black); DIP-γ (magenta) and dpr11 (black); DIP-ζ (cyan) and dpr13 (black). These DIPs were selected because they are MN-specific in the legs. The expression patterns in this and other panels were generated with MiMIC Gal4 insertions (see Supplementary file 1). (C) Expression of four additional DIPs (DIP-δ, DIP-ε, DIP-η, and DIP-θ) in the T1 adult leg (yellow). In addition to MNs, these DIPs are expressed in leg sensory neurons (DIP-δ, DIP-η, and DIP-θ) or muscles (DIP-ε). (D) DIP-δ, DIP-ε, DIP-η, and DIP-θ expression restricted to glutamatergic MNs neurons in the T1 adult leg using a genetic intersectional approach (see Materials and methods). (scale bar: 50 μm). (E) Heat-map summary of DIP-dpr expression patterns in the T1 leg. Each column represents a distinct DIP or dpr expression pattern and each row represents a specific component of the adult leg-neuro-musculature. MN expression is categorized according to their terminal branching in different segments of the leg: Co, Coxa; Tr, Trochanter; Pr Fe, Proximal Femur; Di Fe, Distal Femur; Pr Ti, Proximal Tibia; Di Ti, Distal Tibia. SN expression is categorized according to their expression in sub-types of SNs (Tuthill and Wilson, 2016): Fe Ch, Femur Chordotonal Organ; Br, Bristle SNs; Ta Br, Tarsal Bristle SNs (campaniform sensilla and hairplate SNs were not included in the expression analysis). Muscle expression is not categorized because two of the three lines were broadly expressed in most muscles. (*) dpr1 is expressed in a single muscle fiber entering the Coxa leg segment.
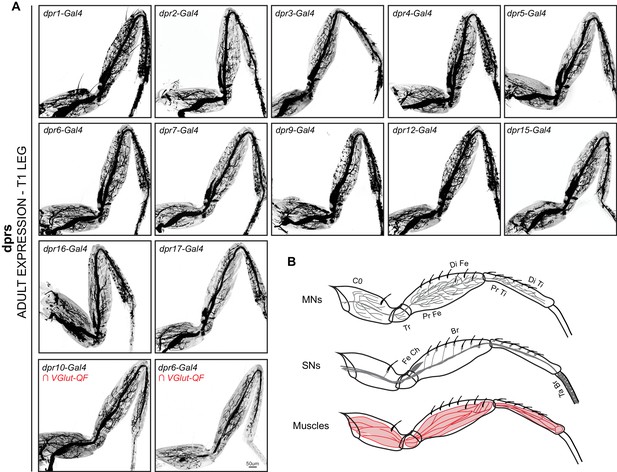
Expression patterns of additional dprs in the Drosophila T1 adult leg.
(A) dpr expression patterns in the T1 adult leg. Bottom row – dpr6 and dpr10 expression was restricted to glutamatergic MNs in the T1 leg using a genetic intersectional approach (see Materials and methods). (scale bar: 50 μm). (B) Schematic representation of MNs, sensory neurons (SNs) and muscles in the T1 leg.
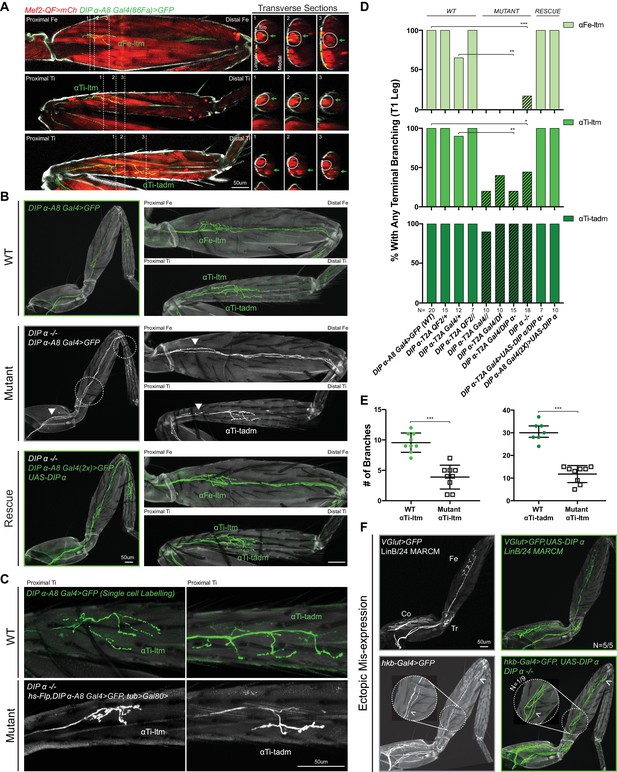
Effects of mutating DIP-α on the terminal branching of α-leg MNs.
(A) Left Column: Proximal-Distal (P–D) oriented Fe and Ti T1 adult leg segments depicting axon muscle-targeting of three DIP-α expressing leg MNs labeled by DIP-α-A8-Gal4(86Fa)>20XUAS-6XGFP (green) (Figure 3—figure supplement 1D) (See Materials and methods). Muscles are labeled using Mef2-QF > 10XQUAS-6XmCherry (red); Grey; cuticle. MNs are named according to the muscle target (αFe-ltm, αTi-ltm, and αTi-tadm) (Soler et al., 2004). Right Columns: Transverse sections of Fe and Ti leg segments at specific locations along the P-D axis, corresponding to the numbered white dotted lines on the left, depicting terminal branching (green arrows) on the Fe and Tiltms (encircled by white dotted lines) and tadm. (scale bar: 50 μm). (B) Terminal branching of the T1 α-leg MNs labeled by DIP-α-A8-Gal4(86Fa)>20XUAS-6XGFP in wild type (WT), DIP-α mutant and rescue contexts. Left; T1 legs; Right; Fe and Ti leg segments (axons; green (WT/rescue) or white (mutant), cuticle; grey). Absence of terminal branching of the α-ltm MNs in the DIP-α mutant T1 leg is indicated by white dotted circles; White arrowheads demarcate axons reaching the vicinity of their muscle targets (refer to Figure 3—figure supplement 1D). (scale bar: 50 μm). (C) Intermediate terminal branching defects in T1 legs displayed by αTi-ltm and αTi-tadm in DIP-α mutants. Single cell labeling of αTi-ltm and αTi-tadm terminal branches in the T1 proximal Ti is shown in WT (green) and DIP-α mutant (white). (scale bar: 50 μm). (D) Quantification of mutant phenotypes (αFe-ltm, light green; αTi-ltm, medium green; and αTi-tadm, dark green) in WT (N = 20), mutant (diagonal lines) and rescue contexts (N = 7 to 20) using a DIP-α null, chromosomal deficiency and MiMIC-T2A-Gal4/QF as indicated. Statistical significance was determined using Fisher’s exact test: *p<0.05; **p<0.01; ***p<0.001 (E) Quantification of number of branches on αTi-ltm and αTi-tadm single-cell samples in WT and DIP-α mutant contexts using genotypes indicated in Figure 1C. Statistical significance was determined using a two-tailed unpaired t-test for αTi-ltm samples, where error bars represent mean ± SD and a Mann-Whitney U test for αTi-tadm samples, where error bars represent median ± interquartile ranges. ***p<0.001 (F) Ectopic expression of DIP-α in LinB/24 leg MNs targeting the Coxa, Trochanter and Distal Fe using OK371-Gal4 MARCM (Top) or an enhancer trap hkb-Gal4 (Bottom) which also labels an additional leg MN targeting the distal Fe (white arrowhead). Normal axon targeting of LinB/24 leg MNs (white) is shown on the left without any terminal branching at the Fe-ltm. However, in a rare case (N = 1/9), ectopic expression of DIP-α using hkb-Gal4 in a DIP-α mutant background caused ectopic branching at the Fe-ltm (white arrowhead within magnified inset). (scale bar: 50 μm).
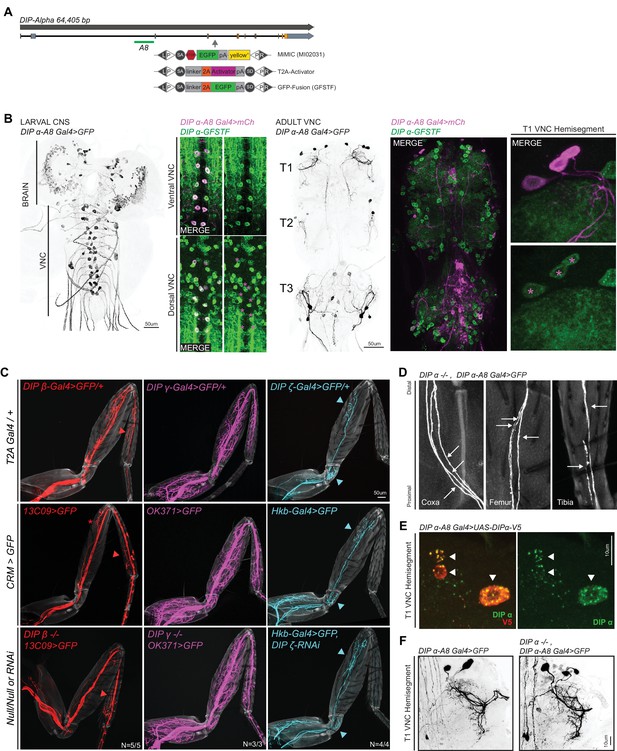
Characterization of DIP-α and other DIP mutants.
(A) Genomic locus of DIP-α showing introns (black line), non-coding exons (grey rectangles) and coding exons (orange rectangles). The location of the DIP-α-A8 enhancer is denoted by a green bar (see Materials and methods). The location of the MiMIC insertion (MI02031) in a coding intron is denoted by a grey arrow. Using recombination mediated cassette exchange (RMCE), MI02031 has been independently swapped into a T2A-binary activator (Gal4/QF) that acts as a ‘gene trap’ and a GFP protein fusion (GFSTF) that acts as a ‘protein trap’ (see Materials and methods) (Venken et al., 2011). (B) Expression of DIP-α-A8-Gal4 > 20XUAS-6XGFP in the larval CNS and adult VNC (black, scale bar: 50 μm). DIP-α-A8-Gal4 is expressed in two segmentally repeated rows of cells along the length of the larval VNC (ventral and dorsal) and in the adult VNC, DIP-α-A8-Gal4 is expressed specifically in three leg MNs in each hemisegment of the thoracic ganglia, in addition to 6–8 cells in the abdominal ganglia (in between the T3 hemisegments). Co-expression of DIP-α-A8-Gal4 > 20XUAS-6XmCherry (Magenta) and DIP-α-GFSTF (detected by anti-GFP – see Materials and methods) in the ventral and dorsal larval VNC as well as the T1 hemisegment of the adult VNC, shows that the DIP-α-A8-Gal4+ cells (marked by magenta asterisks in the dorsal larval VNC and adult T1 VNC hemisegment) express DIP-α. (C) Comparisons of terminal branching of DIP-expressing T1 leg MNs (MiMIC-T2A-Gal4/+; Top Row) in WT (Enhancer-Gal4 >20XUAS-6XGFP; Middle Row) and mutant contexts (bottom row); DIP-β (red), DIP-γ (magenta) or DIP-ζ (cyan) (grey, cuticle). 13C09-Gal4 labels a DIP-β-expressing leg MN in the distal Ti (red asterisk marks a leg MN in the distal Fe that is inconsistently labeled even in WT animals); OK371-Gal4 labels the DIP-γ-expressing leg MNs targeting the majority of adult leg muscles; hkb-Gal4 labels DIP-ζ-expressing leg MNs in the Tr and distal Fe. DIP-expressing leg MN terminal branches that are consistently labeled by the Enhancer-Gal4 (colored arrowheads) are maintained in the mutant contexts. (scale bar: 50 μm). (D) Magnified images of T1 leg segments showing individual axons (white arrows) of the DIP-α mutant leg MNs from Figure 3B. Three axons enter the Coxa (left) and proximal Fe (middle) and two axons enter the proximal Ti (right) (grey, cuticle). (E) Expression of DIP-α in the T1 adult VNC hemisegment of DIP-α-A8-Gal4 > UAS-DIP-α-V5 animals detected by anti-V5 (red) and anti-DIP-α (green) (see Materials and methods). Anti-DIP-α worked to detect DIP-α protein levels only in the cell bodies when overexpressed. White arrowheads denote DIP-α-A8+ leg MNs. (scale bar: 10 μm). (F) No major abberrations were detected in the leg MN cell bodies and dendritic projections (Black) of the α-leg MNs by comparing WT (Left) and mutant (Right) adult T1 VNC hemisegments. (scale bar: 10 μm).
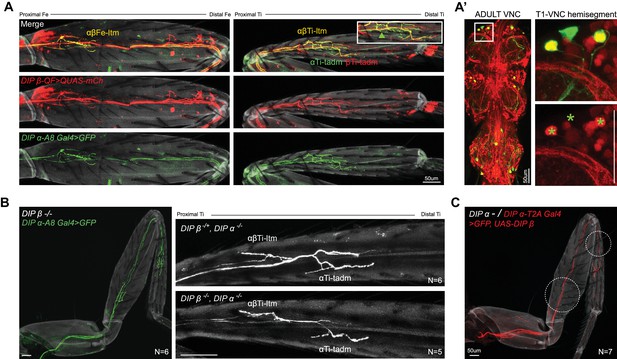
Co-expression and phenotypic analysis of DIP-α and DIP-β in adult leg MNs.
(A) Expression of DIP-β in the α-ltm-leg MNs is confirmed by co-expression of DIP-β-T2A-QF > 10XQUAS-6XmCherry (red) along with DIP-α-A8-Gal4 > 20XUAS-6XGFP (green). (A) In the Fe (left), DIP-β is expressed in the DIP-α-expressing MN innervating the Fe-ltm (denoted as αβFe-ltm in yellow) while DIP-β alone is expressed in leg MNs innervating the distal Fe. In the Ti (right), DIP-β is expressed in the DIP-α-expressing MN innervating the Ti-ltm (denoted as αβTi-ltm in yellow) as well an additional proximal Ti-ltm innervating neuron that does not express DIP-α. Both DIP-α and DIP-β are independently expressed in two tadm-targeting leg MNs (denoted as αTi-tadm in green and βTi-tadm in red) whose terminal branches are closely associated with one another; Magnified inset; green arrowhead points to the αTi-tadm GFP +axon that does not express mCherry. (grey; cuticle). In the adult VNC (A’), co-expression of DIP-β-T2A-QF > 10XQUAS-6XmCherry (Red) with DIP-α-A8-Gal4 > 20XUAS-6XGFP (green) confirms that DIP-β is expressed in only two of three DIP-α-expressing leg MNs as seen in the magnified image of the T1 VNC hemisegment (green asterisks demarcate the locations of the DIP-α-A8-Gal4+ leg MN cell bodies). (scale bar: 50 μm). (B) Left: Terminal branching of the T1 α-leg MNs is unaffected in a DIP-β mutant (green, axons; grey, cuticle). Right: T1 Ti leg segments of a DIP-α homozygous and DIP-β heterozygous mutant (top) in comparison to a DIP-α and DIP-β homozygous double mutant (bottom) showing no increase in the severity of the terminal branching defect seen in αβTi-ltm (Figure 3.C). (white, axons; grey, cuticle). (scale bar: 50 μm). (C) DIP-β cannot rescue the DIP-α mutant branching defect when reintroduced in the mutant DIP-α leg MNs using DIP-α-T2A-Gal4 > UAS-DIP-β. (red, axons; grey, cuticle). Note that mutant αTi-tadm branching was not affected by ectopically expressing UAS-DIP-β. (scale bar: 50 μm).
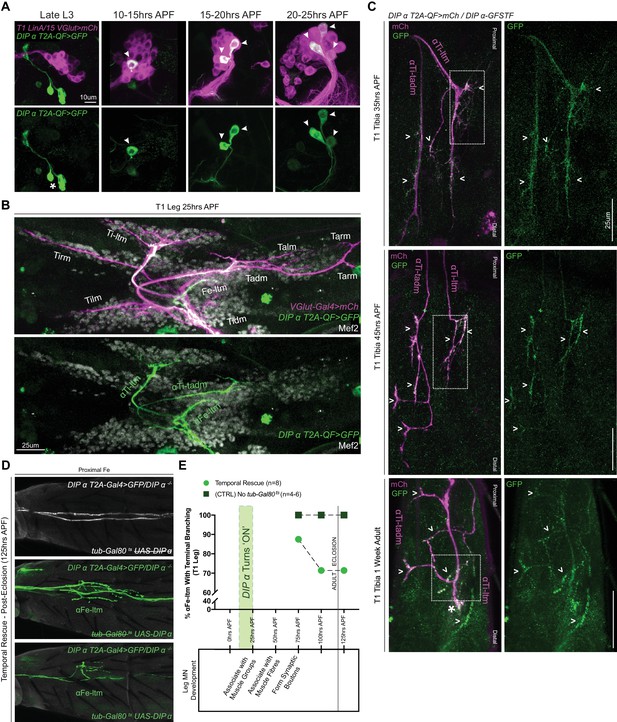
Spatial and temporal characterization of DIP-α expression.
(A) T1 LinA/15 leg MN MARCM clones using OK371-Gal4 > 20XUAS-6XmCherry (magenta) and DIP-α-T2A-QF > 10XQUAS-6XGFP (green) to label leg MN cell bodies in the VNC at multiple developmental time points. At late L3 stages DIP-α expression is not yet ‘ON’ in LinA/15 leg MNs although expression is observed in non-LinA/15 cells (asterisk). Between 10–25 hr APF, the three LinA/15 α-leg MNs (Figure 4—figure supplement 1A) initiate DIP-α expression in a sequential manner, one after the other (arrowheads point to DIP-α+cells in LinA/15 clones) (scale bar: 10 μm). (B) Pupal leg at 25 hr APF stained for all MNs (OK371-Gal4 > 20XUAS-6XmCherry; magenta), immature muscles (Mef2 expression; grey), and DIP-α-expressing MNs (green). (scale bar: 25 μm). (C) Endogenous DIP-α expression in αTi-ltm and αTi-tadm axon termini using GFP-tagged DIP-α-GFSTF (green, detected by anti-GFP, see Materials and methods) and labeled by DIP-α-T2A-QF > 10XQUAS-6XmCherry magenta) at 35 hr APF, 45 hr APF and in 1 week old adults. White arrowheads point to selected regions of mCherry and GFP co-expression. White-dotted boxes denote magnified insets in Figure 4—figure supplement 1D. (scale bar: 25 μm) (D) Temporal rescue at 125 hr APF of axon terminal branching of αFe-ltm in the proximal Fe of T1 adult legs in samples mutant for DIP-α using DIP-α-T2A-Gal4 > 20X-6XGFP, UAS-DIP-α-V5 and tub-Gal80ts (see Supplementary File 2). Top row: Negative control (no UAS-DIP-α-V5) showing absence of αFe-ltm terminal branching in flies that were temperature shifted from 18°C to 30°C at 125 hr APF (axons, white; cuticle, grey). Middle row: Positive control (no tub-Gal80ts) showing complete terminal branching of αFe-ltm in flies that were temperature shifted from 18°C to 30°C at 125 hr APF (axons, green; cuticle, grey). Bottom row: Temporal rescue of terminal branching of αFe-ltm in a DIP-α mutant background in flies that were temperature shifted from 18°C to 30°C at 125 hr APF; Terminal branches are shorter and/or fewer in number compared to the positive controls (axons, green; cuticle, grey). (E) Quantification of T1 leg samples with terminal branching of αFe-ltm in temporally rescued samples (N = 8) (green circles) compared to positive controls (N = 4–6) (no tub-Gal80ts, dark green squares) that were temperature-shifted together at 75 hr, 100 hr or 125 hr APF. Terminal branching of αFe-ltm was seen in 87.5% of samples that were temperature-shifted at 75 hr APF and in 71.42% of samples that were temperature-shifted at 100 hr or 125 hr APF. Terminal branching was always observed in 100% of samples of the positive control and always absent in the negative control (N = 4–6, Figure 4C). Stages of leg MN axon development are indicated below the graph as defined in Figure 1. Initiation of endogenous DIP-α expression in the three WT LinA/15 α-leg MNs is indicated by a vertical green bar at 10 to 25 hr APF. Time of eclosion is indicated by a vertical line at 120 hr/5 days APF.
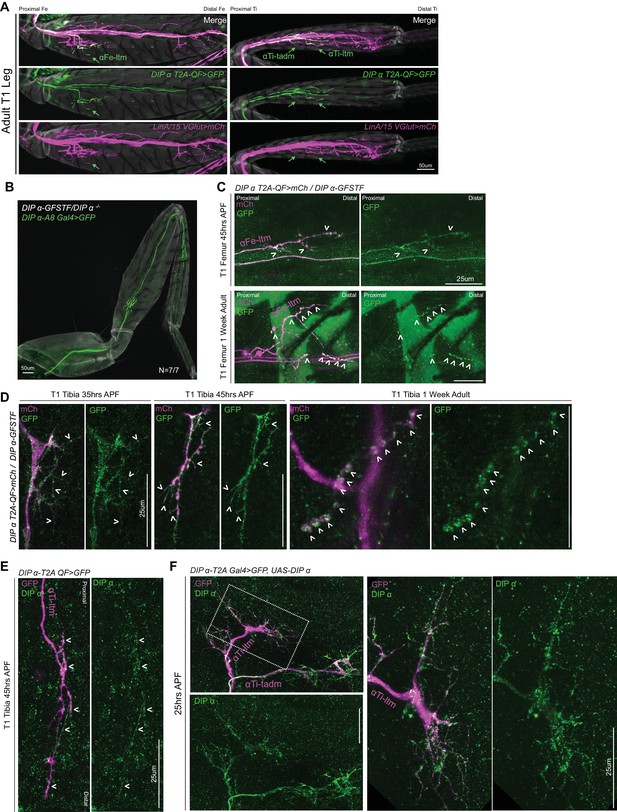
Spatial and temporal characterisation of DIP-α expression.
(A) T1 LinA/15 leg MN cis2MARCM clones (Enriquez et al., 2018) using OK371-Gal4 > 20XUAS-6XmCherry (magenta) and DIP-α-T2A-QF > 10XQUAS-6XGFP (green) to label leg MN axons in the T1 adult Fe and Ti leg segments. Green arrows indicate terminal branching of αFe-ltm, αTi-ltm, and αTi-tadm as belonging to the LinA/15 leg MN lineage in Fe and Ti segments respectively. (scale bar: 50 μm) (B) T1 α-leg MNs labeled by DIP-α-A8-Gal4 > 20XUAS-6XGFP (green) showing normal terminal branching in DIP-α-GFSTF/DIP-α– animals (N = 7/7). (cuticle, grey). (scale bar: 50 μm) (C) Endogenous DIP-α expression in P-D oriented αFe-ltm axon terminals using GFP-tagged DIP-α-GFSTF (green, detected by anti-GFP – see Materials and methods) and labeled by DIP-α-T2A-QF > 10XQUAS-6XmCherry (magenta) at 45 hr APF and in 1 week adults. White arrowheads point to selected regions of mCherry and GFP co-expression. (scale bar: 25 μm). (D) Magnified insets from Figure 4C showing localization of DIP-α::GFP protein expression using DIP-α-GFSTF (green, detected by anti-GFP; see Materials and methods) in fine filopodial projections of αTi-ltm and αTi-tadm labeled by DIP-α-T2A-QF > 10XQUAS-6XmCherry (magenta) at 35 hr and 45 hr APF, and in pre-synaptic regions of the NMJ/synaptic boutons along the terminal branches in 1 week old adults (denoted by white arrowheads) (scale bar: 25 μm). (E) Antibody staining against DIP-α (green) in an αTi-ltm axon labeled by DIP-α-T2A-QF > 10XQUAS-6XGFP (magenta) at 45 hr APF showing low levels of expression in specific axon branches (denoted by white arrowheads). (scale bar: 25 μm). (F) Antibody staining against DIP-α (green) in α-leg MN axons (Left) overexpressing DIP-α and labeled by DIP-α-T2A-Gal4 > 20XUAS-6XGFP, UAS-DIP-α (magenta) at 25 hr APF showing high levels of expression in axon terminals including filopodial projections. White-dotted box denotes magnified inset of αTi-ltm (right) (scale bar: 25 μm).
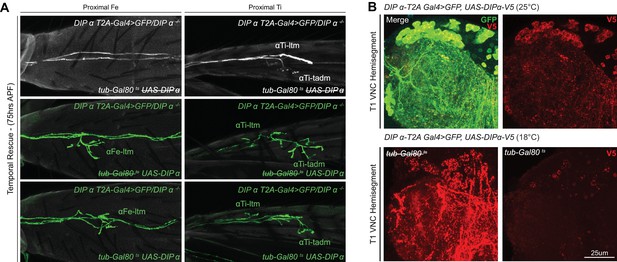
Temporal rescue of DIP-α in a mutant background.
(A) Temporal rescue at 75 hr APF of axon terminal branching of αFe-ltm (left column) and αTi-ltm (right column) in the proximal Fe of T1 adult legs in samples mutant for DIP-α using DIP-α-T2A-Gal4 > 20XUAS-6XGFP, UAS-DIP-α-V5 and tub-Gal80ts (see Materials and methods). Top row: Negative control (no UAS-DIP-α-V5) showing absence of αFe-ltm and αTi-ltm terminal branching in flies that were temperature shifted from 18°C to 30°C at 75 hr APF (axons, white; cuticle, grey). Middle row: Positive control (no tub-Gal80ts) showing complete terminal branching of αFe-ltm but incomplete terminal branching of αTi-ltm and αTi-tadm in flies that were temperature shifted from 18°C to 30°C at 75 hr APF (refer to Figure 3. for WT terminal branching) (axons, green; cuticle, grey). Bottom row: Temporal rescue of terminal branching of αFe-ltm in a DIP-α mutant background in flies that were temperature shifted from 18°C to 30°C at 75 hr APF; αTi-ltm and αTi-tadm were not compared to defective positive controls. (axons, green; cuticle, grey). (B) T1 VNC hemisegments expressing DIP-α-T2A-Gal4 > 20XUAS-6XGFP, UAS-DIP-α-V5 at 25°C (top) and with/without tub-Gal80ts at 18°C (bottom) stained for V5 expression (red). (scale bar: 25 μm).
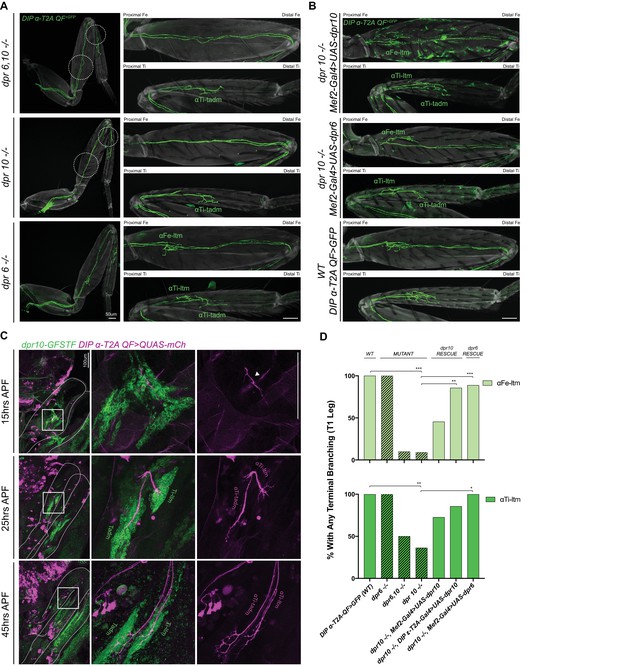
dpr10 Expression in muscles is necessary for terminal branching of the α-leg MNs.
(A) Terminal branching of the T1 α-leg MNs labeled by DIP-α-T2A-QF > 10XQUAS-6XGFP in dpr6 and dpr10 double and single mutants. Left – T1 legs; Right – Fe and Ti leg segments (axons, green; cuticle, grey). Terminal branching of αFe-ltm and αTi-ltm is absent only in the dpr6, dpr10 double mutant and dpr10 single mutant and phenocopies the DIP-α mutant phenotype (white dotted circles), while terminal branching of the α-leg MNs is intact in a dpr6 single mutant. (scale bar: 50 μm) (B) Muscle-specific expression of dpr10 (top) and dpr6 (middle) using Mef2-Gal4 > UAS-dpr10/6 V5 in a dpr10 mutant background in Fe and Ti T1 leg segments showing rescue of terminal branching of αFe-ltm and αTi-ltm labeled by DIP-α-T2A-QF > 10XQUAS-6XGFP. Expression of dpr10 in the muscles with the strong muscle driver, Mef2-Gal4, caused ectopic aberrant induction of DIP-α-T2A-QF > 10XQUAS-6XGFP expression in the cuticle of the leg. Wild-type terminal branching of the α-leg MNs is displayed using DIP-α-T2A-QF > 10XQUAS-6XGFP (bottom). (scale bar: 50 μm) (C) Endogenous dpr10 expression in the developing T1 leg (Left column) using a GFP protein-trap inserted into a coding intron of dpr10 (Figure 5—figure supplement 1D) (detected using a anti-GFP (green) – see Materials and methods) at 15 hr, 25 hr and 45 hr APF. Developing α-leg MNs are concurrently labeled by DIP-α-T2A-QF > 10XQUAS-6XmCherry (magenta) (middle column, merge; right column, DIP-α-T2A-QF > 10XQUAS-6XmCherry). At 15 hr APF (top row), at most only two of three α-leg MNs express DIP-α (immature axon terminals are indicated by a white arrowhead) and dpr10 is broadly expressed in immature adult muscle precursors. By 25 hr APF (middle row) when axons are normally associated with their muscle groups, immature axons of αTi-ltm and αTi-tadm form filopodia in the dpr10 expressing Ti-ltm and tadm. At 45 hr APF (bottom row) when leg MN axons are normally associated with distinct muscle fibers, αFe-ltm (Figure 5—figure supplement 1F), αTi-ltm and αTi-tadm have generated their terminal branches in the dpr10 expressing Ti-ltm and Tadm. (scale bar: 100 μm) (D) Quantification of percentages of T1 leg samples with terminal branching of αFe-ltm (light green) and αTi-ltm (medium green) in WT (N = 15), mutant (diagonal lines) and rescue contexts (N = 7 to 11) using dpr6 and dpr10 double and single null mutations as indicated (see Materials and methods). Statistical significance was determined using Fisher’s exact test.
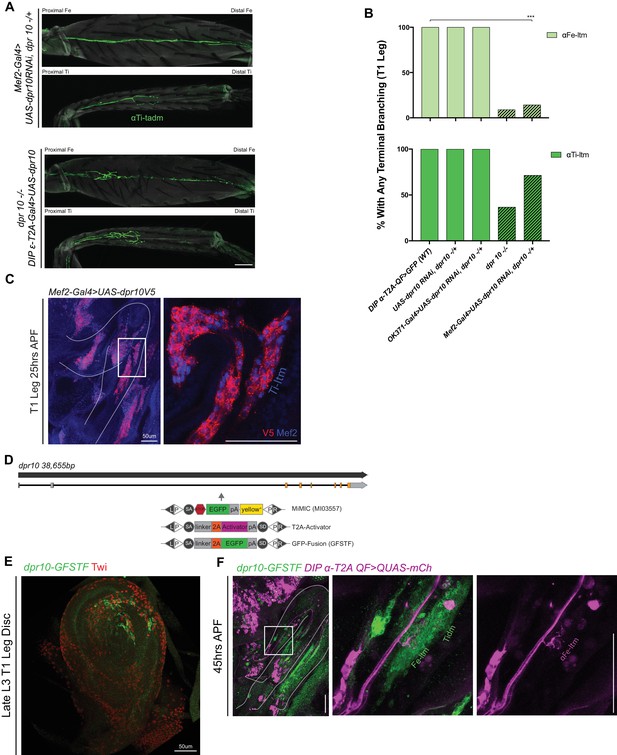
dpr10 expression in muscles is necessary for terminal branching of the α-Leg MNs.
(A) Terminal branching of the T1 α-leg MNs in Fe and Ti segments labeled by DIP-α-T2A-QF > 10XQUAS-6XGFP (axons, green; cuticle, grey). Top – Muscle-specific RNAi knockdown of dpr10 using Mef2-Gal4 > UAS-dpr10 RNAi, dpr10-/+ resulted in the absence of terminal branching of αFe-ltm and αTi-ltm. Bottom – Inducing dpr10 expression with DIP-ε-T2A-Gal4 > UAS-dpr10-V5 rescued the dpr10 mutant terminal branching of αFe-ltm and αTi-ltm. (scale bar: 50 μm) (B) Quantification of percentages of T1 leg samples with terminal branching of αFe-ltm (light green) and αTi-ltm (medium green) in WT samples (N = 15) as compared to negative controls, dpr10 mutant (diagonal lines) and muscle-specific RNAi knockdown of dpr10 (diagonal lines) as indicated. Muscle-specific RNAi knockdown of dpr10 caused a significant reduction in the frequency of αFe-ltm terminal branching (N = 7, ***p<0.001) but not that of αTi-ltm. (C) Muscle-specific expression of dpr10 using Mef2-Gal4 > UAS-dpr10-V5 induces high levels of Dpr10 in the immature T1 adult leg muscles (Left, white curved lines outline the T1 leg and white rectangular box demarcates region of magnification; right, magnified image of the T1 distal Fe and proximal Ti segments) visualised here by staining against V5 (red) and Mef2 (blue) at 25 hr APF (See Materials and methods). (scale bar: 50 μm) (D) Genomic locus of dpr10 showing introns (black line), non-coding exons (grey rectangles) and coding exons (orange rectangles). The location of the MiMIC insertion (MI03557) in the coding intron is denoted by a grey arrow. Using recombination mediated cassette exchange (RMCE), MI02031 was swapped into a T2A-binary activator (Gal4) that acts as a ‘gene trap’ and a GFP protein fusion (GFSTF) that acts as a ‘protein trap’ (see Materials and methods) (Venken et al., 2011). (E) Endogenous dpr10 expression in a subset of immature leg muscle precursors in a late L3 T1 leg imaginal disc using dpr10-GFSTF (Figure 5—figure supplement 1S) (detected using a ch-anti-GFP (green) – see Materials and methods) and stained for Twist (Twi) a muscle-precursor marker (red). (scale bar: 50 μm) (F) dpr10 expression in the developing T1 leg (left) at 45 hr APF using dpr10-GFSTF (Figure 5—figure supplement 1D) (detected using anti-GFP (green) – see Materials and methods) at 45 hr APF showing terminal branching of αFe-ltm labeled by DIP-α-T2A-QF > 10XQUAS-6XmCherry (magenta) in dpr10 expressing Fe-ltm (green). The tidm also expresses high levels of Dpr10 at this stage. (middle column – merge; right column, DIP-α-T2A-QF > 10XQUAS-6XmCherry). (scale bar: 100 μm).
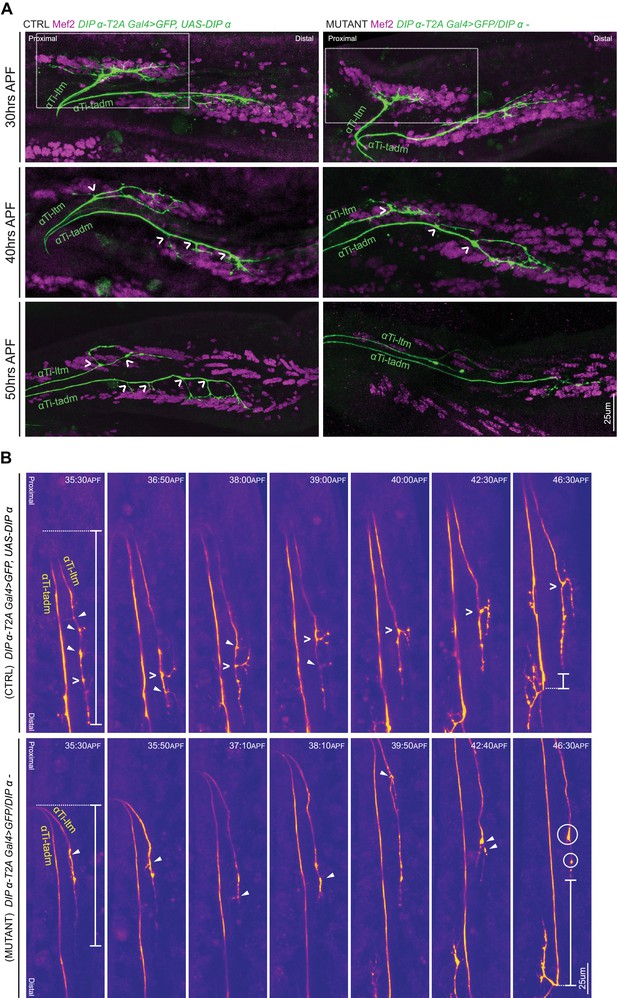
DIP-α is required for terminal axon lengthening and branching 30 to 45 hr APF.
(A) Terminal axon branching of control (left) and DIP-α mutant (right) αTi-ltm and αTi-tadm leg MNs at 30 hr (top), 40 hr (middle) and 50 hr (bottom) APF using DIP-α-T2A-Gal4 > UAS-DIP-α and DIP-α-T2A-Gal4/DIP-α–, respectively. Axons are labeled using DIP-α-T2A-Gal4 > 20XUAS-6XGFP (green) and muscles are labeled with antibody against Mef2 (magenta). White arrowheads demarcate branch points along the axon terminal. At 50 hr APF, mutant αTi-ltm axons lack a prominent contralateral branch and mutant αTi-tadm axons lack four contralateral branches and retain the distal-most branch. White-dotted box denotes magnified inset in Figure 5—figure supplement 1A (scale bar: 25 μm). (B) Snapshots from time-lapse videos (Video 4, Video 5) comparing control (top) and mutant (bottom) αTi-ltm and αTi-tadm axons between ~35 hr and 45 hr APF (time-stamp is located on the top-right corner of each snapshot). Axons are labeled using DIP-α-T2A-Gal4 > 20XUAS-6XGFP (yellow). White open arrowheads demarcate the contralateral branch point on the αTi-ltm axon in the control sample while filled white arrowheads demarcate assorted dynamic filopodial projections along the αTi-ltm axon in both control and mutant samples. The distal-most tip of the αTi-ltm axon is more proximally located in the mutant sample compared to the control at ~35 hr APF (far left), as measured from the axon ‘bend’ at the joint between the distal Femur and proximal Tibia (denoted by white vertical bars) as well as at ~45 hr APF (far right), as measured from the distal most branch of αTi-tadm (denoted by white vertical bars). White circles demarcate globular punctate structures that form on the mutant αTi-ltm axon by ~45 hr APF (far right). (scale bar: 25 μm).
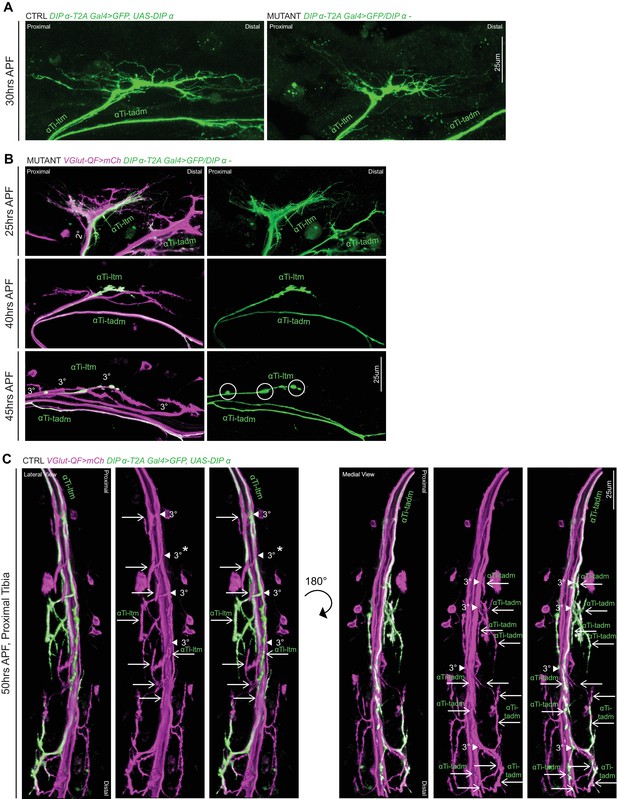
DIP-α is Required for terminal axon lengthening and branching 30 to 45 hr APF.
(A) Magnified inset from Figure 6A showing decreased αTi-ltm terminal axon branching in a DIP-α mutant (right) compared to the control (left) at 30 hr APF. Axons are labeled using DIP-α-T2A-Gal4 > 20XUAS-6XGFP (green) (scale bar: 25 μm). (B) 3D projections of axon terminals of Ti-ltm targeting leg MNs, including αTi-ltm, in a DIP-α mutant background at 25 hr (top), 40 hr (middle) and 45 hr (bottom) APF. Leg MNs are labeled using VGlut-QF >10XQUAS-6xmCherry (magenta) and αTi-ltm is labeled using DIP-α-T2A-Gal4 > 20XUAS-6XGFP (green) (left, merge; right, αTi-ltm). At 25 hr APF, DIP-α mutant αTi-ltm axon terminals are located within their secondary axon bundles (2°) and generate filopodial projections. Between 40–45 hr APF, although mutant αTi-ltm axons sort into tertiary axon bundles (3°), they fail to generate terminal branches. White circles denote globular, punctate structures that form on the mutant αTi-ltm axon by ~45 hr APF. (scale bar: 25 μm). (C) 3D projections of axon terminals of Ti targeting leg MNs, including αTi-ltm (lateral view) and αTi-tadm (medial view), in a control background at 50 hr APF. Leg MNs are labeled using VGlut-QF >10XQUAS-6xmCherry (magenta) and α-leg MNs are labeled using DIP-α-T2A-Gal4 > 20XUAS-6XGFP (green)(left-unlabeled, merge; middle-labeled, VGlut; right-labeled, merge). White arrowheads point to tertiary bundles of MNs targeting the Ti-ltm and tadm and white arrows point to distinct terminal branches. In total there are four tertiary bundles targeting the Ti-ltm (one of which does not belong to the LinA/15 leg MN lineage – white asterix) composed of eight terminal branches, two of which belong to αTi-ltm. In the portion of the tadm pictured here, there are four tertiary bundles composed of approximately thirteen distinct terminal branches, of which nine belong to αTi-tadm.(scale bar: 25 μm).
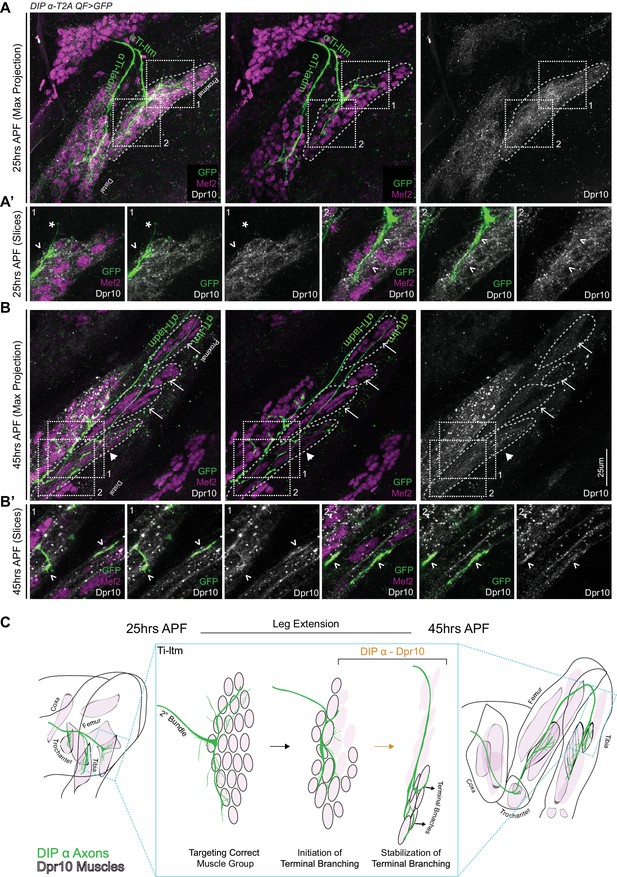
Dpr10 Expression is gradually restricted to distal fibers of the Ti-ltm 30 to 45 hr APF.
(A–B) Dpr10 protein expression (grey) in the developing Ti-ltm and proximal tadm, labeled by Mef2 (magenta) along with αTi-ltm and αTi-tadm axons labeled by DIP-α-T2AQF > 10XQUAS-6XGFP (green) at 25 hr (A) and 45 hr (B) APF. Left: GFP, Mef2 and Dpr10; middle: GFP and Mef2; right: Dpr10. (A’,B’) show magnified single slice images of the dashed boxes indicated in A,B. While Dpr10 is widely expressed in the entire Ti-ltm at 25 hr APF (A) (dashed boundary demarcates immature Ti-ltm), it is later restricted to the distal muscle fibers of the Ti-ltm at 45 hr APF (B) (white arrowhead, dashed boundaries demarcate distinct groups of muscle fibers of the Ti-ltm). Lack of Dpr10 expression in the proximal muscles fibers of the Ti-ltm at 45 hr APF (B) are denoted by white arrows. Dpr10 is continuously expressed in the tadm. (scale bar: 25 μm). At 25 hr APF (A’) there is no correlation between Dpr10 protein and αTi-ltm innervation, denoted by white arrowheads. By 45 hr APF (B’) higher levels of Dpr10 protein are seen in regions innervated by the αTi-ltm terminal branches (white arrowheads). (C) Schematic representation of a DIP-α expressing axon (green) innervating Dpr10 expressing muscle precursors (black outlined pink cells) during the course of development between 25 to 45 hr APF, depicting the restriction of Dpr10 to specific muscle fibers targeted by the DIP-α expressing axon. Leg extension occurs between ~30 to 40 hr APF.
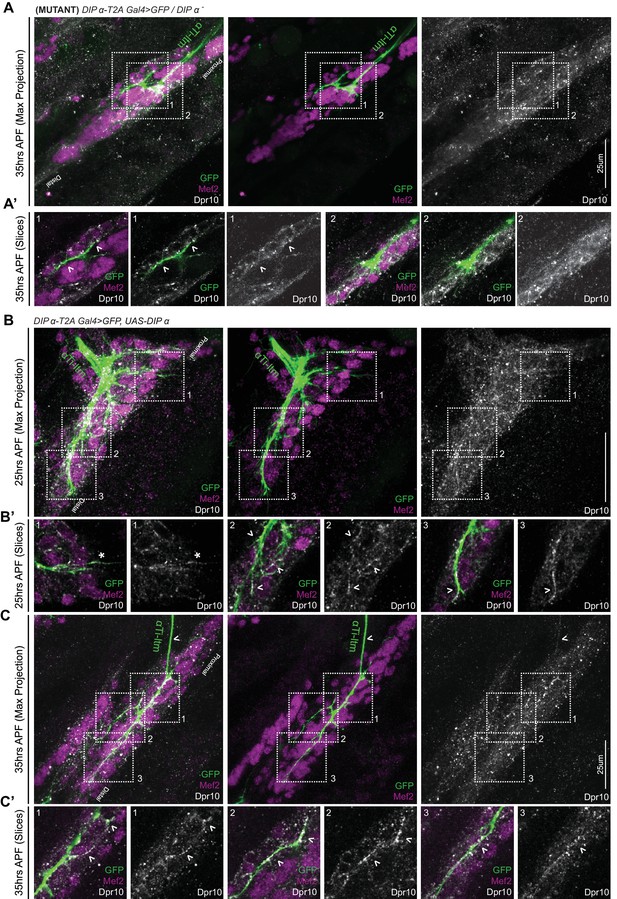
Dpr10 expression in the developing Ti-ltm in DIP-α mutant and overexpression contexts.
(A, A’) DIP-α mutant αTi-ltm axon labeled by DIP-α-T2A-Gal4 > 20XUAS-6XGFP (green) retains its ability to target regions of high Dpr10 expression (grey) in the developing Ti-ltm (labeled by Mef2, magenta) at 35 hr APF. (Left: GFP, Mef2 and Dpr10; middle: GFP and Mef2; right: Dpr10). Numbered white-dotted boxes demarcate locations of magnified single-slice insets (A’) (scale bar: 25 μm). White arrowheads demarcate locations of axon innervation by the DIP-α mutant αTi-ltm. (B–C) Overexpression of DIP-α in α-leg MNs using DIP-α-T2A-Gal4 > UAS-DIP-α (labeled by 20XUAS-6XGFP, green), causes accumulation of Dpr10 protein (grey) at sites of axon innervation in the Ti-ltm (labeled by Mef2, magenta), as well as within the primary axon and filopodial projections of αTi-ltm at 25 hr APF (B–B’) and 45 hr APF (C–C’). Numbered white-dotted boxes demarcate locations of magnified single-slice insets (B’,C’). Left: GFP, Mef2 and Dpr10; middle: GFP and Mef2; right: Dpr10). White asterix demarcates a filopodial projection of αTi-ltm that is not physically associated with the Ti-ltm but nevertheless expresses Dpr10. White arrowheads point to regions of axon innervation in the Ti-ltm or within the main axon of αTi-ltm that express high levels of Dpr10.
Videos
Live imaging of developing LinA/15 leg MNs between ~25 - 37 hr APF.
WT Time lapse in vivo live imaging of developing T1 LinA/15 leg MNs expressing myr::GFP (yellow) between ~25 hr APF (00:00) to ~37 hr APF (12:20) (10 min interval, five fps). In the first frame the Ti-ltm targeting secondary axon bundle is labeled within the white-dotted box demarcating the entire Ti segment. Leg extension is initiated at ~30 hr APF and axons within the secondary bundle begin to defasciculate while filopodial branches maintain physical contact with their muscle targets (Figure 1B–D, Figure 1—figure supplement 1C–E). (scale bar: 50 μm).
Live imaging of developing LinA/15 Ti-ltm targeting leg MNs between ~40 - 50 hr APF.
WT Time lapse in vivo live imaging of developing T1 LinA/15 Ti-ltm targeting leg MNs expressing myr::GFP (yellow) between ~40 hr APF (00:00) to ~50 hr APF (12:20) (10 min interval, five fps). In the first frame the Ti-ltm targeting secondary axon bundle is labeled within the white-dotted box. The generation of stable terminal branches occurs between ~43 to 45 hr APF, at which point leg muscles are being reorganized into distinct muscle fibers (Figure 1B–D, Figure 1—figure supplement 1C–E). (scale bar: 50 μm).
Comparison of WT and DIP-α mutant Ti-ltm targeting leg MNs using live-imaging.
Time lapse in vivo live imaging of Ti-ltm targeting leg MNs, including αTi-ltm, in a DIP-α mutant animal between ~30 hr APF (00:00) to ~38 hr APF (08:30) (10 min interval, five fps). Leg MNs are labeled using VGlut-QF >10XQUAS-6xmCherry (magenta) and αTi-ltm is labeled using DIP-α-T2A-Gal4 > 20XUAS-6XGFP (green) (left: αTi-ltm; right: Merge). In the first frame, both, the Ti-ltm and Ti-tadm, lm (levator muscles), and rm (reductor muscles) targeting secondary axon bundles (magenta), as well as individual αTi-ltm and αTi-tadm axons (green) within these bundles are visible. DIP-α mutant αTi-ltm axons generate dynamic filopodia during leg extension, but show a gradual decline in branching between 30 to 45 hr APF (Figure 6A, Figure 6—figure supplement 1A–B). (scale bar: 50 μm).
Live imaging of WT αTi-ltm leg MN between ~35 hr APF to ~45 hr APF.
Time lapse in vivo live imaging of αTi-ltm leg MNs in control (Video 4) and DIP-α mutant animal (Video 5), using DIP-α-T2A-Gal4 > UAS-DIP-α and DIP-α-T2A-Gal4/DIP-α– respectively, between ~35 hr APF (00:00) to ~45 hr APF (Control: 11:30; Mutant: 11:40) (10 min interval, five fps). α-leg MNs are labeled using DIP-α-T2A-Gal4 > 20XUAS-6XGFP (yellow). αTi-ltm and αTi-tadm axons are labeled in the first frame. The DIP-α mutant αTi-ltm axon fails to generate stable terminal branches, while the control αTi-ltm axon begins to generate a collateral branch at ~38 hr APF which stabilizes and extends in length by ~45 hr APF (Figure 6B). (scale bar: 50 μm).
Live imaging of DIP-α mutant αTi-ltm leg MN between ~35 hr APF to ~45 hr APF.
https://doi.org/10.7554/eLife.42692.020Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (D. melanogaster) | OK371-Gal4 | BDSC #26160 | RRID:BDSC_26160 | |
| Genetic reagent (D. melanogaster) | Vglut-T2A-QF2 | BDSC #60315 | RRID:BDSC_60315 | |
| Genetic reagent (D. melanogaster) | 10 C12-Gal4 | BDSC #47841 | RRID:BDSC_47841 | |
| Genetic reagent (D. melanogaster) | dpn > KDRT > Cre; Act > LoxP > LexA, LexA-myr::GFP; UAS-KD | PMID:24561995 | ||
| Genetic reagent (D. melanogaster) | Mef2-QF2 | BDSC #66469 | RRID:BDSC_66469 | |
| Genetic reagent (D. melanogaster) | 13XLexAop2-6XGFP | BDSC #52265 | RRID:BDSC_52265 | |
| Genetic reagent (D. melanogaster) | 10XQUAS-6XGFP | BDSC #52264; this paper | RRID:BDSC_52264 | VK0027 insertion generated for this paper. |
| Genetic reagent (D. melanogaster) | 10XQUAS-6XmCherry | BDSC #52269; BDSC #52270 | RRID:BDSC_52269; RRID:BDSC_52270 | |
| Genetic reagent (D. melanogaster) | 20XUAS-6X-GFP | BDSC #52261; BDSC #52262; This paper | RRID:BDSC_52261; RRID:BDSC_52262 | 86Fa insertion generated for this paper. |
| Genetic reagent (D. melanogaster) | 20XUAS-6X-mCherry | BDSC #52268 | RRID:BDSC_52268 | |
| Genetic reagent (D. melanogaster) | MiMIC-T2A-Gal4 lines | BDSC #7838; BDSC #76200; This paper | RRID:BDSC_78385; RRID:BDSC_76200 | Additional lines are listed in Supplementary file 1. generated by S.Nagarkar Jaiswal, H.Bellen and M.Courgeon, C. Desplan. |
| Genetic reagent (D. melanogaster) | DIP-α-A8-Gal4 | This paper | attp2 and 86Fa insertions generated for this paper; See Materials and methods | |
| Genetic reagent (D. melanogaster) | DIP-α-T2A-QF2 | This paper | MiMIC Trojan Swaps generated for this paper; See Materials and methods | |
| Genetic reagent (D. melanogaster) | DIP-β-T2A-QF2 | This paper | MiMIC Trojan Swaps generated for this paper; See Materials and methods | |
| Genetic reagent (D. melanogaster) | DIP-α1-7 also referred to as DIP-αnull2 | PMID: 30467079 | Generated by the Zipursky Lab | |
| Genetic reagent (D. melanogaster) | DIP-β1-95 | This paper | Generated by the Zipursky Lab | |
| Genetic reagent (D. melanogaster) | DIP-γ1-67 | PMID: 30467079 | Generated by the Zipursky Lab | |
| Genetic reagent (D. melanogaster) | UAS-DIP-α-V5 | PMID: 30467079 | Generated by the Zipursky Lab | |
| Genetic reagent (D. melanogaster) | UAS-DIP-β | VK0027 insertion generated for this paper. | ||
| Genetic reagent (D. melanogaster) | dpr6,10 - also referred to as dpr6-10L | PMID: 30467079 | Generated by the Zipursky Lab | |
| Genetic reagent (D. melanogaster) | dpr101-29 also referred to as dpr10null | PMID: 30467079 | Generated by the Zipursky Lab | |
| Genetic reagent (D. melanogaster) | dpr61-116 also referred to as dpr6null | PMID: 30467079 | Generated by the Zipursky Lab | |
| Genetic reagent (D. melanogaster) | UAS-dpr10-V5 | PMID: 30467079 | Generated by the Zipursky Lab | |
| Genetic reagent (D. melanogaster) | UAS-dpr6-V5 | PMID: 30467079 | Generated by the Zipursky Lab | |
| Genetic reagent (D. melanogaster) | UAS-dpr10 RNAi VDRC | VDRC# 103511 | ||
| Genetic reagent (D. melanogaster) | tub > FRT .Gal80> | BDSC #38879; BDSC #38880 | RRID:BDSC_38879; RRID:BDSC_38880 | |
| Genetic reagent (D. melanogaster) | QUAS-DSCP-Flp0.2G (attp2) | BDSC #30008 | RRID:BDSC_30008 | |
| Genetic reagent (D. melanogaster) | tub-Gal80ts | BDSC #7108 | RRID:BDSC_7108 | |
| Genetic reagent (D. melanogaster) | DIP-α-GFSTF | BDSC #60523 | RRID:BDSC_60523 | |
| Genetic reagent (D. melanogaster) | dpr10-GFSTF | BDSC #59807 | RRID:BDSC_59807 | |
| Genetic reagent (D. melanogaster) | R13C09-Gal4 | BDSC #48555 | RRID:BDSC_48555 | |
| Genetic reagent (D. melanogaster) | hkb-Gal4 | BDSC #62578 | RRID:BDSC_62578 | |
| Genetic reagent (D. melanogaster) | DIP-ζ RNAi – TriP.HMS01671 | BDSC #38227 | RRID:BDSC_38227 | |
| Genetic reagent (D. melanogaster) | FRT42D | BDSC #1802 | RRID:BDSC_1802 | |
| Genetic reagent (D. melanogaster) | FRT42D tubG80, tubQS | PMID: 29395908 | ||
| Genetic reagent (D. melanogaster) | y,w,hs-Flp1.22, hs-Flp122.2(Chr 2) | Other | Gift from Gary Struhl | |
| Genetic reagent (D. melanogaster) | Mef2-Gal4 (Chr 2) | Other | Bellen Lab, provided by R.Carrillo | |
| Antibody | Rabbit polyclonal Anti-Mef2 | PMID: 7839146 | RRID:AB_2568604 | Generated by B.Paterson; (1:500) |
| Antibody | Sheep polyclonal Anti-GFP | Bio-Rad | Cat# 4745–1051 | (1:500) |
| Antibody | Chicken polyclonal Anti-GFP | Abcam | Cat# ab101863; RRID:AB_10710875 | (1:1000) |
| Antibody | Mouse monoclonal Anti-DIP-α | PMID: 30467079 | Generated by the Zipursky Lab; (1:20) | |
| Antibody | Mouse monoclonal Anti-Dpr10 | PMID: 30467079 | Generated by the Zipursky Lab; (1:500) | |
| Recombinant DNA reagent | T2A-QF2-Hsp70 | PMID:25732830 | RRID:Addgene_62944; RRID:Addgene_62945 | |
| Recombinant DNA reagent | pJFRC28-10XUAS-IVS-GFP-p10 | PMID:22493255 | RRID:Addgene_36431 |
Additional files
-
Supplementary file 1
DIP and dpr MiMIC-T2A-Gal4 lines .
- https://doi.org/10.7554/eLife.42692.023
-
Supplementary file 2
Genotypes used for each figure.
- https://doi.org/10.7554/eLife.42692.024
-
Transparent reporting form
- https://doi.org/10.7554/eLife.42692.025





