FGF21 trafficking in intact human cells revealed by cryo-electron tomography with gold nanoparticles
Figures
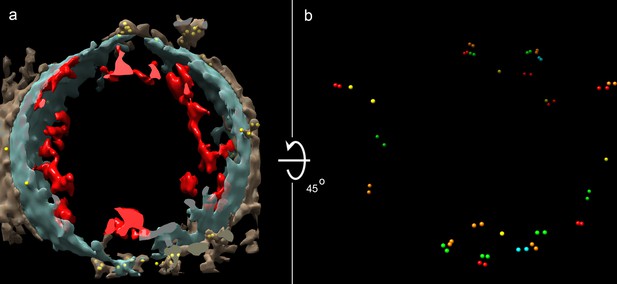
Cryo-ET of a vesicle from human adipocytes membrane preparations, treated with AuNP-FGF21 conjugate.
(a) Isosurface rendering of tomographic reconstruction, with membrane density in blue-gray, density on the outer surface of the membrane in brown, density on the inner surface of the membrane in red, and AuNPs in yellow. (b) Same as (a), with all membrane and membrane-associated density removed, with different colors to distinguish pairs of AuNPs, and with rotation of 45° from the view in (a) for better visualization of AuNPs.
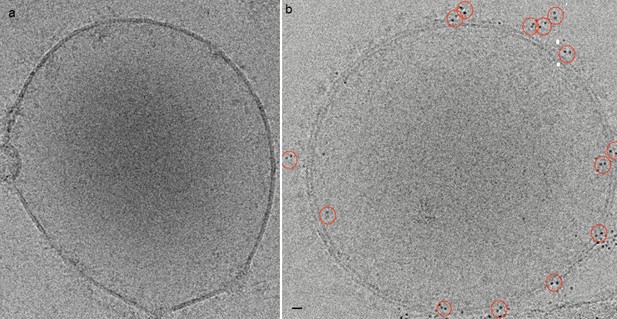
Cryo-EM images of membrane preparations treated with AuNP-FGF21 conjugate.
Vesicles from AuNP-FG21 treated membrane preparations from (a) parental CHO cells, in which neither FGFR1c nor βKlotho are expressed and (b) human primary adipocyte cells, in which FGFR1c and βKlotho are endogenously expressed. Bar 10 nm.
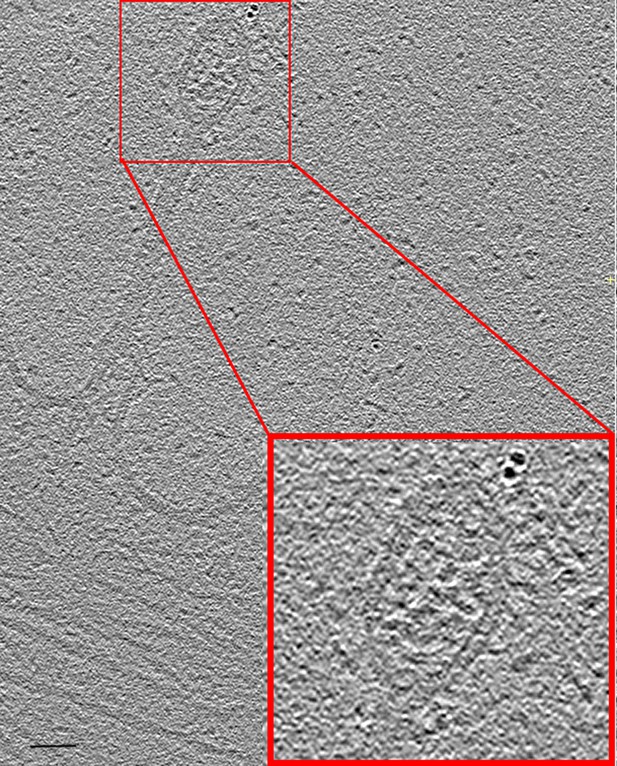
AuNPs characteristic footprint in a tomogram slice.
Tomogram slice showing a pair of gold nanoparticles associated with cell surface. Bar 10 nm. Lower right corner, boxed area zoomed out two fold.
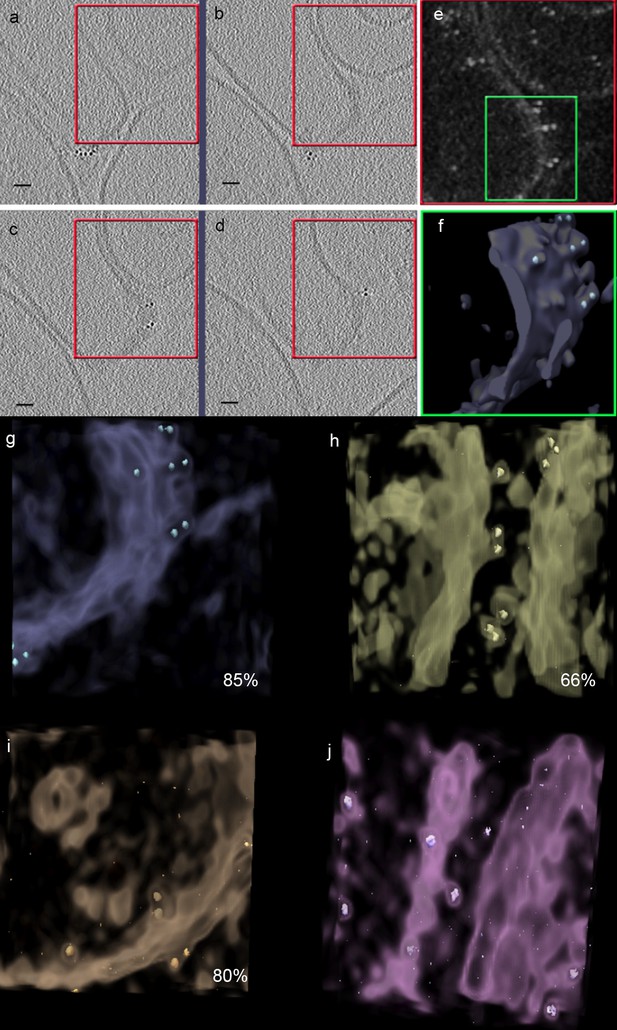
Predominance of AuNP pairs on the surface of vesicles from membrane preparations form CHO cells overexpressing FGFR1c and βKlotho.
Membrane preparations from CHO cells expressing FGFR1c and βKlotho (a–h) or CHO cells expressing only βKlotho (i and j) were treated with AuNP-FGF21 (a-g, and i) or with a AuscFv against βKlotho (h and j). (a–d) Tomogram slices. Bar 10 nm. (e) Solid representation of areas boxed red in a-d. (f) Isosurface of area boxed green in e, rotated 20° to facilitate visualization of pairs and single AuNPs. (g–i) AuNP pair (%) measured as [(number of AuNPs in pairs)/(total number of AuNPs)]*100, where total number of AuNPs >100 for all cases (including (j), where no pairs were observed). AuNPs counted from four to five tomograms in each case.
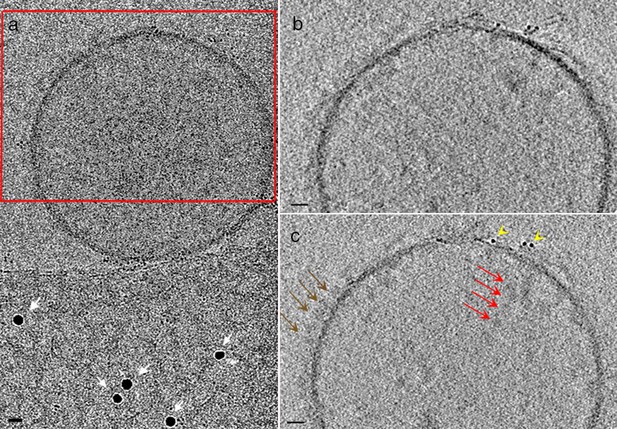
Comparison of tomographic reconstruction using different markers as fiducials.
(a) 0° tilt image of a vesicle from adipocytes membrane preparations treated with AuNP-FGF21. 10 nm gold beads (white arrows) were mixed with the sample prior to application to an electron-microscope grid and plunge-freezing for cryo-EM collection. (b and c) Slices of tomographic reconstruction of red boxed area in (a) using either (b) 10 nm gold beads, or (c) Au144 NPs (Azubel et al., 2017) for alignment. Better-resolved densities on the outer surface of the membrane (brown arrows), on the inner side of the membrane (red arrows), and corresponding to AuNPs (yellow head arrows) are indicated. Bars 10 nm.
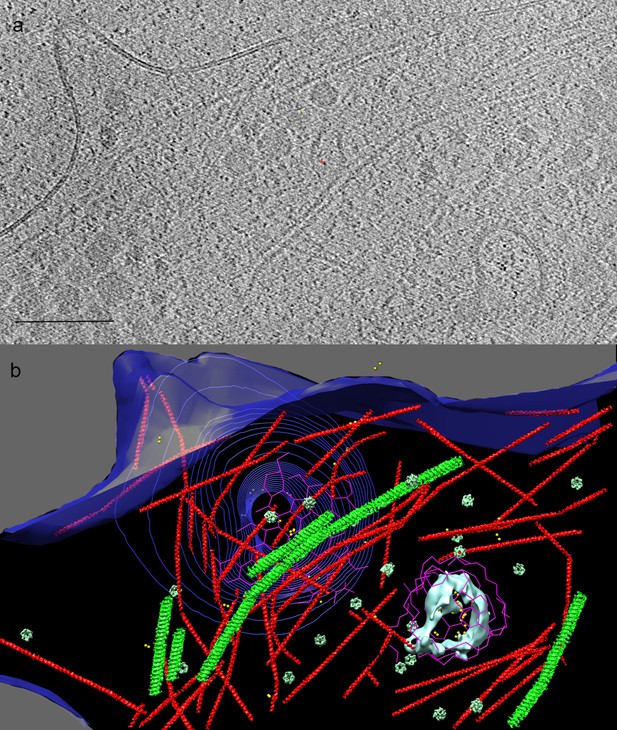
Cryo-ET of human adipocyte cell treated with AuNP-FGF21 conjugate.
(a) Slice of tomogram showing a region near the cell periphery. Bar 100 nm. (b) 3D tomographic data, with the plasma membrane in blue (invagination of the membrane, viewed from inside the cell, represented by contours), isosurface rendering of a coated vesicle membrane in cyan, clathrin in magenta, actin in red and microtubules in green (substituted with helical reconstructions from Figure 4), hexameric rings (putative p97 AAA+ ATPAse) in emerald (substituted with subtomogram averages from Figure 4), and AuNPs in yellow.
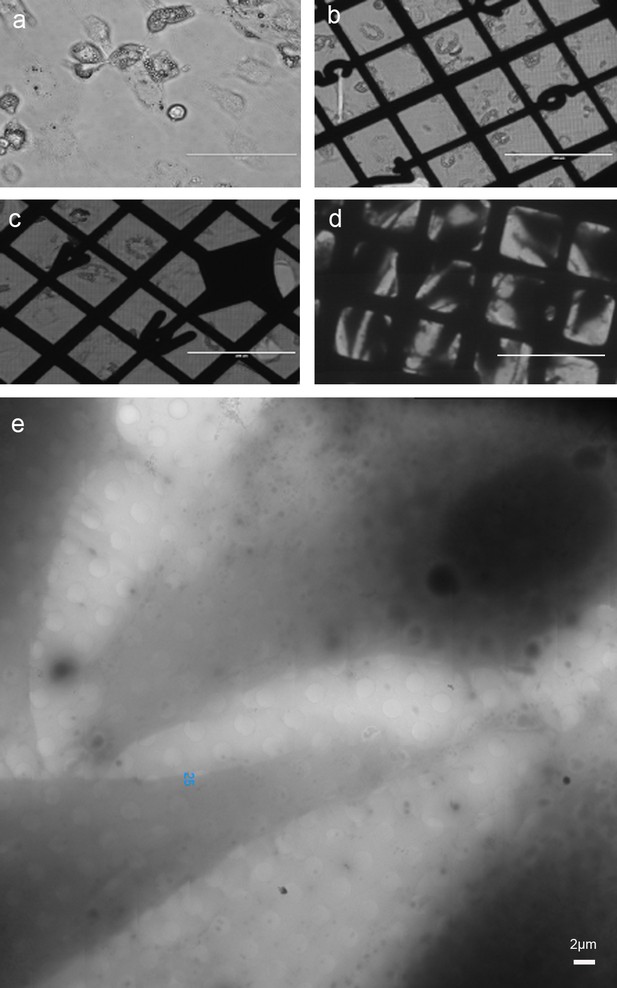
Viability of human adipocyte cells transferred to EM grids, and thinness at the periphery after plunge-freezing.
(a) Cells grown in tissue culture flask. (b) Cells transferred to EM grids and incubated overnight at 37°C in the presence of 5% CO2. (c) Same as (b), followed by 1 h incubation at 4°C. The cells remained attached and flat, indicative of viability. (d) Same as (c) after plunge-freezing. (e) Same as (d) at higher magnification. Bar (a–d) 200 μm, bar (e) 2 µm. (a–c) Light microscopy images, (d–e) cryo-EM images.
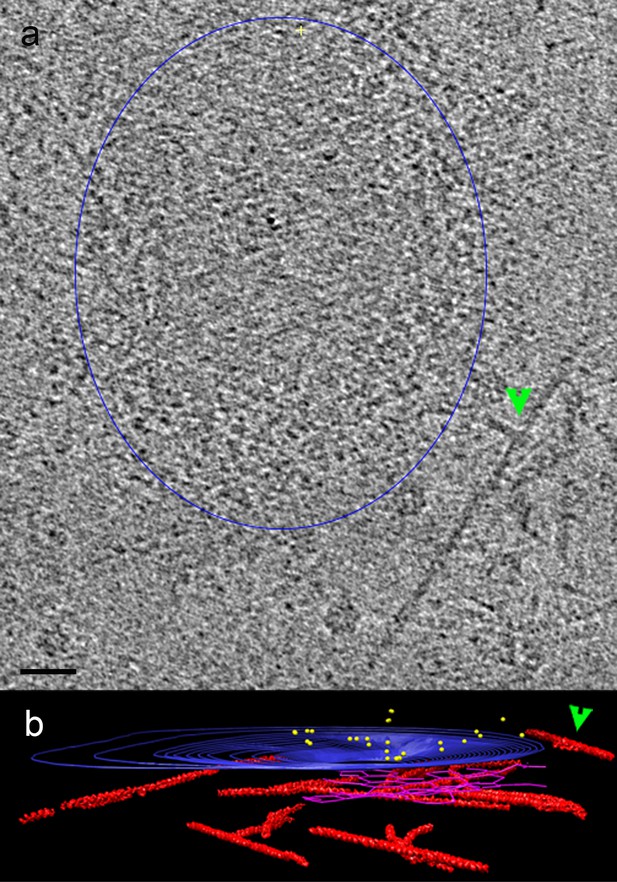
Polymerization of actin filament in a y-shape provides force for membrane deformation (Kaksonen et al., 2006).
(a) Tomogram slice, from cell shown in Figure 2, showing a y-shaped actin filament (green arrow) in the vicinity of an invaginating membrane (segmented in blue). Bar 20 nm. (b) Side view 3D tomographic representation with clathrin net in magenta and actin filaments in red (Hexameric rings and microtubules have been removed for clarity).
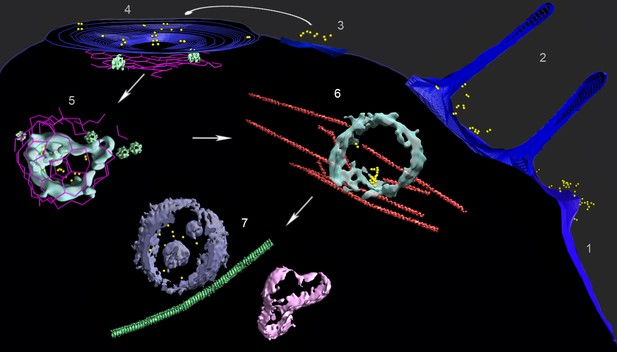
Multiple locations of FGF21-FGFR1c-βKlotho ternary complex in human adipocyte cells.
A composite image from several tomograms, with the cell surface membrane in blue, isosurface renderings of coated vesicle and endosomal membranes in cyan, isosurface renderings of a multivesicular body (MVB) and other vesicle membranes in violet and pink, clathrin in magenta, actin and microtubules in red and green (substituted with helical reconstructions from Figure 4), hexameric rings (substituted with subtomogram averages from Figure 4; putative p97 AAA+ ATPAse) in emerald, and AuNPs in yellow. Tomograms collected following treatment with AuNP-FGF21 for 1 h at 4°C show (1) a lamellopodium decorated with clusters of AuNP pairs, (2) filopodia surrounding clusters of AuNP pairs, (3) clusters of AuNP pairs on the cell surface, (4) AuNP pairs clustered in a coated pit, and (5) a clathrin-coated vesicle. Hexameric rings (putative p97 AAA+ ATPAse) are abundant in the vicinity of clathrin. A tomogram following treatment with AuNP-FGF21 for 1 h at 37°C shows an endosome associated with actin filaments (6) and a tomogram following treatment with AuNP-FGF21 overnight at 37°C shows a microtubule between an MVB and another vesicle (7). The arrows indicate a possible order of events, not an actual sequence; regions numbered 1–7 were taken from different tomograms.
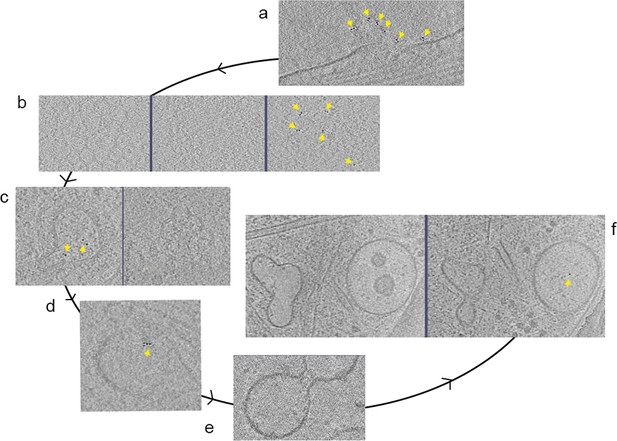
Activation and internalization cycle.
Composite image of slices from several tomograms. Tomograms collected following treatment with AuNP-FGF21 for 1 h at 4°C, show (a) a lamellopodium decorated with clusters of AuNP pairs, (b) AuNP pairs clustered (right panel) on top of an invaginated membrane (middle panel) above a clathrin pit (left panel), and (c) a clathrin-coated vesicle; left panel showing AuNP pairs inside the vesicle, and right panel showing the clathrin cage. (d) Tomogram after 1 h treatment at 37°C, shows an endosome, tightly associated with actin filaments, with AuNP pairs inside, and (e) a vesicle presumably dividing, although merging cannot be ruled out. (f) Tomogram following overnight treatment at 37°C, shows a microtubule between a MVB, with unpaired AuNP inside (right panel) and other vesicle, with no AuNPs inside (putative late endosome). (For a complete mapping of AuNPs see Figure 4). AuNPs indicated by yellow arrows.
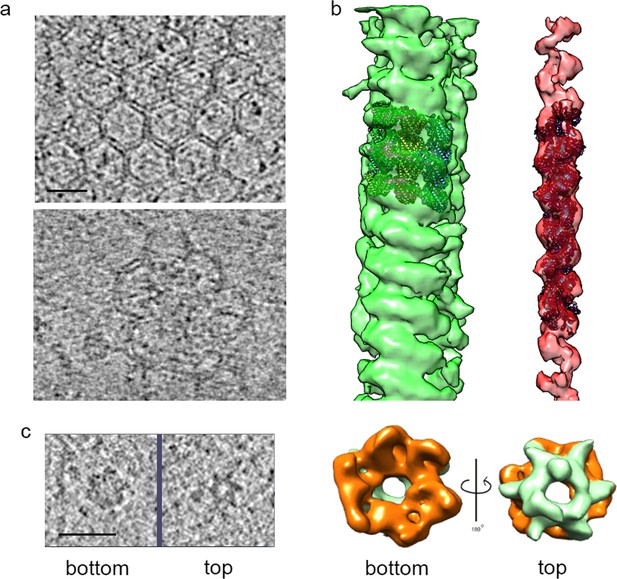
Structures identified in tomograms of human adipocyte cells.
(a) Tomogram slices showing a clathrin net (top panel) and cage (lower panel). (b) Helical reconstructions of densities attributed to microtubules (green) and actin filaments (red) with high-resolution structures (PDB:IDs 3JAK and 3B5U, respectively) manually docked in the densities. (c) Left panel, bottom and top tomogram slices of an individual hexameric ring particle; right panel, hexameric ring subtomogram average rotated by 180°, showing, bottom (orange), and top (emerald) sides. Bars 20 nm.
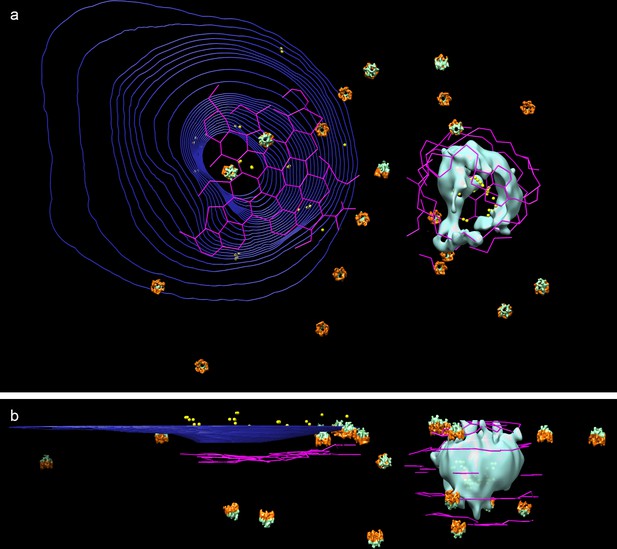
Orientation and proximity of hexameric rings to clathrin nets.
(a) Top view and (b) side view of 3D tomogramographic data from cell shown in Figure 2, with dual color hexameric ring as in Figure 4. (Microtubules and actin filament have been removed for clarity).
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.43146.015





