Neurotransmitter identity is acquired in a lineage-restricted manner in the Drosophila CNS
Figures
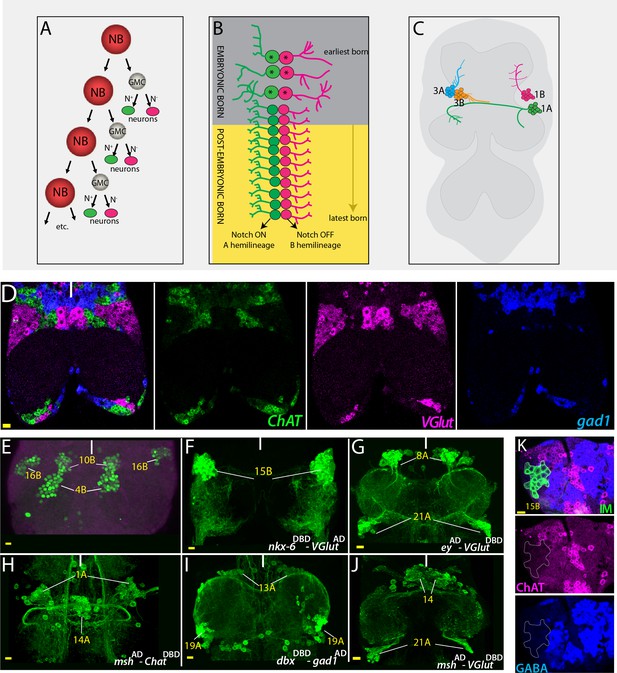
Strategies used to delineate neurotransmitter identity.
(A) A schematic representation of the NB division mode. With each division, the NB renews itself and generates a GMC, which divides via asymmetric cell division to generate two neurons. One of these neurons receives Notch (N) signaling and adopts ‘A’ fate (green), and the other neuron does not receive Notch signaling and adopts the ‘B’ fate (magenta). (B) Repeated NB divisions generate two hemilineages: The Notch ON ‘A’ hemilineage and the Notch OFF ‘B’ hemilineage. Neurons born in late embryonic and postembryonic stages adopt similar fates within a hemilineage whereas early embryonic divisions generate diverse neuronal types (asterisks). (C) A schematic representation of the adult VNC and hemilineages coming from NB1-2 (1A and 1B) and NB7-1 (3A and 3B). Neurons that belong to the same postembyonic hemilineage cluster together and send their cellular processes to the same region, while the neurons of the sibling hemilineage extend their axons to a different region (compare 1A to1B or 3A to 3B). (D) RNA in situ hybridization against neurotransmitter specific genes ChAT (green; cholinergic), gad1(blue; GABAergic), and VGlut (magenta; glutamatergic) reveals mutually exclusive mRNA expression patterns. Note that neurons with the same neurotransmitter cluster together. (E) Hb9 expression (green) marks neurons of three hemilineages (4B, 10B, and 16B) in the adult VNC. (F–J) Split-GAL4 intersectional strategy with genetic tools reporting the expression of lineage-marking transcription factors and neurotransmitter specific genes. Nkx6DBD-VGlutAD marks 15B neurons (F), eyAD-VGlutDBD marks 8A and 21A neurons (G); mshAD-ChATDBD marks 1A and 14A neurons (H); dbxDBD-gad1AD marks 13A and 19A neurons (I); and mshAD-VGlutDBD marks 14A and 21A neurons (J). (K) Antibody labeling against ChAT (magenta) and GABA (blue) does not mark glutamatergic 15B leg motor neurons (green), which were visualized via NB intersected reporter immortalization (IM) of the R10C12 GAL4 driver. Solid lines indicate midline. Scale bar is 10 microns.
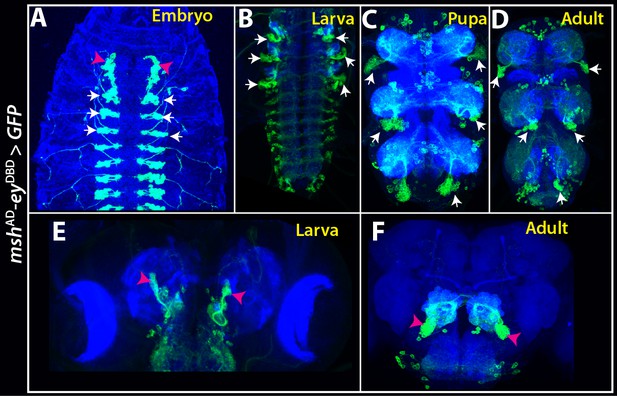
mshAD-eyDBD split GAL4 combination marks NB4-3 and its progeny (white arrows) in the VNC (A–D), and a local antennal lobe lineage, ALv2 (magenta arrowheads) in the brain (A, E, F) across all life stages.
https://doi.org/10.7554/eLife.43701.003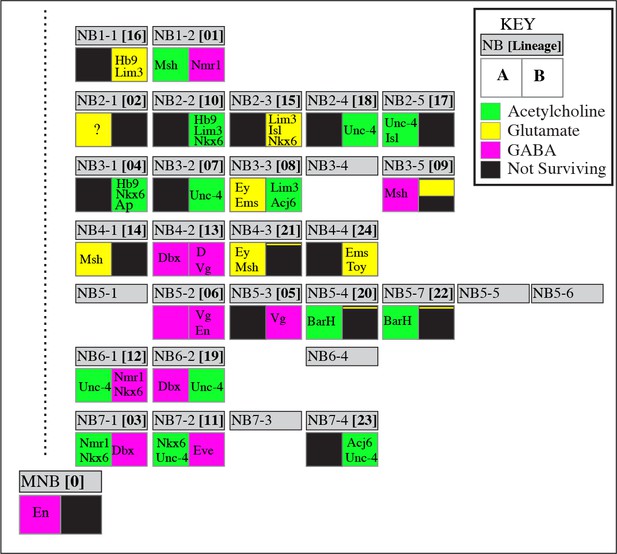
Neurotransmitter map of the adult Drosophila VNC.
NB identity and corresponding postembryonic lineages are shown in gray boxes. The left bottom box represents the ‘A’ hemilineage and the right bottom box represents the ‘B’ hemilineage. Out of 34 major hemilineages that survive to function in the adult nervous system, 14 are cholinergic (green), eight are glutamatergic (yellow) and 12 are GABAergic (magenta). Many hemilineages are eliminated completely by programmed cell death (black boxes) except in a few cases (9B, 21B, and 20/22B) where a small number of neurons survive. Hemilineage specific transcription factor expressions are stated in each box. These transcription factors are expressed in most if not all neurons of the indicated hemilineages. Note that Bar H expression (asterisks) is based on studies in larval stages; its adult expression was not tested due to lack of reagents. Dbx expression in 3B neurons (double asterisks) occurs transiently in larval stages, but does not last into the adult. NBs 5–1, 5–5, 5–6, 6–4, and 7–3 are eliminated by apoptosis during late embryonic development so they do not have postembryonic progeny. NB3-4 generates a few postembryonic motor neurons; their incorporation to the adult VNC is unknown. Note that the correspondence of postembryonic lineages to the NB identity is based on Lacin and Truman (2016), and differs from the map of Birkholz et al. (2015).
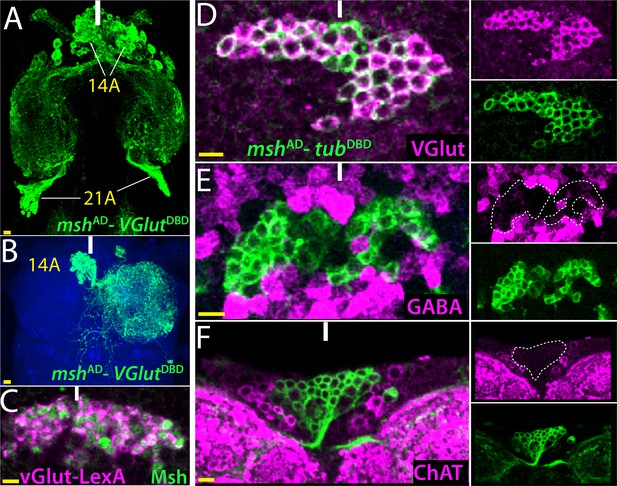
14B neurons are glutamatergic.
(A) The split GAL4 combination of mshAD-VGlutDBD marks 14A and 21A neurons. (B) A lineage flip-out clone generated with mshAD-VGlutDBD visualizes 14A neurons, which innervate the contralateral leg neuropil. (C) VGlut-LexA driver labels 14A neurons, which are revealed by the Msh expression. (D–F) mshAD-tubDBD is used to visualize bilaterally paired 14A clusters; single confocal stack is shown. VGlut antibody labels 14A neurons (D) but GABA and ChAT antibodies fail to mark them (E, F). Images in (A, B) are maximum projections while the rest are from a single confocal stack. Solid lines indicate midline. Scale bar is 10 microns.
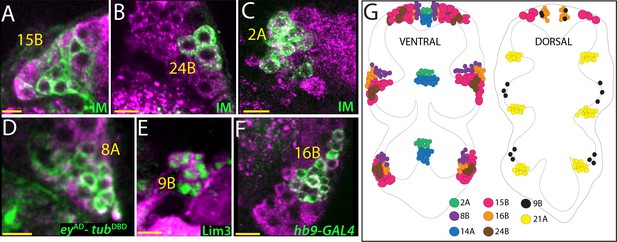
Glutamatergic lineages.
VGlut antibody (magenta in A, B, C, D, and F) and VGlut-LexA reporter (magenta in E) label neurons of 15B, 24B, 2A, 8A, 9B, and 16B hemilineages in the adult VNC. NB intersected reporter immortalization (IM) with the R10C12, VT205490, and R70D06AD-R28H10DBD driver lines used to visualize 15B, 24B and 2A neurons, respectively (A, B, and C). eyAD-tubDBD, Lim3, and hb9-GAL4 expressions used to mark 8A, 9B, and 16B neurons, respectively (D, E, and F). (G) The location of glutamatergic hemilineages shown schematically via color-coded map. Left and right images represent ventral and dorsal halves of the VNC, respectively. Only thoracic lineages are shown. All images are from a single confocal stack. See Figure 4—figure supplement 1 for individual channels. Scale bar is 10 microns.
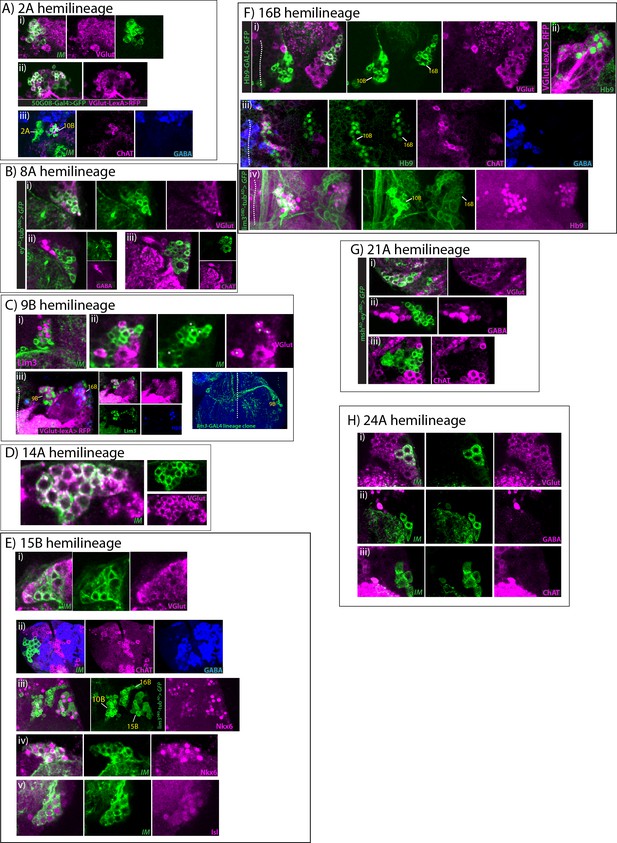
Glutamatergic hemilineages.
All images are from the adult VNCs. IM indicates NB intersected reporter immortalization. (A) 2A hemilineage (i, ii) 2A neurons (green) express VGlut (magenta; i) and VGlut-LexA reporter (magenta; ii). (iii) ChAT (magenta) and GABA (blue) do not label 2A neurons while ChAT labels neighboring 10B neurons. (B) 8A hemilineage eyAD-tubDBD marked 8A neurons (green) are labeled with anti-VGlut (magenta; i), but not with GABA (magenta; ii) or ChAT (magenta; iii) antibodies. (C) 9B hemilineage (i)Lim3 (magenta) marks 9B neurons in NB3-5 progeny (green), which was visualized by reporter immortalization. (ii) A small subset of NB3-5 progeny, 9B neurons, express VGlut (magenta) while the majority of NB3-5 progeny, 9A neurons, are GABAergic (see Figure 5); only four 9B neurons are visible on this focal plane (asterisk). (iii) Lim3+ Hb9- 9B neurons reside next to Lim3+ Hb9+ 16B neurons and neurons in both hemilineages are labeled with VGlut reporter (magenta). Lim3, green; Hb9, blue. (iv) A 9B lineage clone in the T3 segment shown. Lim3-GAL4 was used to generate the clone. 9B neurons extend contralateral axons ventrally. Dashed lines indicate midline. (D) 14A hemilineage VGlut antibody staining (magenta) marks all of the 14A neurons (green), which are visualized by NB4-1 intersected reporter immortalization. (E) 15B hemilineage 15B neurons (green) express VGlut (magenta; i), but do not express ChAT (magenta; ii) or GABA (blue; ii). (iii) lim3DBD-tubAD marks 15B motor neurons (green), which express Nkx6 (magenta). Note some cell express Nkx6 at high levels while some express it at low levels. (iv-v)15B neurons visualized with reporter immortalization (green). They express Nkx6 (magenta; iv) and Isl (magenta; v). (F) 16B hemilineage. (i) hb9-GAL4 marked while 10B neurons do not. (ii) VGlut-LexA (magenta) reporter also marks 16B neurons (green). (iii) Hb9+16B (green) neurons do not express ChAT (magenta) or GABA (blue), while Hb9+10B neurons express ChAT. (iv) lim3DBD-tubAD expression (green) marks Hb9+ 10B and 16B neurons (magenta). (G) 21A hemilineage 21A neurons, which were visualized by the driver mshAD-eyDBD (green), express VGlut (magenta; i), but not GABA (magenta; ii) or ChAT (magenta; iii). (H) 24A hemilineage NB4-4 intersected reporter immortalization used to visualize 24B hemilineage (green), which is composed of leg motor neurons. These neurons express VGlut (magenta; i), but not GABA (magenta; ii) or ChAT (magenta; iii).
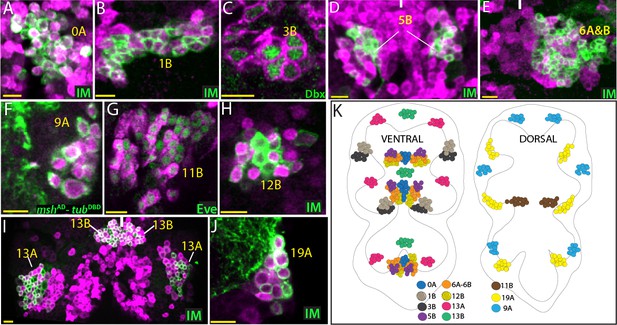
GABAergic lineages.
GABA antibody (magenta) labels neurons of 0A, 1B, 5B, 6A, 6B, 9A, 11B, 12B, 13A, 13B, and 19A hemilineages in the adult VNC (A, B, and D-J). gad1-LexA reporter (magenta) labels 3B neurons in the VNC of a wandering-stage larva (C). NB intersected reporter immortalization (IM) used to visualize 0A, 1B, 5B, 6A, 6B, 12B, 13A, 13B, and 19A hemilineages (A, B, D, E, H, I and J). The GAL4 lines used for these immortalization experiments are listed in Table 1. Dbx, mshAD-tubDBD, and Eve used to identify neurons of 3B, 9A, and 11B, respectively (C, F, and G). (K) The location of GABAergic hemilineages shown schematically via color-coded map. Left and right images represent ventral and dorsal halves of the VNC, respectively. Only thoracic lineages are shown. All images are from a single confocal stack except the image in (E), which is a maximum projection of 20 optical slices (0.5 micron each). See Figure 5—figure supplement 1 for individual channels. Solid lines indicate midline. Scale bar is 10 microns.
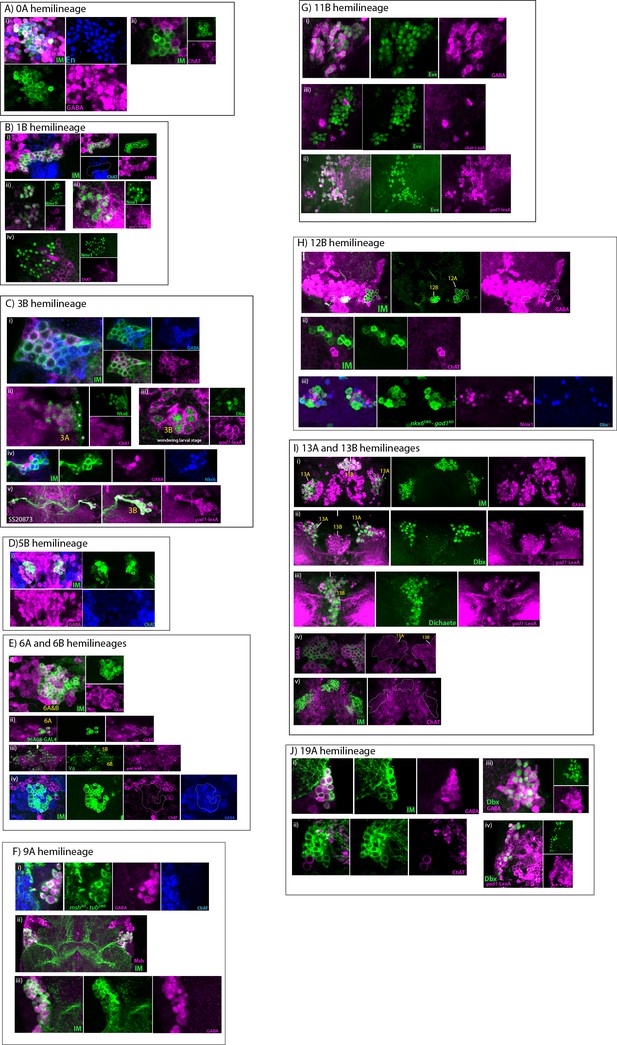
GABAergic hemilineages.
All images are from adult samples except (Ciii), which is from a larval sample. IM indicates NB intersected reporter immortalization. (A) 0A hemilineage (i) 0A neurons (green) express En (blue) and GABA (magenta). (ii) 0A neurons do not express ChAT (magenta). (B) 1B hemilineage (i) 1B neurons (green) are immunopositive for GABA staining (magenta) but negative for ChAT (blue). (ii-iv) GABA antibody (magenta; ii) and gad1-lexA reporter (magenta; iii) label Nmr1 expressing 1B neurons (green), but ChAT antibody (magenta; iv) does not label them. (C) 3B hemilineage (i) NB7-1 postmebyonic progeny composed of cholinergic (magenta) and GABAergic (blue) neurons. (ii) ChAT immunostaining (magenta) marks Nkx6 expressing 3A neurons (green). Note the region marked with asterisks indicate nonspecific surface staining. (iii) Dbx+ immature 3B neurons (green) express gad1-lexA reporter (magenta) in the larval VNC. (iv) Nkx6 expressing 3A neruons (blue) and GABA (magenta) are expressed mostly in a mutually exclusive manner in the NB7-1 progeny (green); note that a few Nkx6+ GABA+ neurons were also detected (not visible at this focal plane). (v) The SS20872 driver marks a subset of 3B neurons (green), which express gad1-lexA (magenta). (D) 5B hemilineage (i) 5B neurons (green) are immunopositive for GABA staining (magenta), but negative for ChAT staining (blue). (E) 6A and 6B hemilineages (i) Both 6A and 6B hemilineages (green) are composed of GABAergic neurons (magenta). (ii) 96A08-GAL4 marks a small subset of 6A neurons (green) expressing GABA (magenta). (iii) Vg (green) expressing 5B and 6B neurons are positive for gad1-lexA reporter expression (magenta). (iv) 6A and 6B neurons (green) do not express ChAT (magenta) but are labeled with GABA (blue). (F) 9A hemilineage (i) mshAD-tubDBD driven reporter expression (green) marks 9A neurons, which are positive for GABA staining (magenta) and negative for ChAT staining (blue). (ii) The majority of the NB3-5 progeny (green) are Msh+ (magenta) 9A neurons. Note that NB3-5 also generates a small number of Msh- 9B neurons which are not visible at this focal plane. (iii) The majority of the NB3-5 progeny (green) are GABAergic neurons (magenta). (G) 11B hemilineage (i-iii) 11B neurons, which are marked with Eve expression (green) express GABA (magenta; i) and gad1-lexA (magenta; ii) but not ChAT (magenta; iii). (H) 12B hemilineage (i–ii) 12B neurons (green) express GABA (magenta; i) but not ChAT (magenta; ii). (i) 12B neurons are located medially compared to 12A neurons, which are GABA negative. (iii) nkx6DBD-gad1AD driver marks a subset of 12B neurons. Nkx6 (green), Nmr1(magenta), and Dbx (blue) are expressed in a partially overlapping manner in 12B neurons. (I) 13A and 13B hemilineages (i) Neurons of both 13A and 13B hemilineages (green) are positive for GABA staining (magenta). (ii-iii) gad1-lexA (magenta) marks Dbx+ 13A neurons (green; ii) and Dichaete+ 13B neurons (green; iii). (iv) GABA levels (magenta) are lower in 13A neurons compared to 13B neurons. (v) 13A and 13B neurons (green) do not express ChAT (magenta). (J) 19A hemilineage (i–ii) 19A neurons (green) express GABA (magenta; i) but not ChAT (magenta; ii). (iii-iv) 19A neurons express Dbx (green), GABA (magenta; iii) and gad1-lexA (magenta; iv).
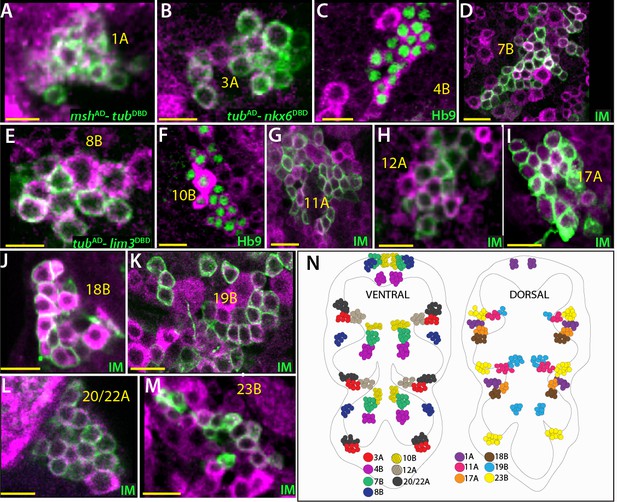
Cholinergic lineages.
ChAT antibody (magenta) labels neurons of 1A, 3A, 4B, 7B, 8B, 11A, 10B, 12A, 17A, 18B, 19B, 23B and 20/22A hemilineages in the adult VNC (A–M). NB intersected reporter immortalization (IM) used to visualize the neurons of 7B, 11A, 12A, 17A, 18B, 19B, 23B and 20/22A hemilineages (D, F, and I-M). The GAL4 lines used for these immortalization experiments are listed in Table 1. mshAD-tubDBD, tubAD-nkx6DBD, tubAD-lim3DBD used to identify neurons of 1A, 3A, and 8B, respectively (A, B, and E). Hb9 expression used to mark 4B and 10B neurons (C and G). (N) The location of cholinergic hemilineages shown schematically via color-coded map. Left and right images represent ventral and dorsal halves of the VNC, respectively. Only thoracic lineages are shown. All images are from a single confocal stack. See Figure 6—figure supplement 1 for individual channels. Scale bar is 10 microns.
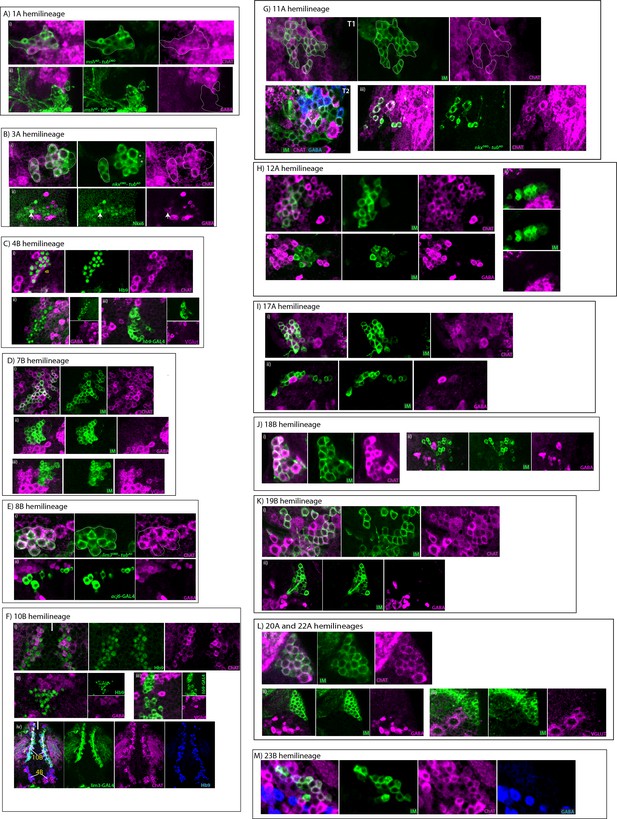
Cholinergic hemilineages.
All images are from adult samples except. IM indicates NB intersected reporter immortalization. (A) 1A hemilineage mshAD-eyDBD (green) used to mark 1A neurons, which express ChAT (magenta; i), but not GABA (magenta; ii). (B) 3A hemilineage (i) 3A neurons marked with nkx6DBD-tubAD (green) express ChAT (magenta). Note a few neurons (asterisks) were negative for ChAT staining. (ii) Nkx6+ 3A neurons (green) mostly lack GABA staining (magenta). A few GABA+Nkx6+ neuron (arrow; one visible at this focal plane) were detected in the region but it is not clear if these neurons are members of 3A or 3B hemilineages. (C) 4B hemilineage (i–ii) Hb9 expression (green) used to mark 4B neurons, which express ChAT (magenta; i) and do not stain with GABA antibody (magenta; ii). (iii) hb9-GAL4 marked 4B neurons (green) do not express VGlut (magenta; iii) (D) 7B hemilineage (i-iii) ChAT staining (magenta; i) marks 7B neurons (green), but GABA (magenta; ii) and VGlut (magenta; iii) immunostaining do not mark them. (E) 8B hemilineage (i,ii) lim3DBD-tubAD split GAL4 combination (i) or acj6-GAL4 (ii) used to mark 8B neurons (green), which express ChAT (magenta; ii) but not GABA (magenta; ii) (F) 10B hemilineage (i-iii) Hb9 expression (green) used to mark 10B neurons, which express ChAT (magenta; i) but not GABA (magenta; ii) or VGlut (magenta; iii). (iv) Lim3 expression (green) marks 10B neurons but not 4B neurons (green). Both 4B and 10B neurons express ChAT (magenta) and Hb9 (blue). Solid line indicates midline. (G) 11A hemilineage NB7-2 intersected reporter immortalization used to visualize its progeny 11A and 11B (green) in a T1 segment (i) and a T2 segment (ii). In the T1, 11B neurons are mostly eliminated by apoptosis, so the clone is composed of mostly 11A neurons, which express ChAT (magenta). In the T2, the cloned is composed of both 11A and 11B neurons, which express ChAT (magenta) and GABA (blue), respectively. See also Figure 5—figure supplement 1G. (iii) nkx6DBD-tubAD marks a subset of 11A neurons (green), which are immunopositive for ChAT staining (magenta). (H) 12A hemilineage (i-iii) 12A neurons (green), identified based on their lateral location in NB6-1 progeny, express ChAT (magenta; i), but they do not express GABA (magenta; ii) or VGlut (magenta; iii). (I) 17A hemilineage (i–ii) 17A neurons (green) express ChAT (magenta; i), but they are negative for GABA staining (magenta; ii). (J) 18B hemilineage (i–ii) 18B neurons (green) express ChAT (magenta; i), but they are negative for GABA staining (magenta; ii). (K) 19B hemilineage (i-iii) 19B neurons (green), identified based on their medial location in NB6-2 progeny, express ChAT (magenta; i), but they do not express GABA (magenta; ii). (L) 20/22A hemilineages (i–ii) 20/22A neurons (green) express ChAT (magenta; i), but they do not express GABA (magenta; ii) or VGlut (magenta; iii). Note that a few VGlut+ motor neurons survive in 20/22B hemilineage but they are not visible at this focal plane. (M) 23B hemilineage 23B neurons (green) are immunopositive for ChAT and negative for GABA (blue).
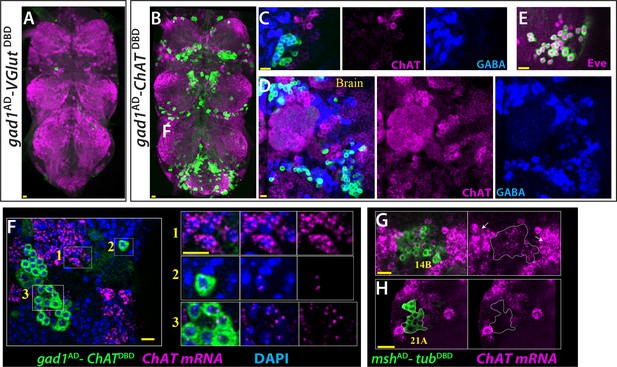
ChAT is transcribed in many Glutamatergic and GABAergic neurons.
(A) gad1AD-VGlutDBD does not mark any neuron reproducibly in the adult VNC. (B–D) gad1AD-ChATDBD mark several clusters of neurons in both the VNC (B, C) and brain (D). These neurons are positive for GABA immunoreactivity (blue) and negative for ChAT immunoreactivity (magenta). (E) The dorsal VNC neurons marked with gad1AD-ChATDBD are members of 11B hemilineage, which express Eve (Magenta). (F) gad1AD-ChATDBD marked neurons (inset 2, 3) contains low levels of ChAT mRNA (Magenta), which are mostly restricted to the nucleus while cholinergic neurons (inset 1) contains copious amount of ChAT mRNA, which are enriched in the cytoplasm. (G–H) mshAD-tubDBD used to mark glutamatergic14B and 21A neurons. (G) 14A neurons (outlined with white dashes) express low levels ChAT mRNA compared to cholinergic neurons with high ChAT expression (arrows). (H) Glutamatergic 21A neurons completely lack ChAT mRNA. Images in (A, B) are maximum confocal projections; the rest of the images are from single confocal plane. Scale bar is 10 microns.
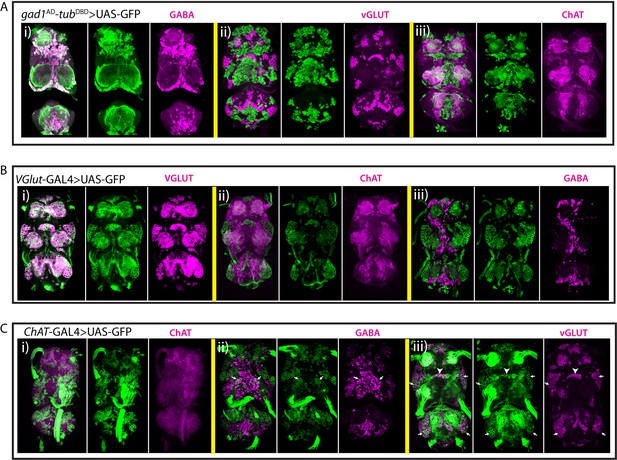
Characterization of neurotransmitter-specific genetic reporters.
(A) gad1AD-tubDBD driven GFP expression (green) marks GABAergic neurons (magenta; i). VGlut (magenta; ii) and ChAT (magenta; iii) are not detected in gad1AD-tubDBD marked neurons. (B) VGlut-GAL4 marked neurons (green) express VGlut (magenta; i), but they do not express ChAT (magenta; ii) or GABA (magenta; iii). (C) ChAT-GAL4 driven reporter expression (green) labels GABA+ (magenta; ii) and VGlut+ (magenta; iii) neurons in addition to ChAT+ neurons (magenta; i). Arrows in (Cii) indicate 5B neurons. Arrows and arrowheads in (Ciii) indicate 15B and 14A neurons respectively. All images are from a single confocal stack except the one in (Ai), which is projection of 20 slices, each 0.5 micron.
Tables
GAL4 lines used for NB intersected reporter immortalization.
https://doi.org/10.7554/eLife.43701.005| Line | Neuroblast | Lineages |
|---|---|---|
| 16A05AD;28H10DBD | NB1-2 | 1A-1B |
| 70D06AD;28H10DBD | NB2-1 | 2A |
| 10C12 | NB2-3 | 15B |
| 65G02 | NB2-4 | 18B |
| 19B03 | NB2-5, NB2-4 | 17A, 18B |
| 21E09AD;16H11DBD | NB3-2 | 7B |
| ems-GAL4 | NB2-2, NB3-3, NB3-5 | 10B, 8A-8B, 9A-9B |
| 59E09 | NB3-5 | 9A-9B |
| VT48571 | NB4-1 | 14A |
| 81C12A;42F01DBD | NB4-2 | 13A-13B |
| 16H11AD-19B03DBD | NB4-3 | 21A |
| VT205490 | NB4-4 | 24A |
| 45D04 | NB5-2 | 6A-6B |
| 54B10 | NB5-3 | 5B |
| 19B03AD;45D04DBD | NB5-4, NB5-7 | 20-22A |
| 81F01 or 70D06AD;42F01DBD | NB6-1 | 12A-12B |
| R76D11 | NB6-2 | 19A-19B |
| R51B04 | NB7-1 | 3A-3B |
| 35B12AD;28H10DBD | NB7-2 | 11A-11B |
| 19B03AD;18F07DBD | NB7-4 | 23B |
| 70D06 | MNB | 0B |
-
For detailed information about these lines, see Lacin and Truman, 2016.
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Antibody | Mouse anti-ChaT monoclonal | DSHB | ChAT4B1 | 1:50 diltuion |
| Antibody | Rabbit anti-GABA polyclonal | Sigma | A2052 | 1:1000 dilution |
| Antibody | Mouse anti-GABA monoclonal | Sigma | A0310 | 1:1000 dilution |
| Antibody | Rabbit antiVGlut polyclonal | Daniels et al., 2004 | 1:10000 dilution | |
| Antibody | Mouse anti-BRP monoclonal | DSHB | nc82 | 1:50 diltuion |
| Antibody | Chicken anti-GFP polyclonal | Life Tech. | A10262 | 1:1000 dilution |
| Antibody | Rabbit anti-DsRED polyclonal | TAKARA | 632496 | 1:500 dilution |
| Antibody | Rabbit anti-Vg polyclonal | Gift from Sean Carroll | 1:500 dilution | |
| Antibody | Guinea pig anti-Ems polyclonal | Gift from U. Walldorf | 1:300 dilution | |
| Antibody | Rabbit anti-Msh polyclonal | Gift from Chris Doe | 1:500 dilution | |
| Antibody | Rabbit anti-Unc-4 polyclonal | Gift from James Skeath | 1:1000 dilution | |
| Antibody | Guinea pig anti-Dbx polyclonal | Gift from James Skeath | 1:1000 dilution | |
| Antibody | Rabbit anti-HB9 polyclonal | Gift from James Skeath | 1:1000 dilution | |
| Antibody | Guinea pig anti-HB9 polyclonal | Gift from James Skeath | 1:1000 dilution | |
| Antibody | Rat anti-Nkx6 polyclonal | Gift from James Skeath | 1:1000 dilution | |
| Antibody | Guinea pig anti-Lim3 polyclonal | Gift from James Skeath | 1:1000 dilution | |
| Antibody | Rat anti-Islet polyclonal | Gift from James Skeath | 1:1000 dilution | |
| Antibody | Rabbit anti-Dichaete polyclonal | Gift from James Skeath | 1:1000 dilution | |
| Antibody | Rabbit anti-Nmr1 polyclonal | Gift from James Skeath | 1:1000 dilution | |
| Antibody | Mouse anti-En monoclonal | DSHB | 4D9 | 1:5 dilution |
| Antibody | Mouse anti-Acj6 monoclonal | DSHB | Acj6 | 1:100 dilution |
| Antibody | Mouse anti-Eve monoclonal | DSHB | 3C10 | 1:25 dilution |
| Antibody | goat anti-rabbit Alexa Fluor 488 | Life Technologies | A-11034 | 1:500 dilution |
| Antibody | goat anti-rabbit Alexa Fluor 568 | Life Technologies | A-11011 | 1:500 dilution |
| Antibody | goat anti-rabbit Alexa Fluor 633 | Life Technologies | A-21070 | 1:500 dilution |
| Antibody | goat anti-rabbit Alexa Fluor 405 | Life Technologies | A-31556 | 1:500 dilution |
| Antibody | goat anti-chicken Alexa Fluor 488 | Life Technologies | A-11039 | 1:500 dilution |
| Antibody | goat anti-mouse Alexa Fluor 488 | Life Technologies | A-11001 | 1:500 dilution |
| Antibody | goat anti-mouse Alexa Fluor 568 | Life Technologies | A-11004 | 1:500 dilution |
| Antibody | goat anti-mouse Alexa Fluor 633 | Life Technologies | A-21050 | 1:500 dilution |
| Antibody | goat anti-mouse Alexa Fluor 405 | Life Technologies | A-31553 | 1:500 dilution |
| Antibody | goat anti-guinea pig Alexa Fluor 488 | Life Technologies | A-11073 | 1:500 dilution |
| Antibody | goat anti-guinea pig Alexa Fluor 568 | Life Technologies | A-11075 | 1:500 dilution |
| Antibody | goat anti-guinea pig Alexa Fluor 633 | Life Technologies | A-21105 | 1:500 dilution |
| Antibody | goat anti-rat Alexa Fluor 488 | Life Technologies | A-11006 | 1:500 dilution |
| Antibody | goat anti-rat Alexa Fluor568 | Life Technologies | A-11077 | 1:500 dilution |
| Genetic reagent (D. melanogaster) | VGlut-GAL4 | Bloomington Drosophila Stock Center | RRID:BDSC_60312 | |
| Genetic reagent (D. melanogaster) | VGlutDBD | Bloomington Drosophila Stock Center | RRID:BDSC_60313 | |
| Genetic reagent (D. melanogaster) | VGlut-LexA | Bloomington Drosophila Stock Center | RRID:BDSC_60314 | |
| Genetic reagent (D. melanogaster) | ChAT-GAL4 | Bloomington Drosophila Stock Center | RRID:BDSC_60317 | |
| Genetic reagent (D. melanogaster) | ChAT-DBD | Bloomington Drosophila Stock Center | RRID:BDSC_60318 | |
| Genetic reagent (D. melanogaster) | ChAT-LexA | Bloomington Drosophila Stock Center | RRID:BDSC_60319 | |
| Genetic reagent (D. melanogaster) | gad1-AD | Bloomington Drosophila Stock Center | RRID:BDSC_60322 | |
| Genetic reagent (D. melanogaster) | gad1-LexA | Bloomington Drosophila Stock Center | RRID:BDSC_60324 | |
| Genetic reagent (D. melanogaster) | Tub-AD | Bloomington Drosophila Stock Center | RRID:BDSC_60295 | |
| Genetic reagent (D. melanogaster) | Tub-DBD | Bloomington Drosophila Stock Center | RRID:BDSC_60298 | |
| Genetic reagent (D. melanogaster) | SS20873 | This study | ||
| Chemical compound, drug | Paraformaldehyde | EMS | 15713 | |
| Chemical compound, drug | Vectashield | Vector | H-1000 | |
| Chemical compound, drug | Prolong Diamond | Molecular Probes | P36961 | |
| Recombinant DNA reagent | pBS-KS-attB2-SA(2)-T2A-Gal4DBD-Hsp70 | addgene | RRID:Addgene_62904 | |
| Recombinant DNA reagent | pBS-KS-attB2-SA(1)-T2A- Gal4DBD-Hsp70 | addgene | RRID:Addgene_62903 | |
| Recombinant DNA reagent | pBS-KS-attB2-SA(0)-T2A-Gal4DBD-Hsp70 | addgene | RRID:Addgene_62902 | |
| Recombinant DNA reagent | pBS-KS-attB2-SA(2)-T2A-p65AD-Hsp70 | addgene | RRID:Addgene_62915 | |
| Recombinant DNA reagent | pBS-KS-attB2- SA(1)-T2A- p65AD-Hsp70 | addgene | RRID:Addgene_62914 | |
| Recombinant DNA reagent | pBS-KS-attB2-SA(0)-T2A-p65AD-Hsp70 | addgene | RRID:Addgene_62912 |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.43701.015





