Regulation of cilia abundance in multiciliated cells
Figures
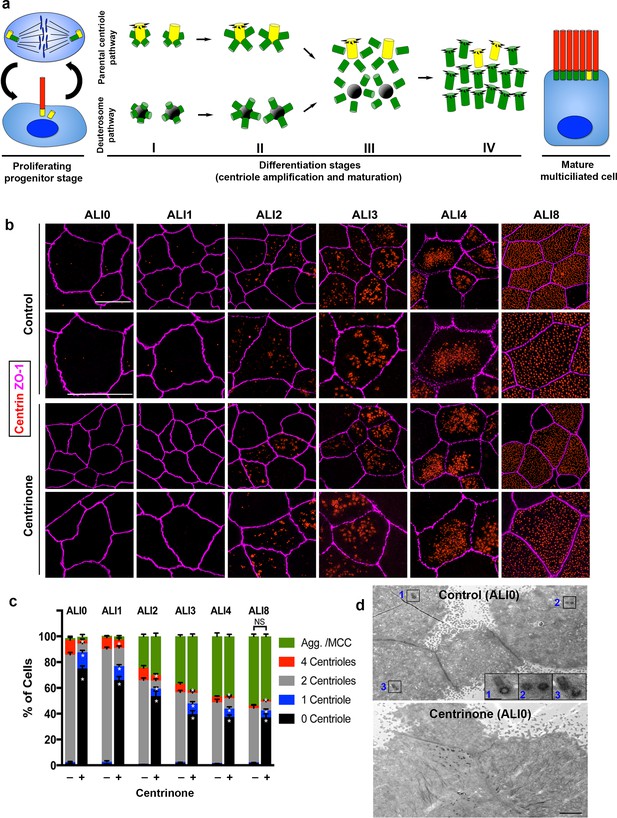
Parental centrioles are dispensable for centriole amplification.
(a) Schematic of the proliferation and differentiation steps of multiciliated respiratory epithelia. The key stages of centriole amplification from parental centrioles and deuterosomes are depicted. (b) Immunofluorescence (3D-SIM) images of control and Centrinone-treated cells. Samples were fixed on the indicated days of air-liquid interface (ALI) culture and stained for centrioles (centrin) and apical cell-cell junctions (ZO-1). Upper panels show lower magnification images, and lower panels highlight a single cell. Scale bar = 10 μm. (c) Quantification of centriole number, and the fraction of the population undergoing centriole amplification, in control and Centrinone-treated MTEC. Ablation of parental centrioles did not affect the initiation of centriolar aggregate (Agg.) formation, the timing of centriole amplification, or the fraction of mature multiciliated cells (MCC) in the population. Results are averages of three independent experiments. More than 3,000 cells were counted per sample for each time point. *p<0.05. (d) Transmission electron microscopy (TEM) of the apical surface of MTEC at ALI0. Parental centrioles were easily found at the apical surface of control cells (20/20 cells examined; insets highlight three sets of PC), but were missing in the majority of Centrinone-treated samples (0 parental centrioles in 17/19 cells). Scale bars = 2 µm (large panels) and 400 nm (insets).
Centrinone-mediated inhibition of Plk4 results in loss of parental centrioles at ALI0.
Samples were serially sectioned from at the cell’s apical surface until the basal membrane above the filter. The thickness of each section was 120 nm. Serial sections were imaged using a transmission electron microscope, and images aligned using Adobe Photoshop and Fiji software. Sections within first 2 µm are shown to illustrate the absence of parental centrioles, which are evident in control cells at or near the apical surface. Scale bar = 4 µm.
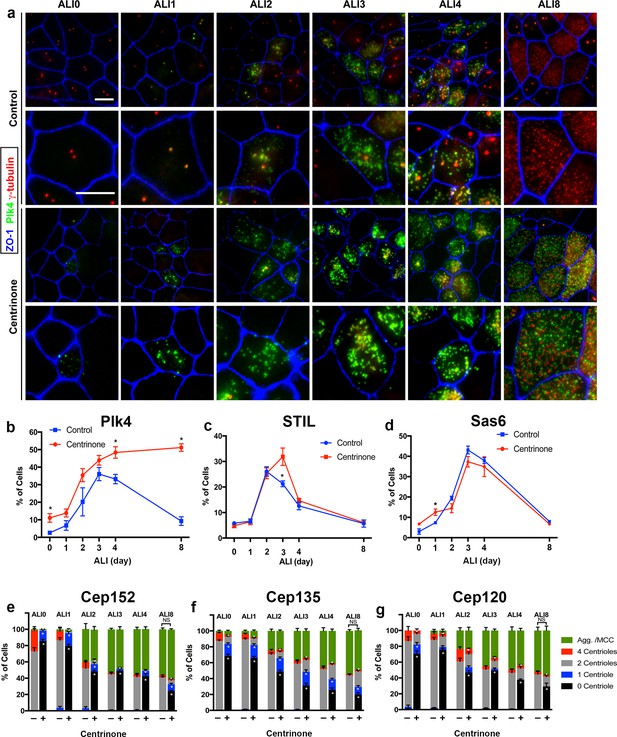
Loss of parental centrioles does not affect dynamics of centriole amplification.
(a) Immunofluorescence images of control and Centrinone-treated cells. Samples were fixed on the indicated days and stained for Plk4, centrioles (γ-tubulin) and apical cell-cell junctions (ZO-1). Upper panels show lower magnification images, and lower panels highlight a single cell. Inhibition of Plk4 kinase activity results in accumulation of Plk4 protein at ALI 0–1, and the protein is still evident at stages (e.g. ALI 8) when it is normally lost. Scale bar = 10 μm. (b) Quantification of the fraction of Plk4-expressing cells during differentiation. Results are averages of two independent experiments. More than 1,500 cells were counted per sample for each time point. *p<0.05. (c) Quantification of the fraction of STIL-expressing cells during differentiation. STIL expression is mostly unchanged upon inhibition of Plk4, with a slight increase in STIL-expressing cells evident at ALI three in Centrinone-treated samples. Results are averages of two independent experiments. More than 1000 cells were counted per sample for each time point. *p<0.05. (d) Quantification of the fraction of Sas6-expressing cells during differentiation. A slight increase in Sas6-expressing cells is evident at ALI 0–1 in Centrinone-treated samples, however the overall pattern of expression is not affected. Results are averages of two independent experiments. More than 1000 cells were counted per sample for each time point. *p<0.05. (e–f) Quantification of centriole number and the fraction of the population undergoing centriole amplification. Cells at the indicated time points were stained for markers of procentriole initiation and growth. Ablation of parental centrioles did not affect the formation of centriolar aggregates (Agg.), the timing of centriole amplification and growth, or the fraction of mature multiciliated cells (MCC) in the population. Results are averages of two independent experiments. More than 2000 cells were counted per sample for each time point. *p<0.05.
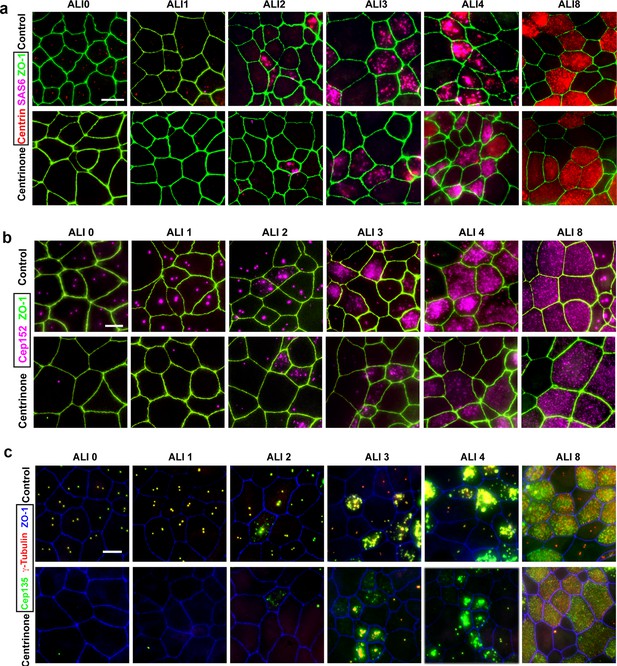
– Analysis of procentriole assembly in control and Centrinone-treated MTEC.
Immunofluorescence images of control and Centrinone-treated cells. Samples were fixed on the indicated days and stained for markers of parental centrioles and early procentriole nucleation as follows: (a) centrin and Sas6, (b) Cep152, and (c) Cep135 and γ-Tubulin. Apical cell-cell junctions were marked using ZO-1. Scale bars = 10 µm.
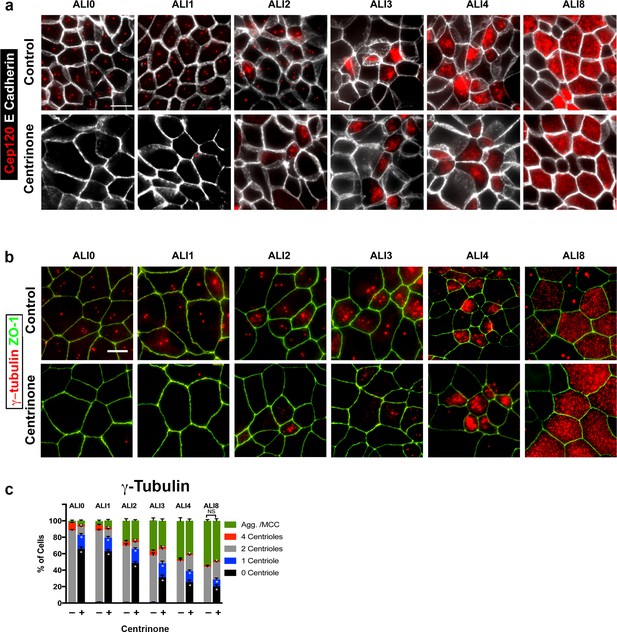
– Analysis of procentriole growth in control and Centrinone-treated MTEC.
Immunofluorescence images of control and Centrinone-treated cells. Samples were fixed on the indicated days and stained for markers of parental centrioles and procentriole growth as follows: (a) Cep120 and (b) γ-Tubulin. Apical cell-cell junctions were marked using ZO-1 or E-cadherin. Scale bars = 10 µm. (c) Quantification of centriole number and the fraction of the population undergoing centriole amplification. Results are averages of two independent experiments. More than 2000 cells were counted per sample for each time point. *p<0.05.
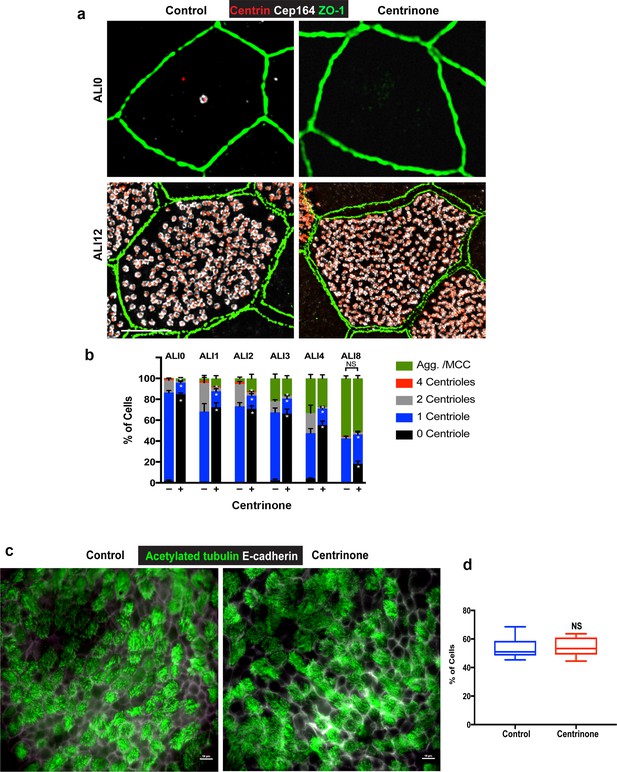
– Analysis of centriole maturation and ciliogenesis in control and Centrinone-treated MTEC.
(a) 3D-SIM images of MTEC at ALI0 and 12 stained for a marker of mature centrioles (distal appendage marker Cep164), centrioles (centrin), and apical cell junctions (ZO-1). Control cells at ALI0 contain a single mature centriole as expected, and these are missing in Centrinone-treated samples lacking the PC. Examination of cells at ALI12 showed no deleterious effects on centriole maturation. Scale bars = 10 µm. (b) Quantification of mature centrioles, and the fraction of the population undergoing centriole maturation, in control and Centrinone-treated MTEC. Ablation of parental centrioles did not affect the timing of centriole maturation. Results are averages of two independent experiments. More than 2000 cells were counted per sample for each time point. *p<0.05. (c–d) Immunofluorescence images of control and Centrinone-treated MTEC at ALI12 stained for cilia (acetylated tubulin) and cell-cell junctions (E-cadherin). Loss of parental centrioles does not disrupt ciliogenesis, further indicating that centriole maturation is unperturbed upon PC loss. Results are averages of three independent experiments. N = 3000 (control) and 3000 (Centrinone).
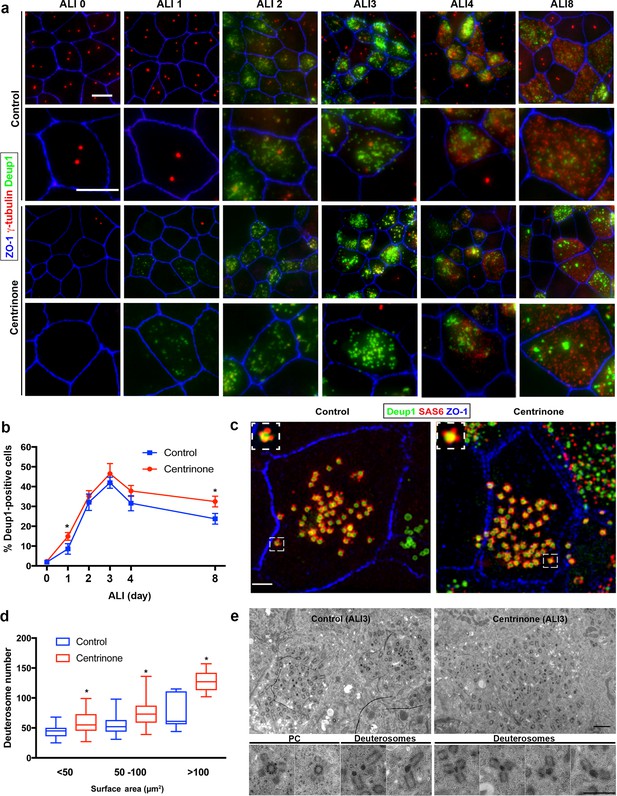
Deuterosome biogenesis is unaffected by loss of parental centrioles.
(a) Immunofluorescence images of control and Centrinone-treated cells stained with antibodies against deuterosomes (Deup1), centrioles (γ-tubulin) and apical cell-cell junctions (ZO-1). Upper panels show lower magnification images, and lower panels highlight a single cell. Loss of PC does not disrupt deuterosome formation. In contrast, they appear earlier than in control cells (e.g. ALI 1) and persist for a longer period of time. Scale bar = 10 μm. (b) Quantification of the fraction of Deup1-expressing cells during differentiation. Results are averages of two independent experiments. More than 1000 cells were counted per sample for each time point. *p<0.05. (c) 3D-SIM images of MTEC at ALI stained for deuterosomes (Deup1), procentrioles (Sas6) and apical cell-cell junctions (ZO-1). Scale bar = 10 μm. (d) Quantification of deuterosome number in control and Centrinone-treated cells. Cells were grouped based on the size of their surface area, and the average number of deuterosomes per cell determined. Loss of PC causes an increase in deuterosome number in each category. N = 87 (control) and 71 (Centrinone). *p<0.05. (e) TEM images of control and Centrinone-treated MTEC at ALI3, confirming the presence of procentriole-forming deuterosomes that appear normal in size and morphology. Scale bars = 2 µm (upper panels) and 800 nm (magnified lower panels).
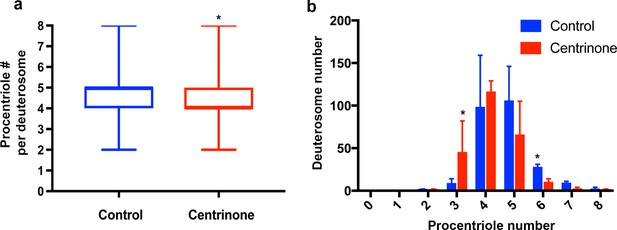
– Deuterosome-mediated procentriole formation is unaffected by loss of parental centrioles.
Quantification of procentriole number per deuterosome in control and Centrinone-treated cells at ALI3. We noted a slight but significant decrease in the average (a) and distribution (b) of procentrioles per deuterosome. Results are averages of three independent experiments. N = 509 (control) and 486 (Centrinone). *p<0.05.
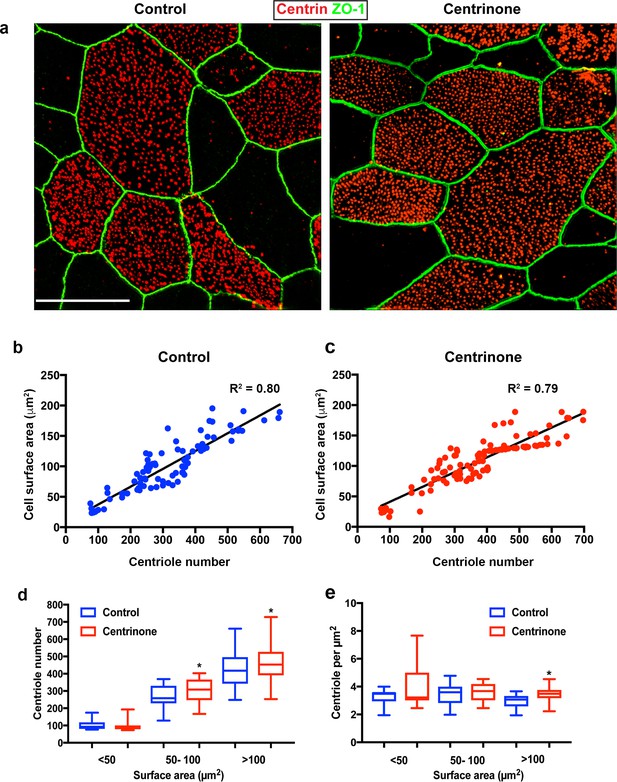
Centriole abundance correlates with surface area and is unaffected by loss of PC.
(a) Example 3D-SIM images of mature multiciliated cells at ALI12 stained with markers of centrioles (centrin) and apical cell junctions (ZO-1). Scale bar = 10 μm. (b–c) Quantification of centriole number in control cells shows a linear relationship with cell surface area, such that larger cells contain more centrioles. Loss of the PC following Centrinone-treatment does not affect this relationship. (d–e) Comparison of abundance and density in mature MCC of varying sizes at ALI12. Loss of the PC did not result in a decrease in average centriole number (d). In fact, there was a slight increase in some cells. Similarly, the density and distribution of these centrioles at the apical surface was generally unchanged (e), although we noted a small but significant increase in the density of centrioles, possibly due to the elevated number per cell. Results are averages of three independent experiments. N = 91 (control) and 114 (Centrinone). *p<0.05.
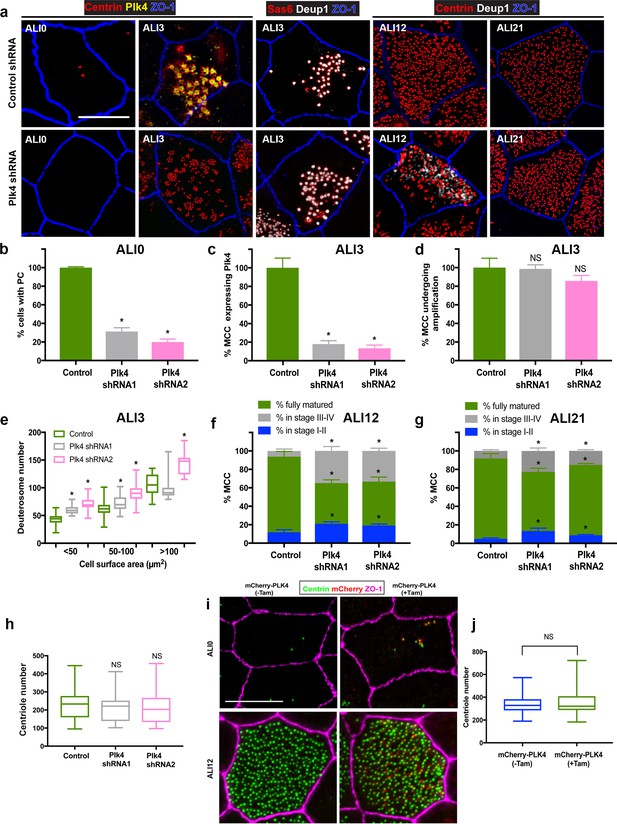
Depletion of Plk4 causes a delay in centriologenesis but does not affect abundance.
(a) Images of MTEC infected with control or Plk4-targeting shRNA. Cells were fixed on the indicated days and stained for centrioles (centrin), endogenous Plk4, procentrioles (Sas6 or centrin), deuterosomes (Deup1) and apical cell junctions (ZO-1). Scale bar = 10 µm. (b) Depletion of Plk4 in basal cells causes loss of parental centrioles by ALI0, quantified using centrin staining of PC (a). Results are averages of three independent experiments. N = 862 (control shRNA), 846 (Plk4 shRNA1) and 1108 (Plk4 shRNA2). *p<0.05. (c–d) Fraction of MCC at ALI3 undergoing centriole amplification in the absence of Plk4. The percentage of cells that initiated centriole amplification, determined using Deup1 and centrin staining, was unchanged (d). However, there was a large decrease in the fraction of MCC with detectable Plk4 (c). Results are averages of two independent experiments. N = 4158 (control shRNA), 1596 (Plk4 shRNA1) and 3456 (Plk4 shRNA2). *p<0.05. (e) Quantification of deuterosome number in control and Plk4-depleted cells. Similar to Centrinone-mediated PC loss, the number of deuterosomes per cell increases in the absence of PC. Results are averages of two independent experiments. N = 43 (control shRNA), 59 (Plk4 shRNA1) and 67 (Plk4 shRNA2) cells. *p<0.05. (f–g) Comparison of centriole amplification stages upon Plk4 depletion at ALI12 (f) and ALI21 (g). Although the proportion of the MCC in the population was unchanged in cells lacking Plk4, a significant fraction of the cells were in Stage I-II (with Deup1-positive deuterosomes still evident) and Stage III-IV (absence of deuterosomes) of centriole amplification (f). Culturing the cells for an additional 9 days (ALI21) resulted in an increase in the percentage of mature MCC with cilia in Plk4-depleted cells (g), highlighting a delay in the maturation process. Results are averages of two independent experiments. N = 815 (control shRNA), 783 (Plk4 shRNA1) and 1346 (Plk4 shRNA2). *p<0.05. (h) Comparison of centriole abundance in control and Plk4-depleted mature MCC at ALI21. Loss of Plk4 and PC did not result in a decrease in average centriole number. N = 43 (control shRNA), 57 (Plk4 shRNA1) and 67 (Plk4 shRNA2). *p<0.05. (i) 3D-SIM images of MTEC generated from Tg::mChPlk4/Rosa26-CreERT2 (described in detail in Materials and methods), stained for exogenous Plk4 (mCherry), centrioles (centrin) and apical cell junctions (ZO-1). Addition of tamoxifen during proliferation resulted in constitutive overexpression of mChPlk4,and caused formation of supernumerary PC by ALI0. Importantly, the protein was continuously expressed and still evident in fully mature MCC at ALI12. Scale bar = 10 µm. (j) Supernumerary parental centrioles and constitutive overexpression of Plk4 do not result in increased centriole abundance in mature MCC. Results are averages of two independent experiments. N = 108(-TAM), and 105 (+TAM).
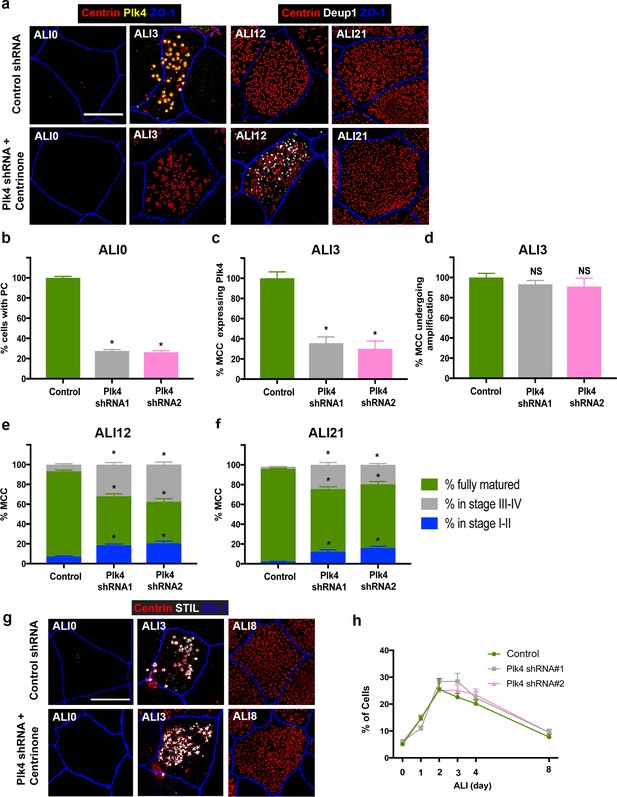
– Combined depletion and Centrinone-mediated inhibition of Plk4 causes a delay in centriologenesis.
(a) 3D-SIM images of MTEC infected with Plk4-targeting shRNA and treated with 1 μM Centrinone. Cells were fixed on the indicated days and stained for centrioles (centrin), endogenous Plk4 and apical cell junctions (ZO-1). Scale bar = 10 µm. (b) Depletion and Centrinone-mediated inhibition of Plk4 in basal cells causes loss of parental centrioles by ALI0, quantified using centrin staining of PC. Results are averages of three independent experiments. N = 3785 (control shRNA), 3017 (Plk4 shRNA1) and 2738 (Plk4 shRNA2). *p<0.05. (c–d) Fraction of MCC at ALI3 undergoing centriole amplification. The percentage of cells that initiated centriole amplification was unchanged (d). However, there was a large decrease in the fraction of MCC with detectable Plk4 (c). Results are averages of three independent experiments. N = 2420 (control shRNA), 2267 (Plk4 shRNA1) and 2158 (Plk4 shRNA2). *p<0.05. (e–f) Comparison of centriole amplification stages upon simultaneous Plk4 depletion and Centrinone treatment. Although the proportion of the MCC in the population was unchanged in cells lacking Plk4, a significant fraction of the cells were in Stage I-II and Stage III-IV of centriole amplification (e). Culturing the cells for an additional 9 days (ALI21) resulted in an increase in the percentage of mature MCC (f), highlighting a delay in the maturation process. N = 2412 (control shRNA), 2202 (Plk4 shRNA1) and 2124 (Plk4 shRNA2). *p<0.05. (g) 3D-SIM images of MTEC infected with Plk4-targeting shRNA and treated with 1 μM Centrinone. Samples were fixed on the indicated days and stained for STIL, centrioles (centrin) and apical cell-cell junctions (ZO-1). Scale bar = 10 μm. (h) Quantification of the fraction of STIL-expressing cells shows no decrease upon loss of Plk4, with a slight increase in STIL-expressing cells evident at ALI three in Plk4-depleted, Centrinone-treated samples. Results are averages of three independent experiments. N = 800 cells per sample for each time point. *p<0.05.
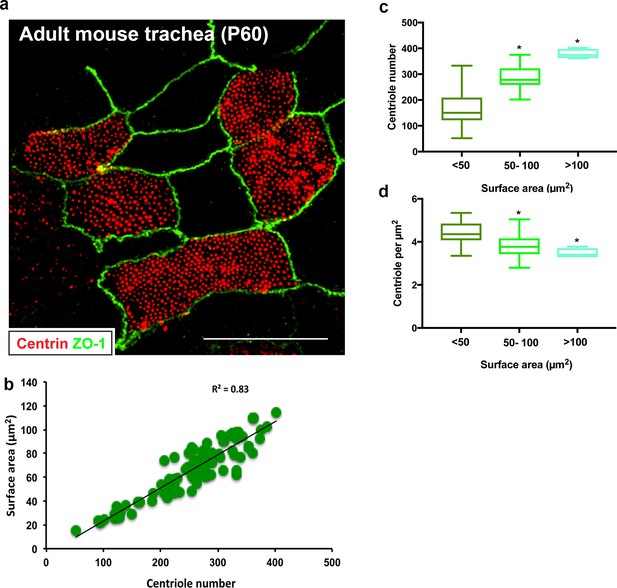
Centriole abundance correlates with surface area in vivo.
(a) Example 3D-SIM image of adult mouse trachea (P60) immunostained for centrioles (centrin) and apical cell junctions (ZO-1). Scale bar = 10 µm. (b–d) Centriole number ranges between 100 and 400 per cell on average and displays a liner relationship with surface area, consistent with the observations in MTEC. Similarly, centriole density decreases slightly as surface area increases. N = 95 cells.
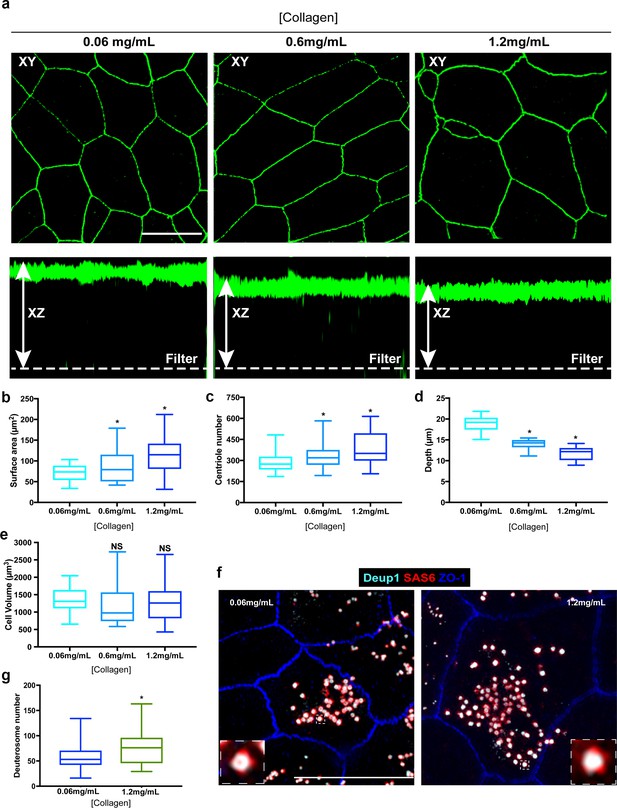
Enlargement of the surface area of progenitor cells results in increased centriole abundance.
(a) Example 3D-SIM images of MTEC grown on varying concentrations of extracellular collagen matrix. Top panels show X-Y orientation, and lower panels the X-Z dimension. Scale bar = 10 µm. (b–e) Analysis of surface area (b), centriole number (c), apical-basal distance (depth, (d) and cell volume (e) in MTEC cultured on differing concentrations of collagen. Increasing the collagen density resulted in a dose-dependent expansion of the apical surface, whereas the cells became shallower, resulting in a similar overall cell volume. Importantly, the increase in surface are at ALI0 resulted in increased centriole number (c). Results are averages of two independent experiments. N = 39 (0.06mg/mL), 48 (0.6 mg/mL) and 42 (1.2mg/mL). *p<0.05. (f) 3D-SIM images of MTEC grown on normal (0.06 mg/mL) and high (1.2 mg/mL) levels of extracellular collagen. Cells at ALI3 were immunostained with antibodies to mark deuterosomes (Deup1), procentrioles (Sas6) and apical cell junctions (ZO-1). Scale bar = 10 µm. (g) Quantification of deuterosome number in MTEC at ALI3 showed a significant increase in cells grown on increased collagen density (with larger surface areas). Results are averages of two independent experiments. N = 45 (0.06mg/mL) and 43 (1.2mg/mL). *p<0.05.
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.44039.015





