Calcium-driven regulation of voltage-sensing domains in BK channels
Figures
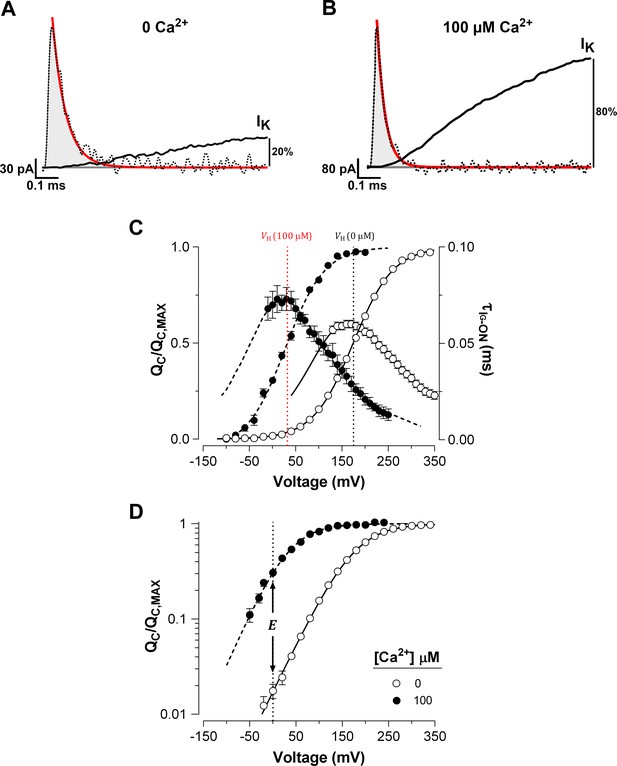
Ca2+ binding strongly affects the activation of VSD in BK channels.
(A–B) Fast component of the ON-gating current (IG-ON) at 0 and 100 μM internal Ca2+ concentration, respectively. The representative IG-ON records were evoked by applying a 160 mV voltage step of 1 ms duration. The first 100 µs of the IG-ON were fitted to a single exponential function (red line; = 58 µs and = 30 µs). The area under the curve described by the monoexponential fit (gray areas) was integrated to obtain the charge displaced between closed states (). For comparison, the initial time course of the macroscopic K+ current (IK) activation (solid black line) obtained at 0 and 100 μM Ca2+ were superimposed on the IG-ON under the same internal Ca2+ conditions. The IK were evoked by a 15 ms pulse to 160 mV. The IK is described by an exponential function with initial delay: = 0.16 ms and = 3.37 ms at 0 Ca2+; and = 0.08 ms and = 0.66 ms at 100 μM Ca2+. After 1 ms, the IK increased to about 20% and 80% of its steady-state amplitude in 0 Ca2+ and 100 μM Ca2+, respectively. (C) Voltage-dependence of the and of the gating current time constants () at 0 Ca2+ (open circles) and 100 μM Ca2+ (filled circles). Gating charge-voltage relationships () were obtained by integrating the fast component for each ON IG trace (from −90 mV to 350 mV). Boltzmann fitting to the experimental data (mean ± SEM) is indicated by solid line at ‘zero’ Ca2+ ( = 174.5 ± 2.4 mV, = 0.60 ± 0.01, = 25) and by a dashed line at 100 µM Ca2+ ( = 31.9 ± 4.5 mV, = 0.66 ± 0.01, = 7). Right ordinate shows the time constants data (mean ± SEM) of the exponential decays of IG-ON () plotted against the voltage. The best fit to a two-state model of the VSD activation where the was constrained to the values found for the relation () is indicated by solid lines at ‘zero’ Ca2+ ( = 3.73 ms−1, = 76.10 ms−1 and = 0.29) and dashed line at 100 µM Ca2+ ( = 7.28 ms−1, = 6.98 ms−1 and = 0.36). The corresponding at each internal Ca2+ concentration is indicated by a vertical line. (D) Semi-logarithmic plot of the curves at 0 Ca2+ and saturating Ca2+ concentration (100 µM). The allosteric parameter determines the vertical displacement of the 0 mV intercept (dashed vertical line) of the curve at 100 µM Ca2+ relative to the 0 Ca2+ condition.
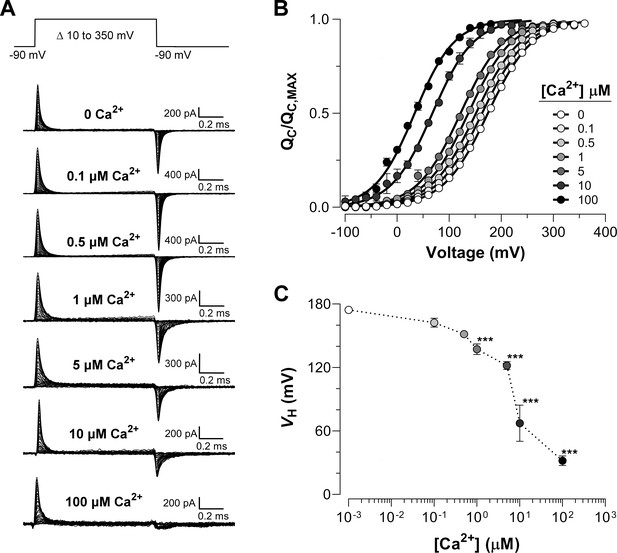
Ca2+-dependent effects on VSD activation in BK channels.
(A) Representative gating current (IG) recordings at different internal Ca2+ concentrations (from 0 to 100 μM). IG was evoked by the indicated voltage protocol of 1 ms duration. Representative gating current records are from different patches with the exception of 0 and 100 μM Ca2+. (B) Gating charge-voltage relationships () were obtained by integrating the fast component for each ON IG trace. Normalized gating charge data (mean ± SEM) were fitted using a single Boltzmann function (solid lines). The fit parameters are as follows: ‘zero’ Ca2+ ( = 174.5 ± 2.4 mV, = 0.60 ± 0.01, = 25); 0.1 µM Ca2+ ( = 162.4 ± 4.2 mV, = 0.59 ± 0.01, = 5); 0.5 µM Ca2+ ( = 151.6 ± 1.3 mV, = 0.60 ± 0.01, = 5); 1 µM Ca2+ ( = 137.1 ± 5.1 mV, = 0.61 ± 0.01, = 5); 5 µM Ca2+ ( = 121.9 ± 3.8 mV, = 0.63 ± 0.01, = 5); 10 µM Ca2+ ( = 67.3 ± 17.1 mV, = 0.64 ± 0.08, = 4); 100 µM Ca2+ ( = 31.9 ± 4.5 mV, = 0.66 ± 0.01, = 7). (C) obtained from the curves as a function of Ca2+ concentration (mean ± SEM). Ca2+ binding produces a leftward shift in : 0.1 µM Ca2+ ( = −12.1 ± 3.5 mV); 0.5 µM Ca2+ ( = −22.9 ± 1.8 mV); 1 µM Ca2+ ( = −37.1 ± 3.5 mV); 5 µM Ca2+ ( = −50.3 ± 4.7 mV); 10 µM Ca2+ ( = −107.1 ± 17.1 mV); 100 µM Ca2+ ( = −142.6 ± 4.5 mV). One-way ANOVA followed by Dunnett’s post-hoc test analysis was used to assess statistical significance of the Ca2+-induced shifts in (***p<0.001).
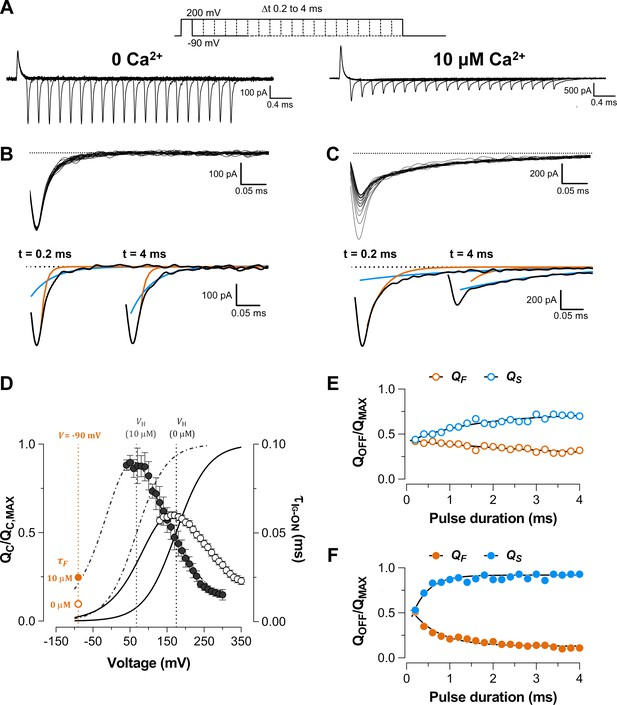
Ca2+ increases the slow component of the OFF gating currents.
(A) Recording of gating current (IG) evoked by 200 mV pulses of different durations (from 0.2 to 4 ms) at 0 and 10 μM [Ca2+] conditions, respectively. (B–C) The top panels show the superimposed traces of the IG-OFF recorded at −90 mV evidencing a decrease in amplitude and a slower decay of the OFF current as the duration of the pulse increases at 10 μM Ca2+. The dashed line represents the baseline for each experiment. IG-OFF were fitted with an exponential function of two-components (fast and slow components). IG-OFF traces at 0.2 ms and 4 ms pulse duration are displayed for each Ca2+ condition. Orange and blue lines correspond to fast and slow components of the two-exponential fits, respectively: (B) ‘zero’ Ca2+ ( = 10 μs and = 44 μs) and (C) 10 μM Ca2+ ( = 25 μs and = 212 μs). (D) Plots of the gating currents time constants measured at 0 Ca2+ (open symbols, solid line) and 10 μM Ca2+ (filled symbols, dot-dashed line). Data are well described using the equations of the two-sate model (see Materials and methods) with the following parameters: = 3.73 ms−1, = 76.10 ms−1 and = 0.29 at 0 Ca2+and = 3.98 ms−1, = 10.61 ms−1 and = 0.37 at 10 μM Ca2+. The fast OFF time constants at −90 mV for 0 Ca2+ (10 μs, orange open circles) and 10 μM Ca2+ (25 μs, orange filled circles) from panels B and C are shown. Time constants extrapolated to −90 mV from the two-state model are close to the experimental measurements: 3 μs and 21 μs for 0 Ca2+ and 10 μM Ca2+, respectively. Boltzmann fit of the curves for 0 Ca2+ (solid line) and 10 μM Ca2+ are also drawn (dot-dashed line). (E–F) The relative amplitude of the OFF-charge components, the fast (, orange symbols) and slow (, blue symbols) charge components were plotted against the pulse duration and fitted with an exponential function representing the time course of the opening of the channel: (E) ‘zero’ Ca2+ (open circles, = 1.8 ms) and (F) 10 μM Ca2+ (filled circles, = 536 μs).
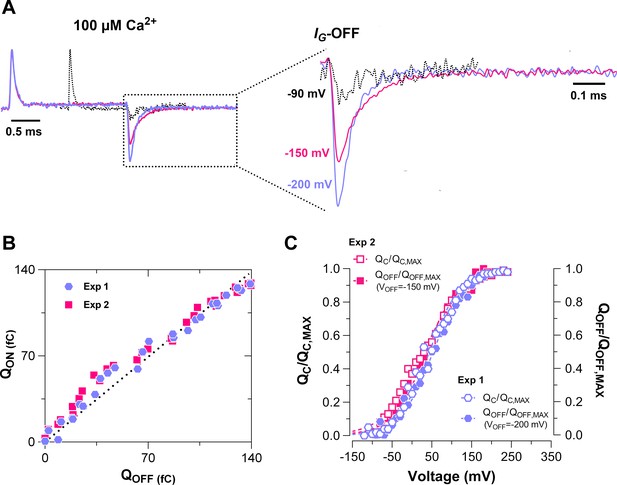
Voltage-dependent movement of the ON and OFF gating charge has a similar behavior at saturating Ca2+ conditions.
(A) Normalized gating current (IG) trace at 160 mV voltage step for three different experiments recordings in 100 μM of internal Ca2+ conditions. The IG-160 mV trace represented by the black dashed line corresponds to the representative IG at 100 μM Ca2+ in Figure 2A. In this record, the IG-ON was evoked by 1 ms voltage steps of 10 mV, going from −90 to 350 and the OFF-gating currents (IG-OFF) recorded at −90 mV of 1 ms duration. For the IG represented by the purple and pink lines, the IG-ON was evoked by 2 ms voltage steps of 10 mV, going from −90 to 350 and the IG-OFF recorded at −200 mV (Experiment 1) and −150 mV (Experiment 2) of 2 ms duration, respectively. For comparison, only the IG trace at 160 mV voltage step of each experiment was shown . (B) plotted against are linearly related in Experiment 1 (Exp 1: purple symbols) and Experiment 2 (Exp 2: pink symbols). was obtained by integrating the fast component for each ON IG trace ( = ), and the was determined by integrating IG-OFF for each pulse. (C) Normalized ON gating charge data (open symbols: ) and OFF gating charge data (filled symbols: ) were fitted using a single Boltzmann function for each experiment: ( = 44.5 mV, = 0.59) and ( = 52.8 mV, = 0.62) for the Exp 1 (VOFF = −200 mV); and ( = 29.5 mV, = 0.53) and ( = 35.3 mV, = 0.62) for the Exp 2 (VOFF = −150 mV).
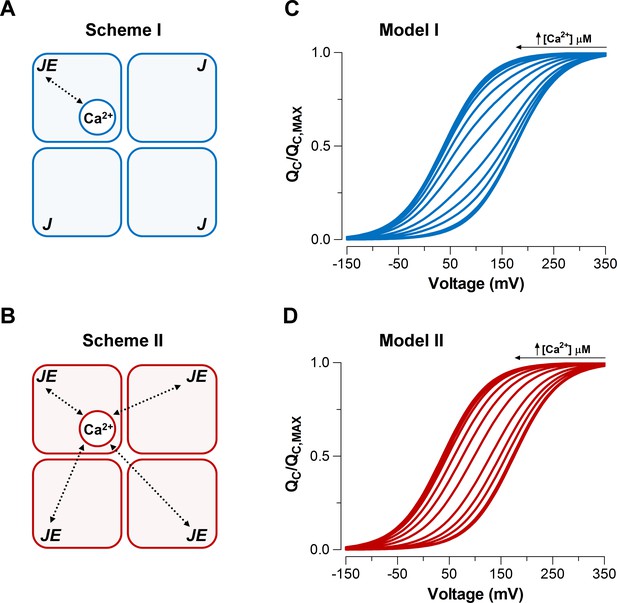
Model-dependent behavior of the curves based on the CTD-VSD interaction mechanisms according to the fractional occupancy of Ca2+-binding sites.
(A–B) Cartoons representing two interaction schemes between voltage sensors and Ca2+-binding sites (modified from Horrigan and Aldrich, 2002). Scheme I (A) assumes that Ca2+-binding only affects the voltage sensor of one α-subunit (), whereas Scheme II (B) predicts that binding of Ca2+ to one α-subunit will affect VSD in all subunits equally, increasing the voltage sensor equilibrium constants () -fold in all four subunits (, when the four Ca2+ sites are occupied). In both schemes, a single Ca2+-binding site is considered in each α-subunit. (C–D) Predictions of relationships at different internal calcium concentration (from 0 to 10 mM) by two distinctive interaction mechanisms between Ca2+-binding sites and voltage sensors (Scheme I and Scheme II), respectively. curves were generated using Equation 4 (blue: Model I) or Equation 6 (red: Model II), and the following set of parameters: = 0.58, = 0.018, = 11 µM and = = 25.
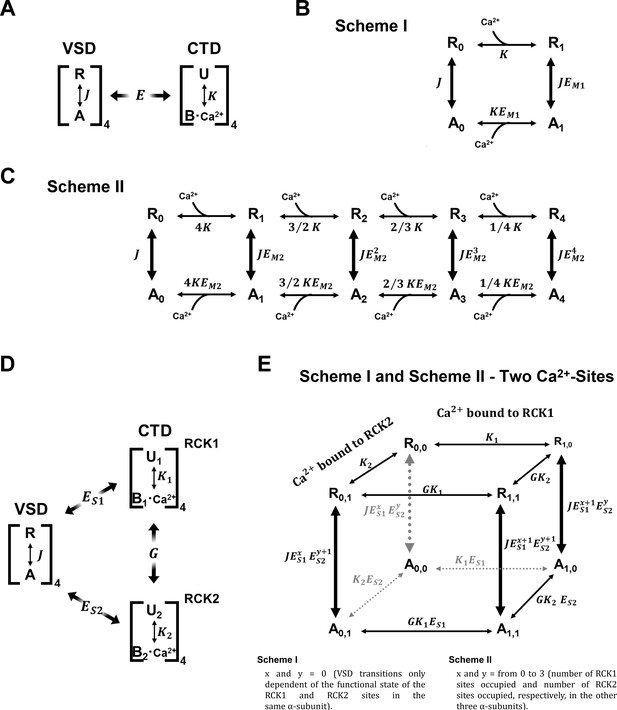
Kinetic models of the VSD activation according to the CTD-VSD interaction schemes.
(A) Sub-scheme describing calcium and voltage allosteric interaction for closed channels. The VSD transit between two resting (R) and active (A) configuration governed by the equilibrium constant , where each Ca2+ site undergoes unbound (U) - Ca2+ bound (B) transitions governed by the equilibrium constant . The allosteric factor accounts for the coupling between the calcium and voltage sensors (CTD-VSD). (B–C) VSD kinetic models in the presence of Ca2+ according to CTD-VSD interaction schemes I and II (Figure 3A–B), respectively, where the vertical transitions (R-A) represent the VSD movement and the horizontal transitions (R-R and A-A) are Ca2+-binding reactions when the VSD is in the resting or active conformation. For Scheme I (B), each VSD can undergo R0-A0 or R1-A1 transitions depending on the unbound or bound state of the Ca2+ site in the α-subunit, respectively. Thus, the R1-A1 equilibrium is defined by increased -fold (). For Scheme II (C), the R0-A0 to R4-A4 transitions represent the VSD equilibrium with 0, 1, 2, 3, and four occupied Ca2+ sites in the channel. Thus, for each Ca2+ bound the equilibrium constant increase -fold reaching to when the four Ca2+ sites are occupied. The thickness of the arrows indicates the probability of transitions. (D) General sub-scheme of the CTD-VSD interaction including two Ca2+ sites for each CTD (RCK1 and RCK2 sites). For each RCK1 and RCK2 site the unbound-Ca2+ bound transitions are governed by the equilibrium constants and . The factor describes the cooperativity between the sites within the same α-subunit; and the and factors define the allosteric coupling between the RCK1 and RCK2 sites and the VSD, respectively. (E) Schematic representation of VSD kinetic model according to the extended version of the Scheme I (B) and Scheme II (C) accounting for both RCK1 and RCK2 Ca2+-sites on each α-subunit. The VSD transitions in Scheme I only depend on the unbound or bound state of the RCK sites within the same α-subunit: unliganded (R0,0-A0,0), RCK1 site occupied (R1,0-A1,0), RCK2 site occupied (R0,1-A0,1) and both sites occupied (R1,1-A1,1). For sake simplicity, Scheme II, only depicts VSD transitions described for the Scheme I. In this case, x and y represent the number of RCK1 sites occupied and number of RCK2 sites occupied, respectively, in the other three α-subunits (x = from 0 to 3 and y = from 0 to 3).
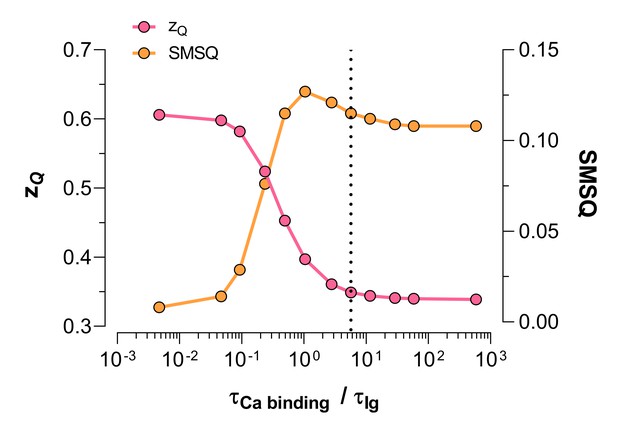
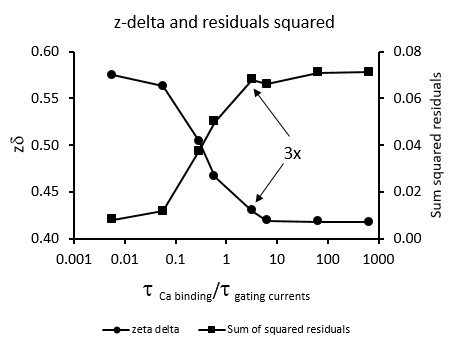
The resting-active transition of the voltage sensor is fast enough to assume no Ca2+ re-equilibration during gating currents measurements.
Simulation of, as a function of the time constant ratios of Ca2+ binding and gating currents are shown. Gating currents were simulated for a BK Scheme I (Figure 3A). The Ca2+ binding rate constant was = 1.81×108 M-1s-1 (Hou et al., 2016) and the unbinding rate constant = 1150. s-1 was calculated using a = 6.4×10-6 M (Table 1). Voltage-dependent rates for the voltage sensor activation were taken from Figure 1. Gating current were simulated using different calcium-binding time constants: scaled with different x-factors ranging from 0.01 to 1000. We fitted the simulated gating current at 10 μM Ca2+ to a single exponential using the first 100 μs of the gating current decay. The maximum gating current time constant was used to calculate the time constant ratios shown in the horizontal axis of the figure. The vertical dashed line marks the time constant ratio calculated for x = 1. The data obtained by integration of the area under the exponential function were fitted using a Boltzmann function. The sum of the residuals squared, SMSQ, was used as an indication of the goodness of the fit. The figure shows that the fit was good (low SMSQ) and the experimental value (0.61) was recovered for calcium-binding/gating current time constant ratio 0.04 or less; that is fast calcium re-equilibration, which is obtained for a calcium-binding rated constant one hundred times faster than the reported by Hou et al. (2016). We conclude that the good fit to a single Boltzmann sigmoidal function to our experimental curves is not due to fast calcium re-equilibration but due to the failure of Model I to describe our data. This conclusion is the same for analysis based on the first 30 μs of the simulated gating currents.
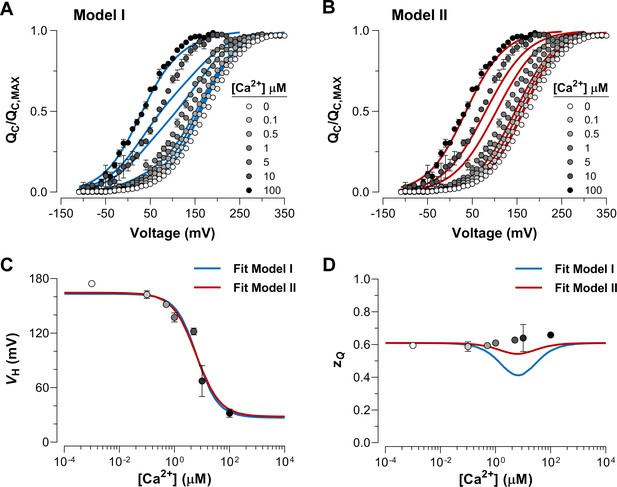
Dose-dependent effect of Ca2+ on voltage sensor activation is predicted by a Ca2+-VSD interaction mechanism in which Ca2+-binding affects equally the VSD in all four α-subunits.
(A–B) The experimental data were fitted using the two possible allosteric interaction mechanisms between voltage and calcium sensors described by Scheme I and Scheme II. The blue and red lines represent the global fits by Model I and Model II, respectively. The allosteric factor ( and ) was constrained to the value obtained from the individual fitting of the curves at 0 and 100 µM Ca2+ (experimental () equal to 26.4, see Table 1). The , and parameters were allowed to vary freely. Note that the allosteric factor for Model I () and Model II () have different interpretations, since whereas given that the four voltage sensor will be altered in 2.3-fold ( = 2.27) with each additional Ca2+ bound. (C–D) The Ca2+-dependence of - (C) and - (D) curves are superimposed with the and values predicted by Model I (blue line) and Model II (red line).
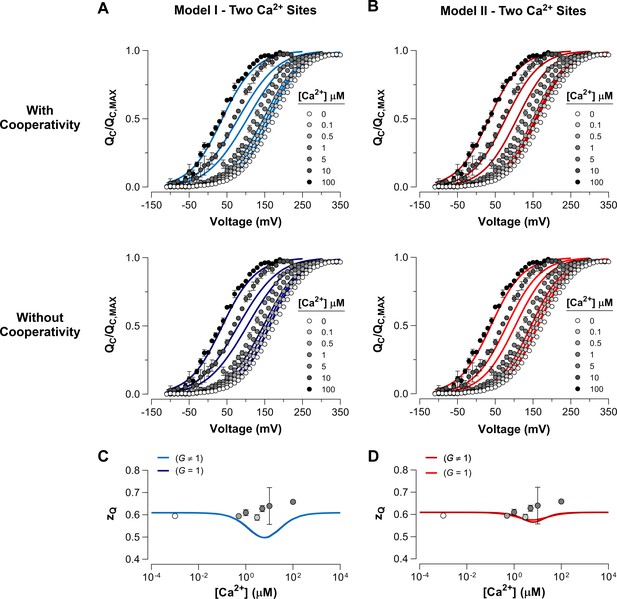
Fitting of experimental data by the Ca2+-VSD interaction mechanisms including two Ca2+-binding sites per α-subunit.
(A–B) The blue and red lines represent the global fits to the experimental curves by Model I (A) and Model II (B), respectively, with two Ca2+ sites in each α-subunit. For each model a cooperativity factor between the Ca2+ sites within the same subunit ( ≠ 1) (Upper) or no cooperative interactions between the Ca2+ sites ( = 1) (Bottom) was introduced. The estimated parameters for each model are shown in Table 1. The Ca2+-dependence of - data are superimposed with the values predicted by Model I (C) and Model II (D). The statistical analysis using one-way ANOVA followed by Dunnett’s post-hoc test showed no significant effect of Ca2+ on (p>0.05).
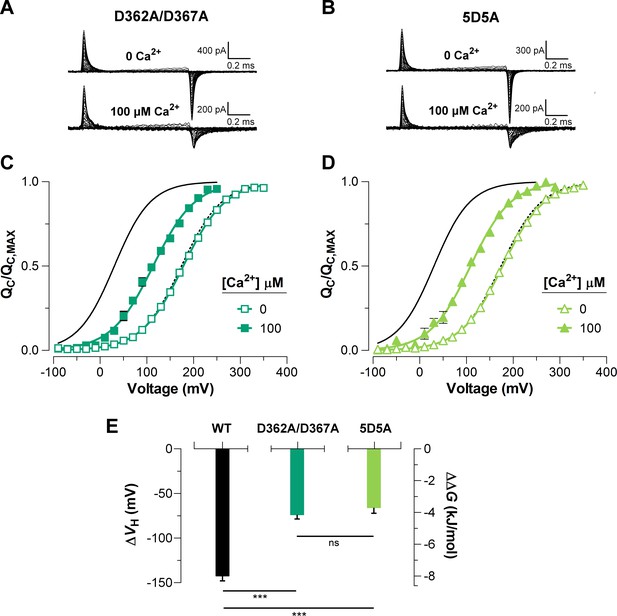
The high-affinity Ca2+-binding sites contribute equally to the allosteric coupling between calcium and voltage sensors in BK channels.
(A–B) Representative gating current (IG) recordings at 0 and 100 μM of [Ca2+]i for the RCK1 site mutant (D362A/D367A) and the RCK2 site mutant (5D5A), respectively. (C–D) Gating charge-voltage curves () were obtained at 0 Ca2+ (open symbols) and 100 μM Ca2+ (filled symbols) for D362A/D367A and 5D5A mutants, respectively. Boltzmann fitting to the experimental data (mean ± SEM) is indicated by solid lines ((D362A/D367A) = 178.0 ± 2.7 mV, = 0.58 ± 0.01, = 12 and (5D5A) = 176.4 ± 4.6 mV, = 0.58 ± 0.01, = 17 at ‘zero’ Ca2+; (D362A/D367A) = 104.2 ± 7.3 mV, = 0.56 ± 0.02, = 7 and (5D5A) = 110.8 ± 6.7 mV, = 0.58 ± 0.02, = 6 at 100 µM Ca2+). For comparison, all plots include the Boltzmann fit of the curves for WT at 0 Ca2+ (dashed black line) and 100 μM Ca2+ (solid black line). (E) Quantification of the shift in the curves and the free energy change induced by 100 μM Ca2+. The non-parametric t-test was used to evaluate statistical significances between WT BK channel and the RCK sites mutants (***p<0.001).
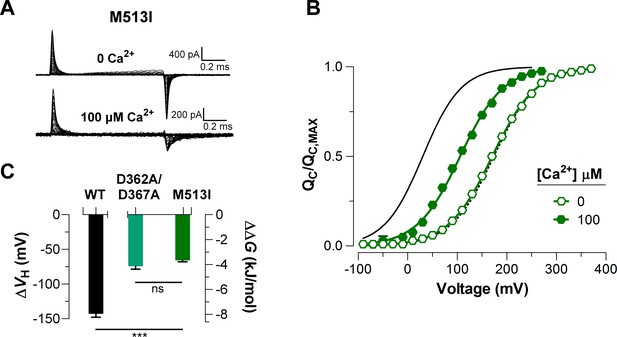
Mutations abolishing Ca2+-sensing by the RCK1 binding-site reduce the Ca2+-induced effect on voltage sensors activation similarly.
(A) Representative gating current (IG) recordings at 0 and 100 μM [Ca2+]i for the RCK1 site mutant M513I. (B) Gating charge-voltage curves were obtained at 0 Ca2+ and 100 μM Ca2+ (open and filled symbols) for the M513I mutant. Boltzmann fit to the experimental data (mean ± SEM) is indicated by solid lines ((M513I)= 170.4 ± 4.4 mV, = 0.58 ± 0.01, = 17 at ‘zero’ Ca2+ and (M513I)= 105.0 ± 6.3 mV, = 0.62 ± 0.03, = 4 at 100 µM Ca2+). For comparison, the plot includes the Boltzmann fit of the curves for WT at 0 Ca2+ and 100 μM Ca2+ (dashed and solid black line). (C) Quantification of the shift in the curves and the free energy change induced by 100 μM Ca2+. A non-parametric t-test was used to compare WT and RCK1-site mutants BK channels (***p<0.001).
Boltzmann sigmoidal curve fits of the Q(V) curves simulated for a BK model where the occupancy of the calcium binding site of one subunit alters the voltage sensor of only the same subunit.
Using the on binding rate constant of Hou et al., 2016 kb = 1.81⋅108 M-1s-1 and the unbinding rate constant ku = 650 s-1, calculated from the calcium dissociation constant of 3.6⋅10-6. We fitted the simulated the gating current at 15 μM Ca2+ to a single exponential using the first 30 μs of the gating current decay. Q(V) data obtained by integration of the area under the exponential function were fitted using a Boltzmann function. The sum of squared residual indicates that the fit is good for the calcium-binding time constant one hundred times shorter than gating current time constant. Also, we recover the zd value (0.58) we used for the simulation, only when the Ca2+ binding is 20 to 200 times faster than the voltage sensor kinetics. The high sum of squared residuals and the low zδ value obtained for calcium binding time constant three or more times slower than the gating currents time constant (pointed with arrows) indicates that the Q(V) curve is not a simple Boltzmann sigmoidal function.
Tables
Parameters for the best fits of the data using different Ca2+-VSD interaction models.
https://doi.org/10.7554/eLife.44934.013| One Ca2+-site per α-subunit | Two Ca2+-sites per α-subunit | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model I | Model II | Model I | Model II | ||||||
| Parameters | Parameters | With cooperativity | Without cooperativity | With cooperativity | Without cooperativity | ||||
| zJ | 0.61 | 0.61 | zJ | 0.61 | 0.61 | 0.61 | 0.61 | ||
| J0 | 0.020 | 0.018 | J0 | 0.019 | 0.019 | 0.021 | 0.019 | ||
| E | 26.4* | 2.27* | ES1 | 4.57* | 1.46* | ||||
| ES2 | 5.35* | 1.52* | |||||||
| KD (µM) | 6.4 | 6.1 | KD1 (µM) | 3.2 | 4.9 | 837.7 | 5.9 | ||
| KD2 (µM) | 631.7 | 6.9 | 6.6 | 5.9 | |||||
| G | 56.1 | 1* | 120.4 | 1* | |||||
| AIC | −948.4 | −1150.9 | −1088.9 | −1090.3 | −1162.0 | −1147.5 | |||
| Li | 4*10−47 | 0.004 | 1*10−16 | 3*10−16 | 1 | 7*10−4 | |||
| wi | 4*10−47 | 0.004 | 1*10−16 | 3*10−16 | 0.995 | 0.001 | |||
-
*Fixed parameters in the model fitting. AIC values correspond to Akaike Information Criterion to select the best fit model. ℒi and wi are the relative likelihood and the weight of each model within the set of candidate models.
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.44934.014





