Bed nucleus of the stria terminalis regulates fear to unpredictable threat signals
Figures
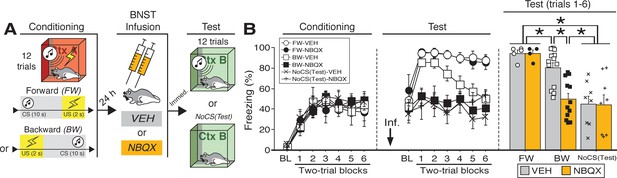
Reversible inactivation of the BNST attenuates conditioned fear expression to a backward, but not forward, CS.
(A) Behavioral schematic. (B) Freezing behavior at conditioning and retrieval testing. For conditioning, the left panel depicts mean percentage freezing during the 5 min baseline (BL) and across each conditioning block (each 136 s block is comprised of two trials; conditioning trials consist of freezing during the 10 s CS followed by the 58 s interstimulus interval). For retrieval testing, the center panel shows mean percentage freezing at the 5 min baseline (BL) and across each test block (each 140 s block is comprised of two trials; trials consist of freezing during the 10 s CS followed by the 60 s interstimulus interval). The right panel shows mean percentage freezing during the first half of the test (trials 1–6; corresponding to 420 s of behavior). All data are represented as means ± s.e.m [FW-VEH (n = 5); FW-NBQX (n = 4); BW-VEH (n = 13); BW-NBQX (n = 12); NoCS(Test)-VEH (n = 8); NoCS(Test)-NBQX (n = 8)]; * = p < 0.05.
-
Figure 1—source data 1
- https://doi.org/10.7554/eLife.46525.008
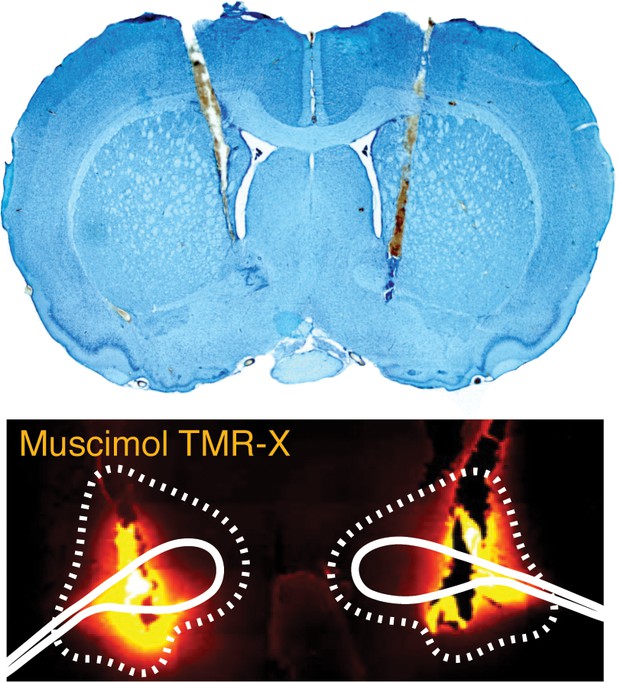
Representative bilateral cannula placements in the BNST.
Photomicrograph (10×) of a thionin-stained coronal section depicting representative cannula tracts in the BNST (top panel). Fluorescent image (gold filter) of a coronal section (10×) showing spread of drug in BNST (BNST outlined in dotted line) (bottom panel).

Bilateral cannula placements for BNST microinfusions.
Schematic depicting cannula placements for Figure 1. Symbols (split by each group) correspond to injector tips (approximate borders of BNST are shown by red dotted outline; approximate borders of BNST are shown by red dotted outline).
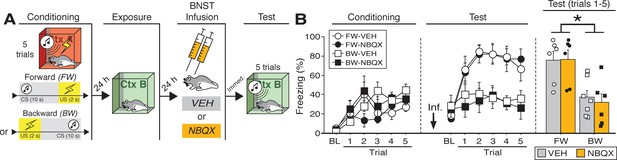
Effects of BNST inactivation on freezing to a forward vs. backward CS trained with five trials.
(A) Behavioral schematic. (B) Freezing behavior during conditioning and retrieval testing. For conditioning, the left panel depicts mean percentage freezing during the 3 min baseline (BL) and across each conditioning block (each 136 s block is comprised of two trials; conditioning trials consist of freezing during the 10 s CS followed by the 58 s interstimulus interval). For retrieval testing, the center panel shows mean percentage freezing at the 3 min baseline (BL) and across each test trial (each trial consists of freezing during the 10 s CS followed by the 60 s interstimulus interval). The right panel shows mean percentage freezing after baseline (trials 1–5; corresponding to 350 s of behavior). All data are represented as means ± s.e.m [FW-VEH (n = 6); FW-NBQX (n = 6); BW-VEH (n = 7); BW-NBQX (n = 6)]; * = p < 0.05.
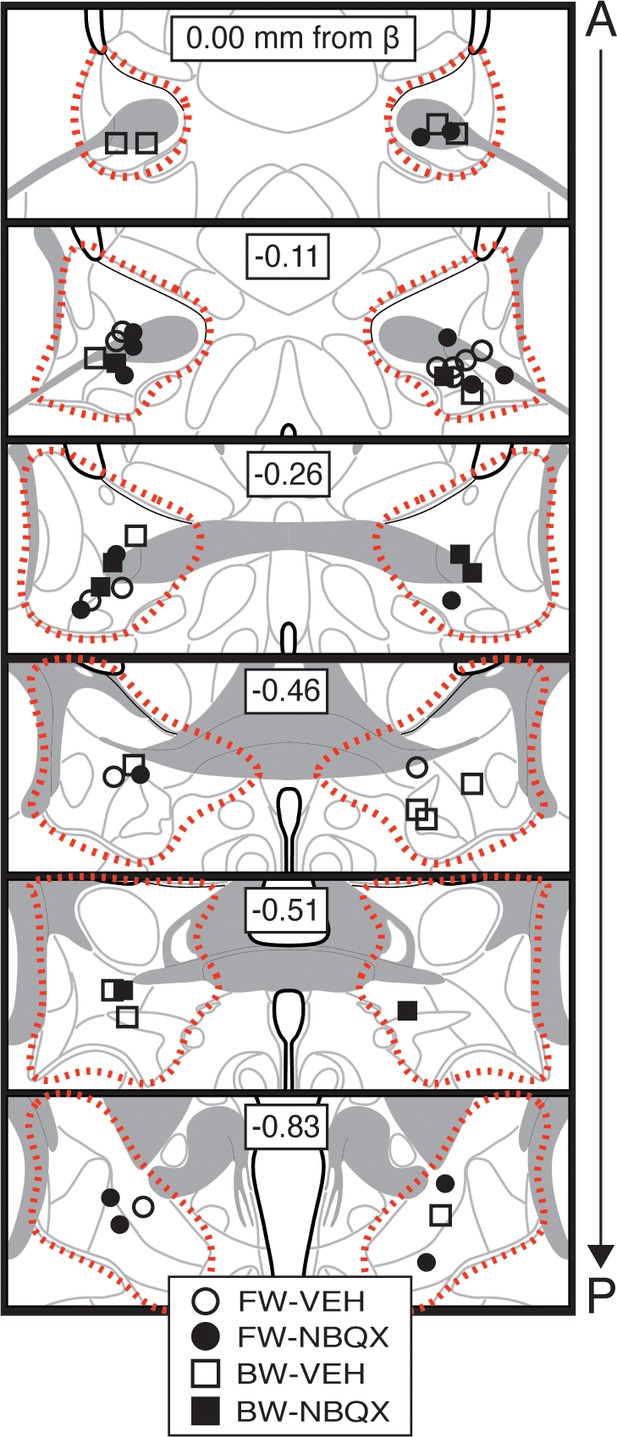
Bilateral cannula placements for BNST microinfusions.
Schematic depicting cannula placements for Figure 1—figure supplement 3. Symbols (split by each group) correspond to injector tips (approximate borders of BNST are shown by red dotted outline).
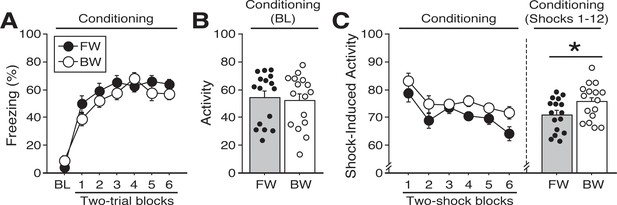
Shock-induced activity during conditioning to a forward vs. backward CS.
(A) Mean percentage of freezing at baseline (BL; 5 min) and across conditioning blocks (each 136 s block is comprised of two trials; trials consist of freezing during the 10 s CS followed by the 58 s interstimulus interval). (B) Mean activity values across the 5 min BL (no shock present). (C) The left panel shows mean shock-induced activity (during shocks) at conditioning [averaged into 4 s (two-shock) blocks]. The right panel shows mean shock-induced activity across all trials. All data are represented as means ± s.e.m [FW (n = 16); BW (n = 16)]; * = p < 0.05.
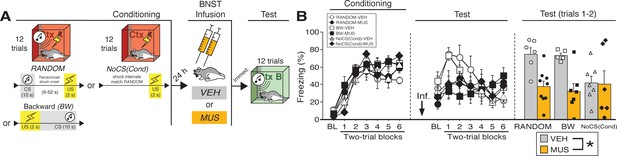
BNST inactivation attenuates freezing to a discrete CS paired with random onset of shock.
(A) Behavioral schematic. (B) Freezing behavior during conditioning and retrieval testing. For conditioning, the left panel depicts mean percentage freezing during the 5 min baseline (BL) and across each conditioning block (each block is comprised of two trials; trials consist of freezing during the 10 s CS followed by the 58 s interval for the BW animals, whereas blocks for the other groups include equivalent time periods which include the CS [if present] and inter-CS intervals [excluding the duration of the shock]). For retrieval testing, the center panel shows mean percentage freezing at the 5 min baseline (BL) and across each test block (each 140 s block is comprised of two trials; trials consist of freezing during the 10 s CS followed by the 60 s interval). The right panel shows mean percentage freezing across the first two test trials (after BL; corresponding to 140 s of behavior). All data are represented as means ± s.e.m [RANDOM-VEH (n = 6); RANDOM-MUS (n = 9); BW-VEH (n = 5); BW-MUS (n = 7); NoCS(Cond)-VEH (n = 7); NoCS(Cond)-VEH (n = 6)]; * = p < 0.05.
-
Figure 2—source data 1
- https://doi.org/10.7554/eLife.46525.011
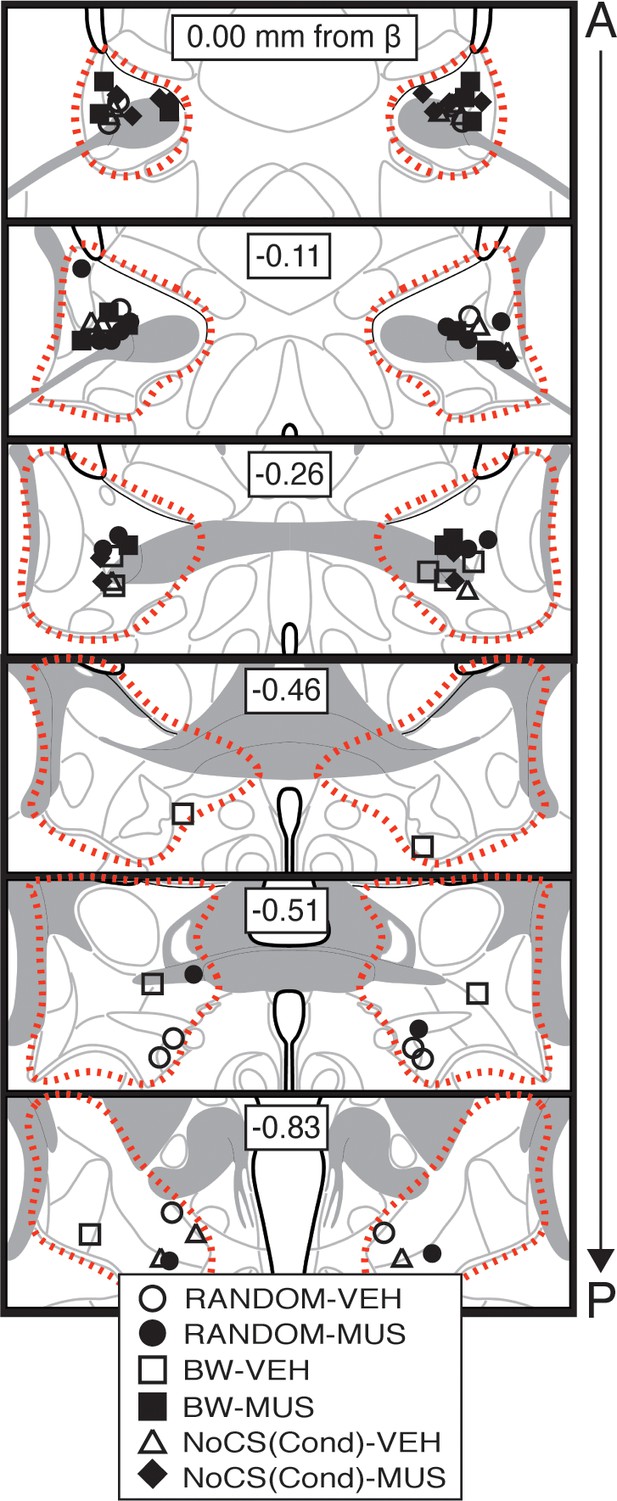
Bilateral cannula placements for BNST microinfusions.
Schematic depicting cannula placements for Figure 2. Symbols (split by each group) correspond to injector tips (approximate borders of BNST are shown by red dotted outline).
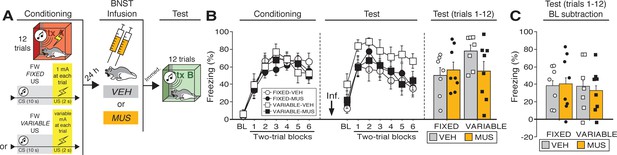
Temporary inactivation of the BNST does not prevent conditioned fear expression to a forward CS paired with a US of fixed or variable intensity.
(A) Behavioral schematic. (B) Freezing behavior during conditioning and retrieval testing. For conditioning, the left panel depicts mean percentage freezing during the 5 min baseline (BL) and across each conditioning block (each 136 s block is comprised of two trials; trials consist of freezing during the 10 s CS followed by the 58 s post-shock interval). For retrieval testing, the center panel shows mean percentage freezing at the 5 min baseline (BL) and across each test block (each 140 s block is comprised of two trials; trials consist of freezing during the 10 s CS followed by the 60 s interval). The right panel shows mean percentage freezing across all test trials (after BL; corresponding to 840 s of behavior). (C) Freezing percentages across all twelve trials, with BL levels of freezing subtracted from these values. All data are represented as means ± s.e.m [FIXED-VEH (n = 7); FIXED-MUS (n = 8); VARIABLE-VEH (n = 7); VARIABLE-MUS (n = 8)].
-
Figure 3—source data 1
- https://doi.org/10.7554/eLife.46525.014

Bilateral cannula placements for BNST microinfusions.
Schematic depicting cannula placements for Figure 3. Symbols (split by each group) correspond to injector tips (approximate borders of BNST are shown by red dotted outline).
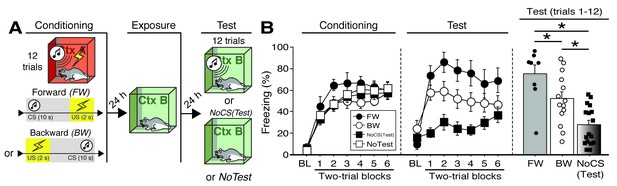
CS-evoked freezing in rats utilized for Fos analyses.
(A) Behavioral schematic. (B) Freezing during conditioning and retrieval testing. For conditioning, the left panel depicts mean percentage freezing during the 5 min baseline (BL) and across each conditioning block (each 136 s block is comprised of two trials; conditioning trials consist of freezing during the 10 s CS followed by the 58 s interstimulus interval). For retrieval testing, the center panel shows mean percentage freezing at the 5 min baseline (BL) and across each test block (each 140 s block is comprised of two trials; trials consist of freezing during the 10 s CS followed by the 60 s interval). The right panel shows mean percentage freezing after BL (corresponding to 840 s of behavior). Animals were sacrificed for Fos analyses 90 min after trial 1. All data are represented as means ± s.e.m [FW (n = 8); BW (n = 14); NoCS(Test) (n = 17); NoTest (n = 8)]; * = p < 0.05.
-
Figure 4—source data 1
- https://doi.org/10.7554/eLife.46525.016
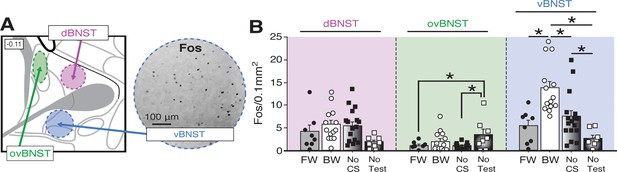
Fos expression in the BNST following exposure to a temporally predictable or uncertain CS.
(A) Schematic depicting regions counted within the BNST (left panel). The right panel shows example of Fos expression in the ventral BNST (vBNST). (B) Mean Fos-positive cells per 0.1 mm2 for each of the quantified regions. All data are represented as means ± s.e.m [FW (n = 8); BW (n = 14); NoCS(Test) (n = 17); NoTest (n = 8)]; * = p < 0.05.
-
Figure 5—source data 1
- https://doi.org/10.7554/eLife.46525.018
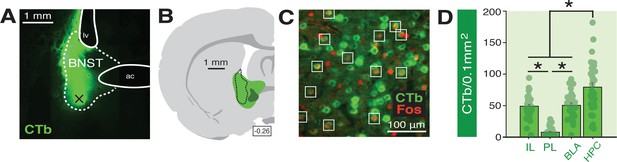
Functional tracing in afferents targeting the BNST.
(A) Coronal section (10×) showing representative fluorescence of a CTb infusion (green) into the BNST (dotted outline; ‘ac’=anterior commissure, ‘lv’=lateral ventricle). Black ‘X’ denotes approximate lowest point of the infusion (as an example of how injection sites are documented in Figure 6—figure supplement 1). (B) Coronal schematic (−0.26 mm from bregma) showing the approximate largest (green) and smallest (dark green) areas of CTb spread in the BNST for animals included in the analyses; the black dotted outline represents the extent of spread of CTb in the BNST in the image shown in panel A. (C) Example CTb-positive (green) and Fos-positive (red; nuclei) cells in a coronal section (40 μm) of the IL; open white squares denote double-labeled cells. (D) Mean number of BNST-targeting CTb-positive cells (per 0.1 mm2) for each of the quantified regions (shows FW, BW, and NoTest animals corresponding to Figure 7). All data are represented as means ± s.e.m (for each region, n = 30); * = p < 0.05.
-
Figure 6—source data 1
- https://doi.org/10.7554/eLife.46525.021
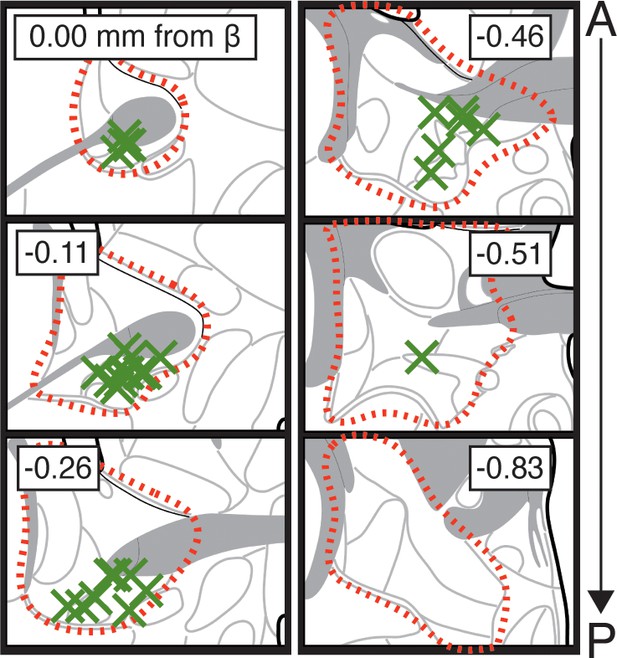
CTb injection sites in BNST.
Approximation of the most ventral and centermost sites of unilateral microinjection of CTb (green X’s) for all animals shown in Figures 6 and 7 (red outline approximates borders of BNST).
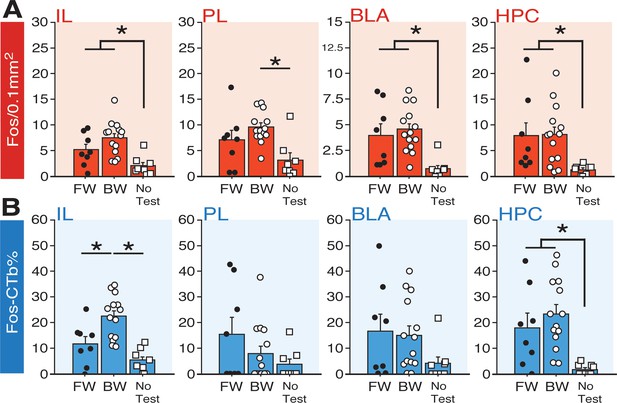
Fos expression in BNST-targeting cells of the prefrontal cortex, amygdala, and hippocampus following exposure to a forward or backward CS.
(A) Mean number of Fos-positive cells (per 0.1 mm2) for each of the quantified regions. (B) Mean percentage (per 0.1 mm2) of Fos-positive and CTb-positive cells divided by the total number of CTb-positive cells for each region. All data are represented as means ± s.e.m [FW (n = 8); BW (n = 14); NoTest (n = 8)]; * = p < 0.05.
-
Figure 7—source data 1
- https://doi.org/10.7554/eLife.46525.023
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.46525.024





