Molecular determinants in Frizzled, Reck, and Wnt7a for ligand-specific signaling in neurovascular development
Figures
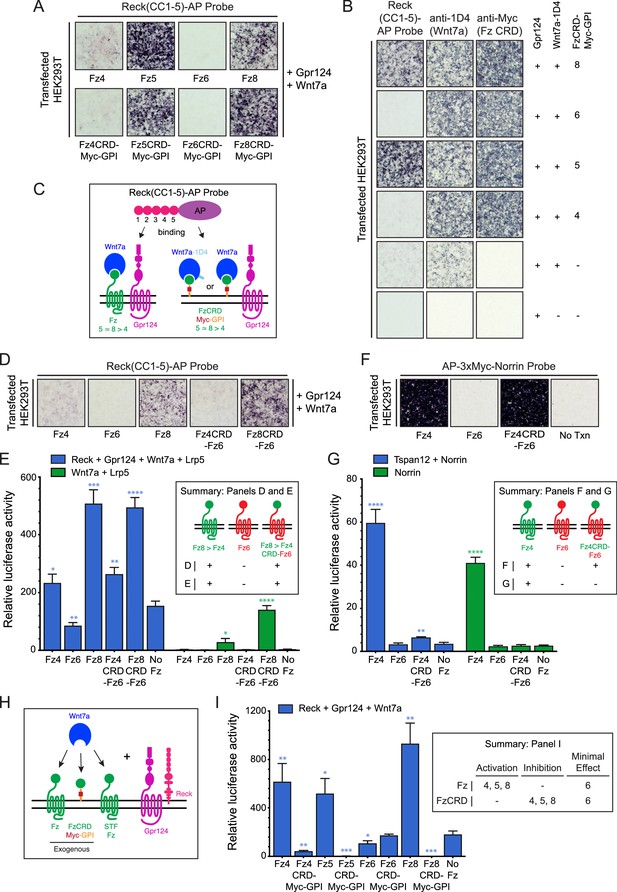
Frizzled CRD specificity for Reck-Gpr124-Wnt7a binding and signaling.
(A) Reck(CC1-5)-AP binding to live HEK293T cells transfected with Gpr124, Wnt7a, and full-length Fz (top) or Gpr124, Wnt7a, and FzCRD-Myc-GPI (bottom). (B) Reck(CC1-5)-AP binding as in (A), with Gpr124, Wnt7a, and the indicated FzCRD-Myc-GPI targets, together with anti-Myc and anti-1D4 controls. (C) Summary of the AP binding assay in (A) and (B). (D) Reck(CC1-5)-AP binding as in (A), with WT or chimeric Frizzleds. (E) Beta-catenin signaling assay using STF cells transfected with Wnt7a, Gpr124, Reck, and Lrp5 (left) or Wnt7a and Lrp5 (right), together with WT or chimeric Frizzleds. Inset: summary of AP binding (D) and STF signaling (E). In this and subsequent figures, bars represent mean ± SD. Statistical significance, determined by the unpaired t-test, is represented by * (p<0.05), ** (p<0.01), *** (p<0.001), and **** (p<0.0001). The statistical comparisons in (E), (G), and (I) are to the ‘No Fz’ control. (F) AP-3xMyc-Norrin binding assay as in (A), with WT or chimeric Frizzleds. (G) Beta-catenin signaling assay using STF cells transfected with Tspan12 and Norrin (left) or Norrin (right), together with WT or chimeric Frizzleds. Inset: summary of AP binding (F) and STF signaling (G). (H) Schematic of the FzCRD-Myc-GPI competition experiment in (I). (I) The effect of FzCRD-Myc-GPI competition on beta-catenin signaling by Reck, Gpr124, and Wnt7a. Inset: summary of STF signaling.
-
Figure 1—source data 1
Relative luciferase activity for STF experiments in Figure 1 panels E, G, and I.
- https://doi.org/10.7554/eLife.47300.003
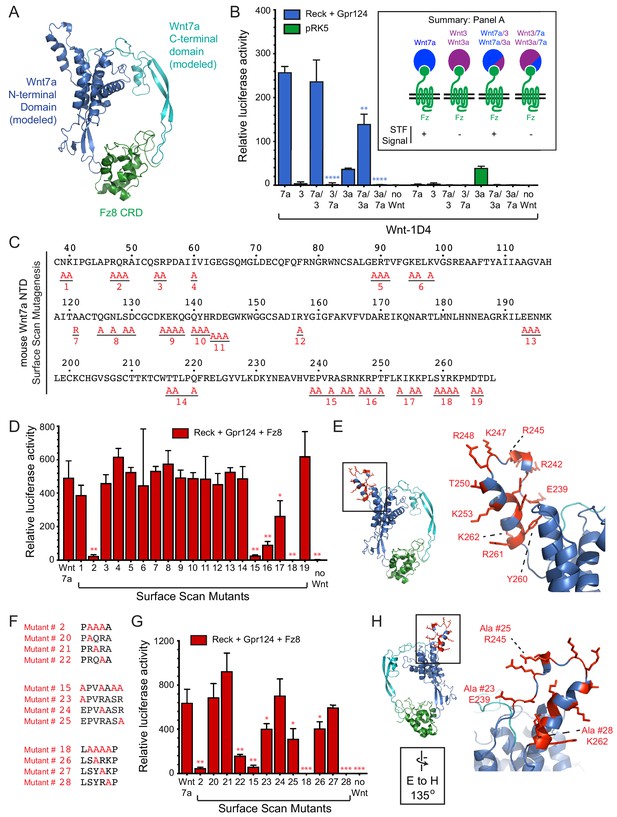
Wnt7a regions that are required for Reck/Gpr124-stimulated signaling.
(A) Backbone model of Wnt7a (N-terminal domain, blue; C-terminal domain, cyan) bound to Fz8 CRD (green) based on the Wnt-CRD crystal structure of Janda et al. (2012). The N-terminal domain of Wnt7a consists of ~270 amino acids (some of which were not resolved in the crystal structure), and the C-terminal domain consists of ~80 amino acids. (B) Beta-catenin signaling assay using STF cells transfected with Reck and Gpr124 (left) or pRK5 vector control (right), together with the indicated Wnts. Inset: summary of STF signaling. Statistical comparisons in (B), (D), and (G) are to WT Wnt7a. (C) Amino acid sequence of the N-terminal domain of mouse Wnt7a, with alanine scanning mutants indicated. (D) Beta-catenin signaling assay using STF cells transfected with Reck, Gpr124, and Fz8, together with WT or mutant Wnt7a. (E) Left, backbone model of Wnt7a bound to Fz8 CRD as in (A), with amino acids that are critical for Wnt7a signaling shown in red. Right, the boxed region is displayed at higher magnification. (F) Single alanine substitution mutants of Wnt7a, indicated in red. (G) Beta-catenin signaling assay as in (D) with WT or the indicated Wnt7a mutants. (H) Left, backbone model of Wnt7a bound to Fz8 CRD as in (A) except rotated 135 degrees, with amino acids that are critical for Wnt7a signaling shown in red. Right, the boxed region is displayed at higher magnification.
-
Figure 2—source data 1
Relative luciferase activity for STF experiments in Figure 2 panels B, D, and G.
- https://doi.org/10.7554/eLife.47300.008
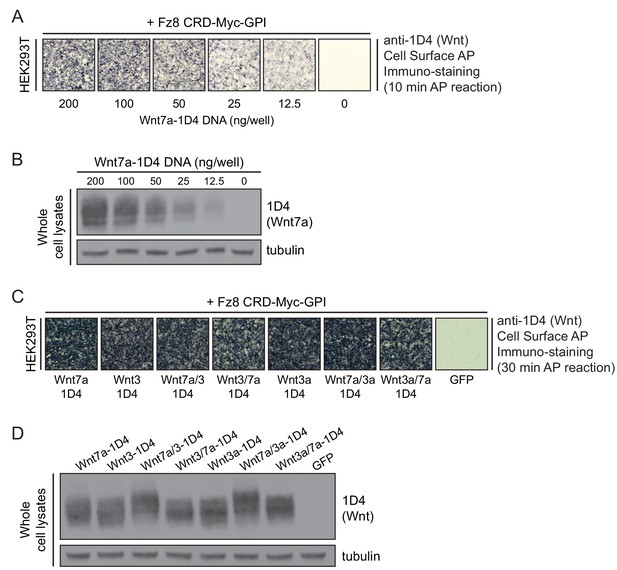
Production of intact and chimeric Wnt proteins for Reck- and Gpr124-mediated signaling.
(A) Detecting WT Wnt7a-1D4 proteins on the surface of HEK293T cells co-transfected with Fz8CRD-Myc-GPI and the indicated concentrations of Wnt7a-1D4 plasmid DNA. Live cells were immuno-stained with anti-1D4, fixed, and then incubated with anti-mouse antibody conjugated to AP. (B) Immunoblot of post-nuclear supernatants from HEK293T cells transfected with the indicated concentrations of Wnt7a-1D4 plasmid DNA, probed with mAb 1D4 and anti-tubulin. (C) Detecting Wnt proteins on the surface of HEK293T cells transfected with Fz8CRD-Myc-GPI, together with Wnt7a-1D4, Wnt3-1D4, Wnt3a-1D4, or their chimeric derivatives. Cell-surface AP immuno-staining was performed as described in (A). (D) Immunoblot of post-nuclear supernatants from HEK293T cells transfected with Wnt7a-1D4, Wnt3-1D4, Wnt3a-1D4, or their chimeric derivatives, probed with mAb 1D4 and anti-tubulin.
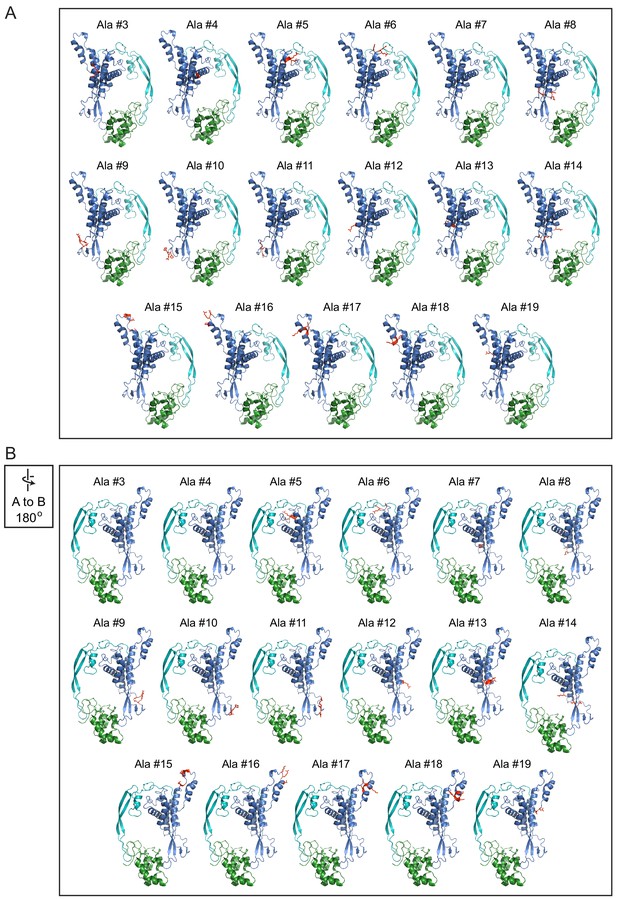
Locations of alanine mutations on the Wnt7a-Fz8CRD model.
Front (A) and back (B; rotated 180 degrees) views of the Wnt7a-Fz8CRD model with alanine mutations shown in red.
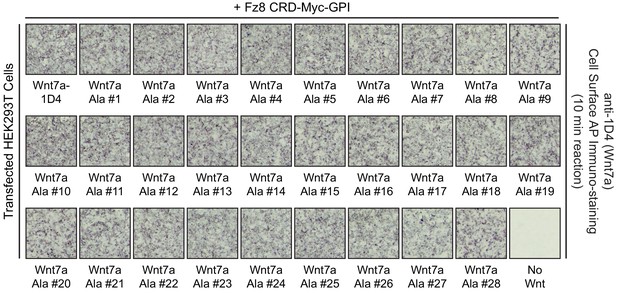
Cell-surface CRD binding by Wnt7a alanine mutants.
Detecting WT and mutant Wnt7a-1D4 proteins on the surface of HEK293T cells co-transfected with Fz8CRD-Myc-GPI and the indicated WT or mutant Wnt7a-1D4 plasmids. Live cells were immuno-stained with anti-1D4, fixed, and then incubated with anti-mouse antibody conjugated to AP.
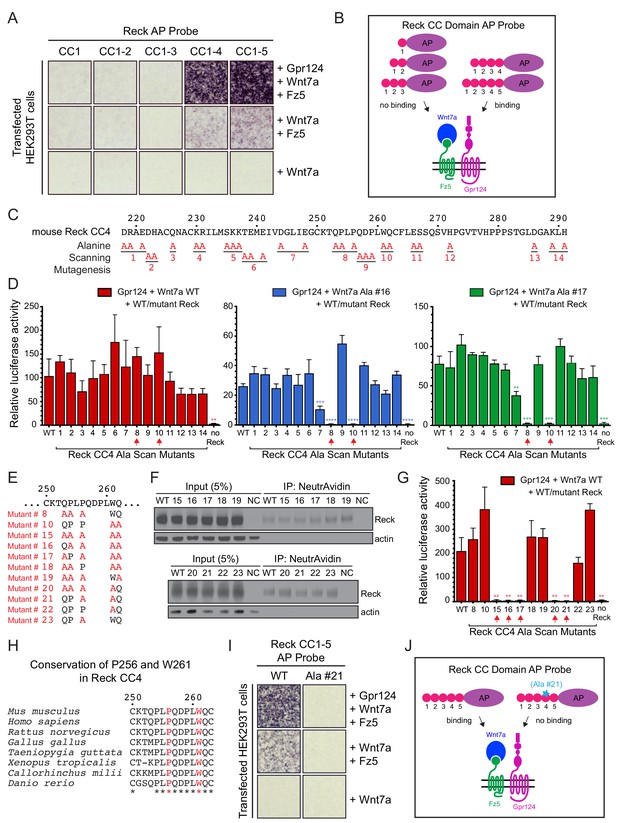
Reck CC4 is necessary for multi-protein complex formation and signaling with Gpr124, Wnt7a, and Fz.
(A) Reck(CC1)-, (CC1-2)-, (CC1-3)-, (CC1-4)-, or (CC1-5)-AP binding to live HEK293T cells transfected as indicated at right. (B) Schematic of the AP binding assay in (A). (C) Amino acid sequence of mouse Reck CC4, with alanine scanning mutants indicated. (D) Beta-catenin signaling assay using STF cells transfected with Gpr124 and WT Reck or the indicated Reck CC4 mutant, in combination with WT Wnt7a (left), Wnt7a Ala #16 (middle), or Wnt7a Ala #17 (right). Red arrows, Reck CC4 Ala #8 and Ala #10 transfections. Statistical comparisons in (D) and (G) are to WT Reck. (E) Sequence of mouse Reck CC4 in the region of Ala#8 and Ala#10, with additional alanine substitution mutants indicated in red. (F) HEK293T cells were transfected with WT Reck or the indicated Reck mutants. Post-nuclear supernatants (input) and surface biotinylated proteins (captured with NeutrAvidin agarose) were immunoblotted for Reck and actin. (G) Beta-catenin signaling assay using STF cells transfected with Gpr124, Wnt7a, and WT Reck or the indicated Reck CC4 mutant. Red arrows, Reck CC4 mutants that eliminate signaling. (H) Alignment and conservation of the Reck CC4 region shown in (E) across vertebrates, generated by Clustal Omega. (*) denotes fully conserved residues. P256 and W261 are highlighted in red. (I) Reck(CC1-5)-AP and Reck(CC1-5 Ala #21)-AP binding to live HEK293T cells transfected as indicated at right. (J) Schematic of the AP binding assay in (I).
-
Figure 3—source data 1
Relative luciferase activity for STF experiments in Figure 3 panels D and G and Figure 3—figure supplement 1 panel B.
- https://doi.org/10.7554/eLife.47300.011
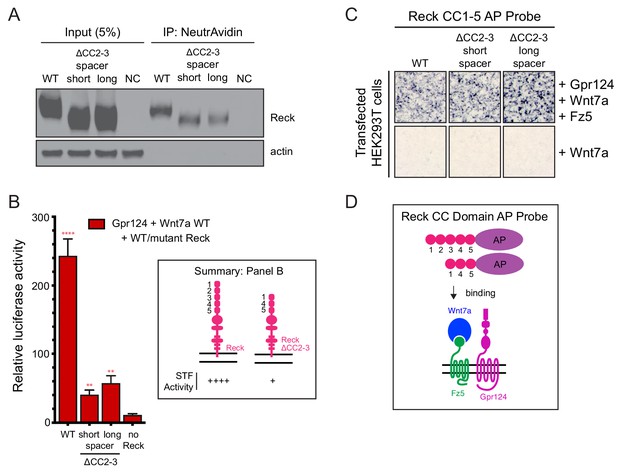
Tests of Reck ΔCC2-3 for Wnt7a/Fz/Gpr124 signaling and complex formation.
(A) HEK293T cells were transfected with WT Reck or the indicated Reck deletion mutants. Post-nuclear supernatants (input) and surface biotinylated proteins (captured with NeutrAvidin agarose) were immunoblotted for Reck and actin. (B) Beta-catenin signaling assay using STF cells transfected with Gpr124, Wnt7a, and WT Reck or the indicated Reck deletion mutants. Inset: summary of STF signaling. (C) Reck(CC1-5)-AP and Reck(CC1-5, Δ2-3)-AP binding to live HEK293T cells transfected as indicated at right. (D) Schematic of the AP binding assay in (C).
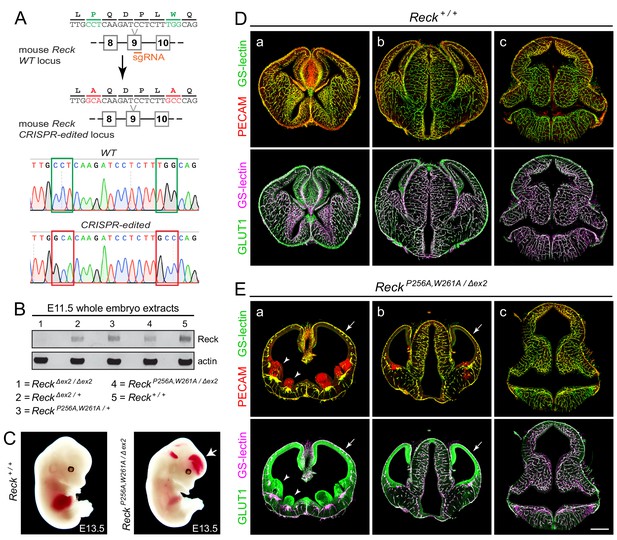
Embryos with ReckP256A/W261A have severe defects in CNS angiogenesis that match the defects in Gpr124 null embryos.
(A) CRISPR/Cas9 strategy for introducing P256A and W261A into Reck exon 9 in the mouse germline and sequencing chromatograms from cloned genomic PCR products from WT (top) vs. ReckP256A,W261A alleles (bottom). (B) Immunoblot of proteins from E11.5 embryos of the indicated genotypes, probed with anti-Reck and anti-actin antibodies. (C) Gross appearance of WT vs. ReckP256A,W261A/Δex2 E13.5 embryos. The arrow points to intracranial bleeding in the ReckP256A,W261A/Δex2 embryo. (D,E) Coronal sections of WT vs. ReckP256A,W261A/Δex2 E13.5 embryos immunostained for PECAM and GLUT1, and stained for GS-lectin. Sections are at the levels of (a) the ganglionic eminences, (b) the thalamus (center) flanked by the cerebral cortices, and (c) the hindbrain. In (E), arrows point to the avascular and hypoplastic cerebral cortex, and arrowheads point to the avascular and hypoplastic ganglionic eminences. Scale bar, 500 μm.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (M. musculus) | ReckΔex2 | PMID: 20691046 | RRID:MGI:4830344 | |
| Genetic reagent (M. musculus) | ReckP256A,W261A | this paper | Please find details under Materials and methods (Gene Targeting) | |
| Cell line (H. sapiens) | HEK/293T | ATCC | Cat. #: CRL-3216; RRID:CVCL_0063 | |
| Cell line (H. sapiens) | Super TOP Flash (STF) luciferase reporter cell line | PMID: 15035989 | ||
| Antibody | Rabbit polyclonal anti-Glut1 | Thermo Fisher Scientific | Cat. #: RB-9052-P1; RRID: AB_177895 | 1:400 dilution |
| Antibody | Rat monoclonal anti-PECAM/CD31 | BD Biosciences | Cat. #: 553370; RRID: AB_394816 | 1:400 dilution |
| Antibody | Isolectin GS-IB4 (GS Lectin), Alexa 488 conjugate | Thermo Fisher Scientific | Cat. #: I21411, RRID: AB_2314662 | 1:400 dilution |
| Antibody | Rabbit polyclonal anti-6xMyc | PMID: 28803732 | 1:10,000 dilution | |
| Antibody | Rat monoclonal anti-alpha tubulin | Thermo Fisher Scientific | Cat# MA1-80017; RRID: AB_2210201 | 1:10,000 dilution |
| Antibody | Mouse monoclonal anti-actin | Millipore Sigma | Cat. #: MAB1501; RRID: AB_2223041 | 1:10,000 dilution |
| Antibody | Rabbit monoclonal anti-Reck | Cell Signaling | Cat. #: 3433S; RRID: AB_2238311 | 1:2000 dilution |
| Antibody | Alkaline phosphatase horse anti-mouse IgG antibody | Vector Laboratories | Cat. #: AP-2000; RRID:AB_2336173 | 1:10,000 dilution |
| Antibody | Goat polyclonal anti-rabbit IgG (H + L) cross-adsorbed secondary antibody, Alexa 488, 594, and 647 conjugates | Thermo Fisher Scientific | Cat. #s: A-11008, RRID: AB_143165; A-11012, RRID: AB_2534079; A-21244, RRID: AB_2535812 | 1:400 dilution |
| Antibody | Goat polyclonal anti-rat IgG (H + L) cross-adsorbed secondary antibody, Alexa 488, 594, and 647 conjugates | Thermo Fisher Scientific | Cat. #s: A-11006, RRID: AB_2534074; A-11007, RRID: AB_2534075; A-21247, RRID: AB_141778 | 1:400 dilution |
| Antibody | IRDye 800CW goat anti-mouse IgG (H + L) secondary antibody | LI-COR | Cat. #: 925–32210; RRID:AB_2687825 | 1:10,000 dilution |
| Antibody | IRDye 680RD goat anti-rabbit IgG (H + L) secondary antibody | LI-COR | Cat. #: 925–68071; RRID:AB_2721181 | 1:10,000 dilution |
| Antibody | IRDye 680RD goat anti-rat IgG (H + L) secondary antibody | LI-COR | Cat. #: 926–68076; RRID:AB_10956590 | 1:10,000 dilution |
| Oligonucleotides | ReckP256A,W261A guide RNA: caagatcctctttggcagtg | this paper | Please find details under Materials and methods (Gene Targeting) | |
| Oligonucleotides | ReckP256A,W261A SSODN HDR template: gttgatggtctcattgagggttgtaagacccagcccttggcacaagatcctcttgcccagtgttttctcgaaagctcacagtcggttcaccctgga | this paper | Please find details under Materials and methods (Gene Targeting) | |
| Recombinant DNA reagents | Mouse Frizzled CRD-GPI cDNA | PMID: 17158104 | ||
| Recombinant DNA reagents | Mouse Norrin, Wnts, and Frizzleds cDNA | PMID: 23095888 | ||
| Recombinant DNA reagents | Mouse Tspan12 cDNA | PMID: 30478038 | ||
| Recombinant DNA reagents | Mouse Reck cDNA | PMID: 28803732 | ||
| Recombinant DNA reagents | Mouse Gpr124 cDNA | PMID: 28803732 | ||
| Recombinant DNA reagents | Frizzled chimera cDNA | this paper | Please find details under Materials and methods (Plasmids) | |
| Recombinant DNA reagents | Wnt7a chimera and mutant cDNA | this paper | Please find details under Materials and methods (Plasmids) | |
| Recombinant DNA reagents | Reck mutant cDNA | this paper | Please find details under Materials and methods (Plasmids) | |
| Recombinant DNA reagents | Reck AP fusion cDNA | this paper | Please find details under Materials and methods (Plasmids) | |
| Commercial assay or kit | Dual-Luciferase Reporter Assay System | Promega | Cat. #: E1910 | |
| Chemical compound, drug | BluePhos phosphatase substrate solution (5-bromo-4-chloro-3-indolyl phosphate/tetrazolium) | Kirkegaard and Perry Laboratories | Cat. #: 50-88-00 | |
| Chemical compound, drug | EZ-Link Sulfo-NHS-LC-Biotin | Thermo Fisher Scientific | Cat. #: 21335 | |
| Chemical compound, drug | Nitro blue tetrazolium/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP) substrate | Roche | Cat. #: 11383213001 | |
| Software, algorithm | ImageJ | https://imagej.nih.gov/ij | ||
| Software, algorithm | Adobe Photoshop CS6 | https://adobe.com/photoshop | ||
| Software, algorithm | Adobe Illustrator CS6 | https://adobe.com/illustrator | ||
| Software, algorithm | GraphPad Prism 7 | http://www.graphpad.com | ||
| Other | FuGENE HD Transfection Reagent | Promega | Cat. #: E2311 | |
| Other | Pierce NeutrAvidin agarose resin | Thermo Fisher Scientific | Cat. #: 29200 | |
| Other | Fluoromount G | EM Sciences | Cat. #: 17984–25 | |
| Other | Protease Inhibitor | Roche | Cat. #: 11836170001 |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.47300.013





