RIM is essential for stimulated but not spontaneous somatodendritic dopamine release in the midbrain
Figures
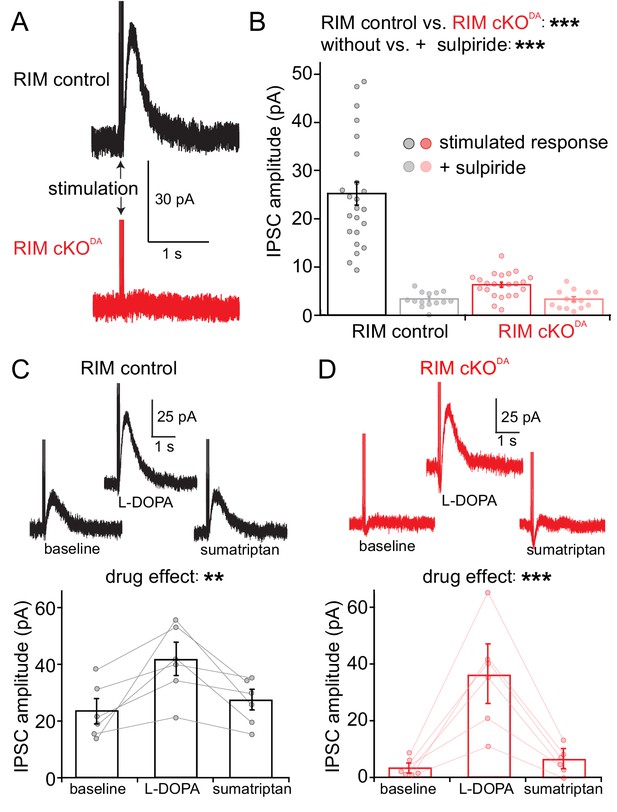
RIM is essential for stimulated somatodendritic dopamine release.
Somatodendritic release was characterized in substantia nigra dopamine neurons. Release was induced using a monopolar electrode and measured by recording D2 receptor IPSCs in control mice (RIM control) and in mice with conditional knockout of RIM specifically in dopamine neurons (RIM cKODA). (A, B) Example traces (A) and quantification (B) of IPSCs in RIM control and RIM cKODA mice with vs. without the presence of the D2 receptor antagonist sulpiride, n = 23 cells/6 mice in RIM control, and n = 23/6 in RIM cKODA, significance was calculated by two-way ANOVA and is reported in panel B (RIM control vs. RIM cKODA: F(1) = 57.63, p < 0.001; stimulated response vs. + sulpiride: F(1) = 60.71, p < 0.001), and was followed by Bonferroni post-hoc analysis (RIM control stimulated response vs. RIM control + sulpiride t = 9.70, p < 0.05; RIM cKODA stimulated response vs. RIM cKODA + sulpiride: t = 1.33, p > 0.05; RIM control stimulated response vs. RIM cKODA stimulated response: t = 9.62, p < 0.05). (C, D) Example traces (top) and quantification (bottom) of IPSCs stimulated in RIM control slices (C) or RIM cKODA slices (D) before and after treatment with L-DOPA (10 µM) and subsequent application of sumatriptan (1 µM, to inhibit dopamine release from serotonin terminals), n = 6 cells/6 mice in each group, significance was calculated by repeated measures ANOVA and is reported in panels C and D, (C: F = 10.44, p = 0.01 D: F = 22.75, p < 0.005), and was followed by Tukey’s multiple comparison test (C: baseline vs. L-DOPA p < 0.05, L-DOPA vs. sumatriptan p < 0.05; D: baseline vs. L-DOPA p < 0.05, L-DOPA vs. sumatriptan p < 0.05). Data in B-D are shown as mean ± standard error of mean (SEM) and small circles represent individual cells.
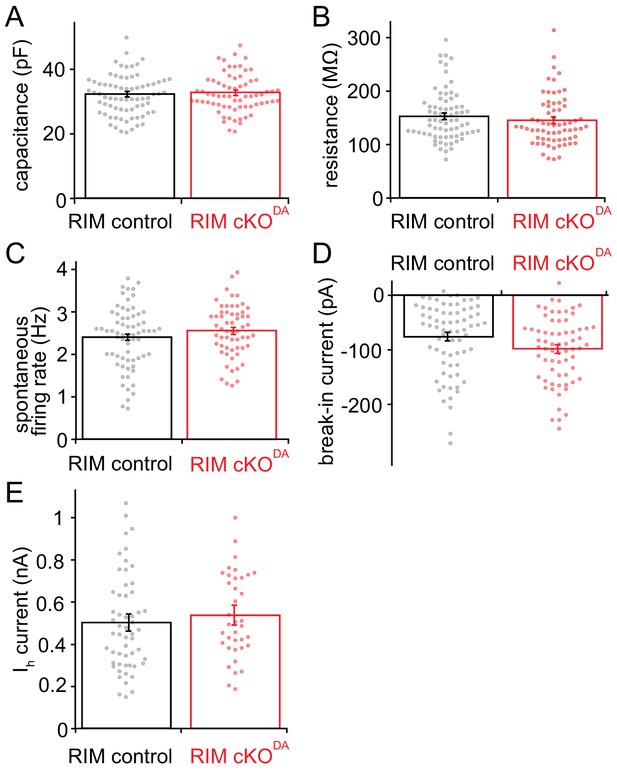
Comparison of dopamine neuron properties in RIM control and RIM cKODA mice.
(A) Cell capacitance measured in RIM control and RIM cKODA slices (Student’s t-test, p = 0.85, RIM control n = 73 cells/12 mice, RIM cKODA n = 68/12). (B) Cell resistance measured in RIM control and RIM cKODA slices (Student’s t-test, p = 0.42, RIM control n = 73/12 and RIM cKODA n = 67/12). (C) The spontaneous firing rates recorded in cell-attached mode from RIM control and RIM cKODA neurons (Student’s t-test, p = 0.17, RIM control n = 67/12 and RIM cKODA n = 59/12). (D) Currents measured upon break-in to whole cell recording mode in dopamine neurons in RIM control and RIM cKODA slices (Student’s t-test, p = 0.12, RIM control n = 71/12 and RIM cKODA n = 67/12). (E) Ih currents of dopamine neurons from RIM control and RIM cKODA mice (Student’s t-test, p = 0.45, RIM control n = 55/12 and RIM cKODA n = 37/9). Data in A-E are shown as mean ± SEM and small circles represent individual cells.
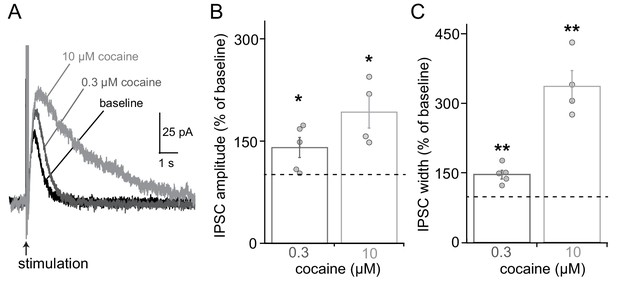
Cocaine enhances the IPSC amplitude and prolongs the IPSC decay.
A characteristic feature of D2-IPSCs is the termination of the signal by uptake of dopamine through the dopamine transporter rather than through diffusion of dopamine. (A) In slices from RIM control animals, the bath application of 300 nM cocaine causes increases in IPSC amplitude and IPSC half-width. A higher concentration of cocaine (10 µM) augments these effects with a larger impact on IPSC half-width. (B, C) Quantification of IPSC amplitude (B) and half width (C) upon application of cocaine. RIM control n = 5 cells/5 mice for 300 nM cocaine and n = 4/4 for 10 µM cocaine. 300 nM cocaine increased the IPSC amplitude to 141% of baseline (indicated by the dotted line, Student’s t-test 300 nM cocaine vs. baseline: t = 2.77, p = 0.05) while in 10 µM cocaine this increase was 192% (Student’s t-test 10 µM cocaine vs baseline: t = 3.94, p < 0.05). The width at half peak amplitude was increased to 147% of baseline in 300 nM cocaine (Student’s t-test 300 nM cocaine vs. baseline: t = 5.31, p < 0.01) and 337% in 10 µM cocaine (Student’s t-test 10 µM cocaine vs. baseline: t = 7.01, p < 0.01). Data in B and C are shown as mean ± SEM and small circles represent individual cells.
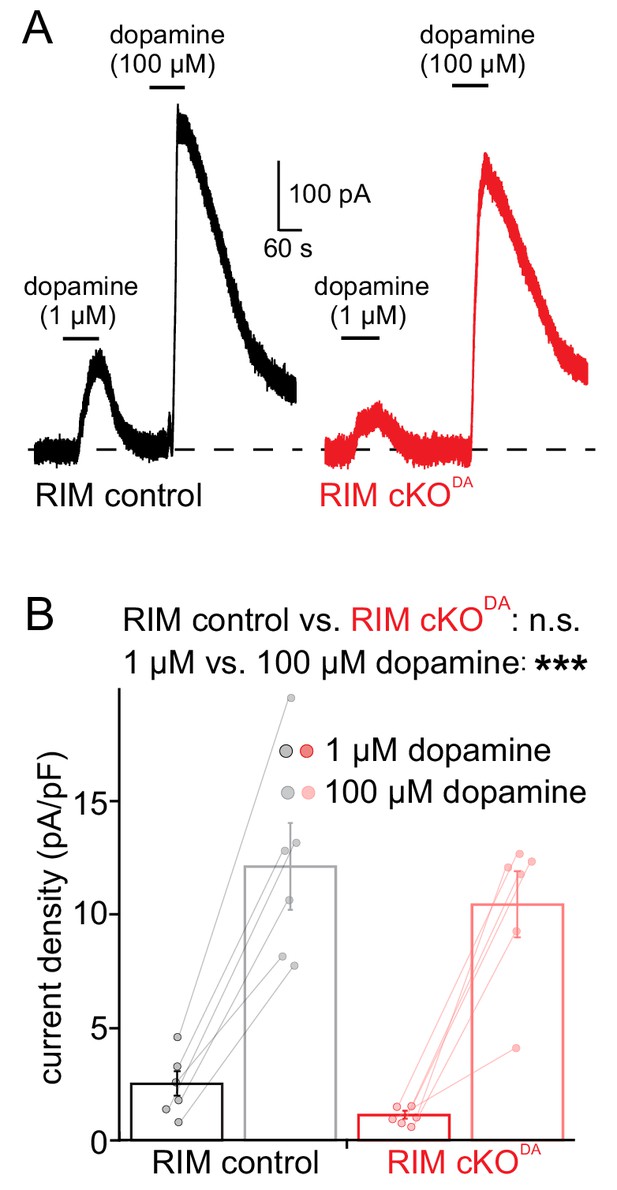
Sensitivity of D2 receptors is not altered by RIM removal.
(A, B) Example traces (A) and quantification (B) of the D2 receptor current densities induced by the bath application of 1 µM and 100 µM dopamine from RIM control (n = 6 cells/6 mice) and RIM cKODA (n = 6/4) mice, analyzed using two-way repeated measures ANOVA (1 µM vs. 100 µM F(1, 10) = 99.08, p < 0.0001; RIM control vs. RIM cKODA F(1, 10) = 1.295, p = 0.28) and followed by Sidak’s multiple comparison test (RIM control 1 µM vs. 100 µM t = 7.16, p < 0.001; RIM cKODA 1 µM vs. 100 µM t = 6.92, p < 0.001). Data in B are shown as mean ± SEM and small circles represent individual cells.
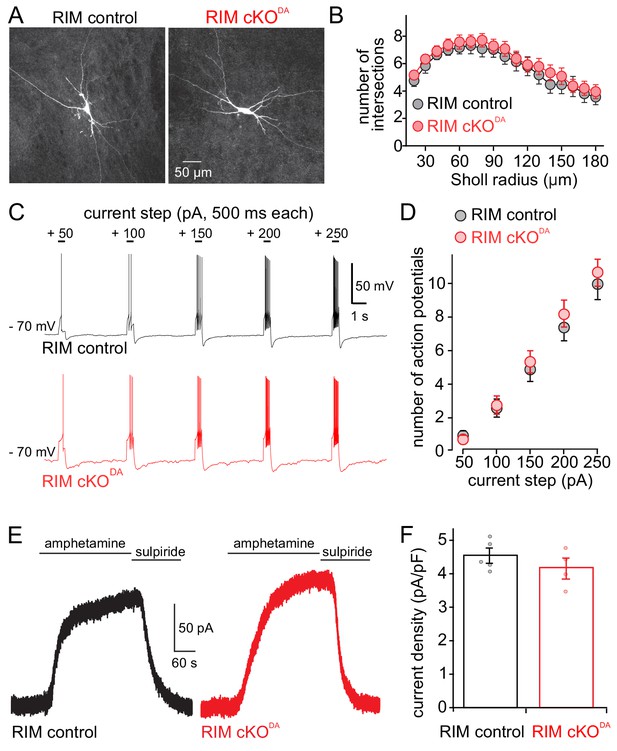
Dopamine neuron shape, excitability and amphetamine induced dopamine release are unaffected by RIM knockout.
(A, B) Example images (A) and Sholl analysis (B) of individual neurobiotin-filled dopamine neurons in RIM control (n = 21 cells/6 animals) and RIM cKODA (n = 24/6) slices. RIM control and RIM cKODA neurons in B were compared using two-way ANOVA (F (1, 43) = 0.53, p = 0.47). (C, D) Neuron excitability was tested by hyperpolarizing cells to − 70 mV and applying progressively larger 500 ms long positive current steps. Example traces (C) and quantification (D) of action potential firing recorded in current clamp are shown. The number of action potentials during each step was quantified in RIM control (n = 27/6) and RIM cKODA (n = 27/6), and then compared using two-way ANOVA (current step size effect F(4, 240) = 71.50, p < 0.0001; RIM control vs. RIM cKODA F(1, 240) = 0.85, p = 0.36). (E, F) Example traces (E) and quantification of current density (F) from RIM control and RIM cKODA mice of D2 receptor currents produced by bath application of amphetamine (10 or 30 µM, which causes the reverse transport of dopamine into the extracellular space); n = 5/5 in RIM control, 4/4 in RIM cKODA, compared by Student’s t-test (t = 1.12, p = 0.30). Data in B, D and F are shown as mean ± SEM and small circles in F represent individual cells.
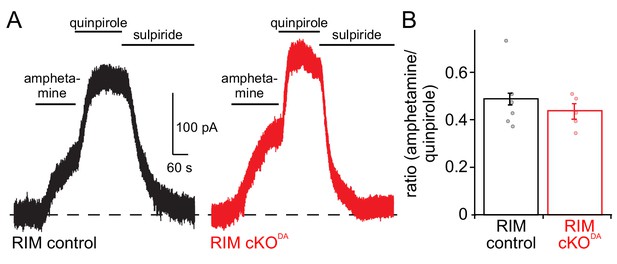
The amphetamine response is not saturated.
(A, B) Example trace (A) and quantification (B) of the ratio between D2 receptor currents induced by the bath application of amphetamine and subsequent application of quinpirole in RIM control (n = 6 cells/4 mice) and RIM cKODA (n = 5/5) mice compared by Student’s t-test (t = 0.82, p = 0.44). Data in B are shown as mean ± SEM and small circles represent individual cells.
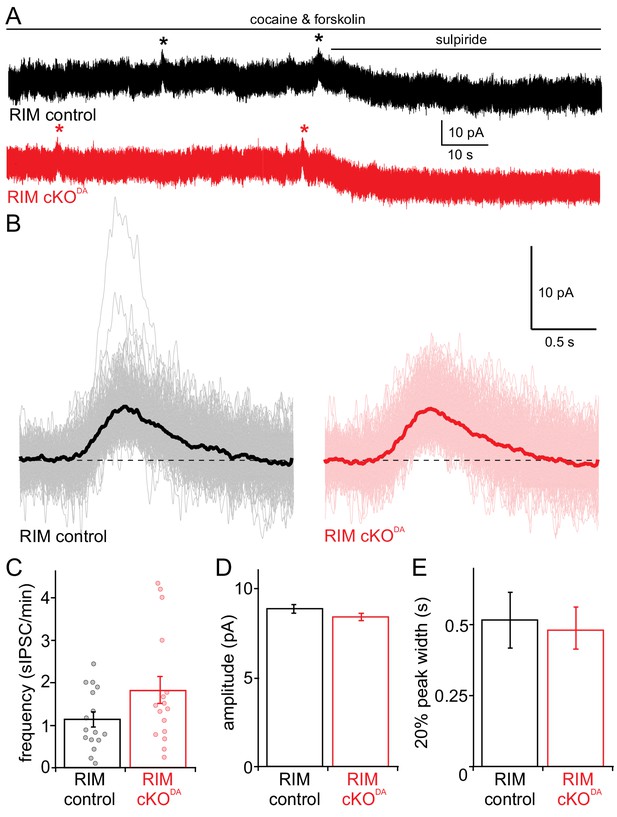
Spontaneous dopamine release is unaffected by RIM knockout.
(A, B) Recording of spontaneous D2 receptor IPSCs (sIPSC) in RIM control and RIM cKODA mice. Example traces (A) and aligned events (B, averages in bold) of D2 receptor IPSCs produced by spontaneous dopamine release from RIM control and RIM cKODA slices in the presence of cocaine (300 nM) and forskolin (1 µM) followed by addition of the D2 receptor antagonist sulpiride (RIM control n = 161 events/16 cells/6 mice, RIM cKODA n = 185/16/6). (C) The frequency of spontaneous IPSCs was quantified per cell in RIM control (n = 16 cells/6 mice) and RIM cKODA (n = 16/6) slices, and the groups were compared by Student’s t-test (t = 1.79, p = 0.09). (D, E) The amplitude (D) and 20% peak width (E) of the spontaneous IPSCs were analyzed and compared between all events of RIM control and RIM cKODA mice using Student’s t-test (n as in B; amplitude: t = 1.32, p = 0.19; half peak width: t = 1.46, p = 0.14). Data in C-E are shown as mean ± SEM and small circles in C represent individual cells.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Genetic reagent (mouse) | B6.SJL-Slc6a3tm1.1(cre)Bkmn/J | Bäckman et al., 2006 | RRID:IMSR_JAX:006660 | |
| Genetic reagent (mouse) | Rims1tm3Sud/J | Kaeser et al., 2008 | RRID:IMSR_JAX:015832 | |
| Genetic reagent (mouse) | Rims2tm1.1Sud/J | Kaeser et al., 2011 | RRID:IMSR_JAX:015833 |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.47972.009





